Guided Self-Assembly of ES-Derived Lung Progenitors into Biomimetic Tube Structures That Impact Cell Differentiation
Abstract
:1. Introduction
2. Material and Methods
2.1. PDMS Device Manufacture
2.2. Scanning Electron Microscopy
2.3. Human Embryonic Stem Cell Culture
2.4. Lung Directed Differentiation
2.5. Extracellular Matrix Screening
2.6. Cell Seeding onto PDMS Architecture
2.7. Immunocytochemistry
2.8. Quantification of Cell Seeding Area and Cell Metrics
2.9. Statistics
2.10. Ethical Considerations
3. Results
3.1. Culture of Progenitor Cells in Replica Moulded PDMS Tubes Reliably Induced Self-Organization into Tube Structures
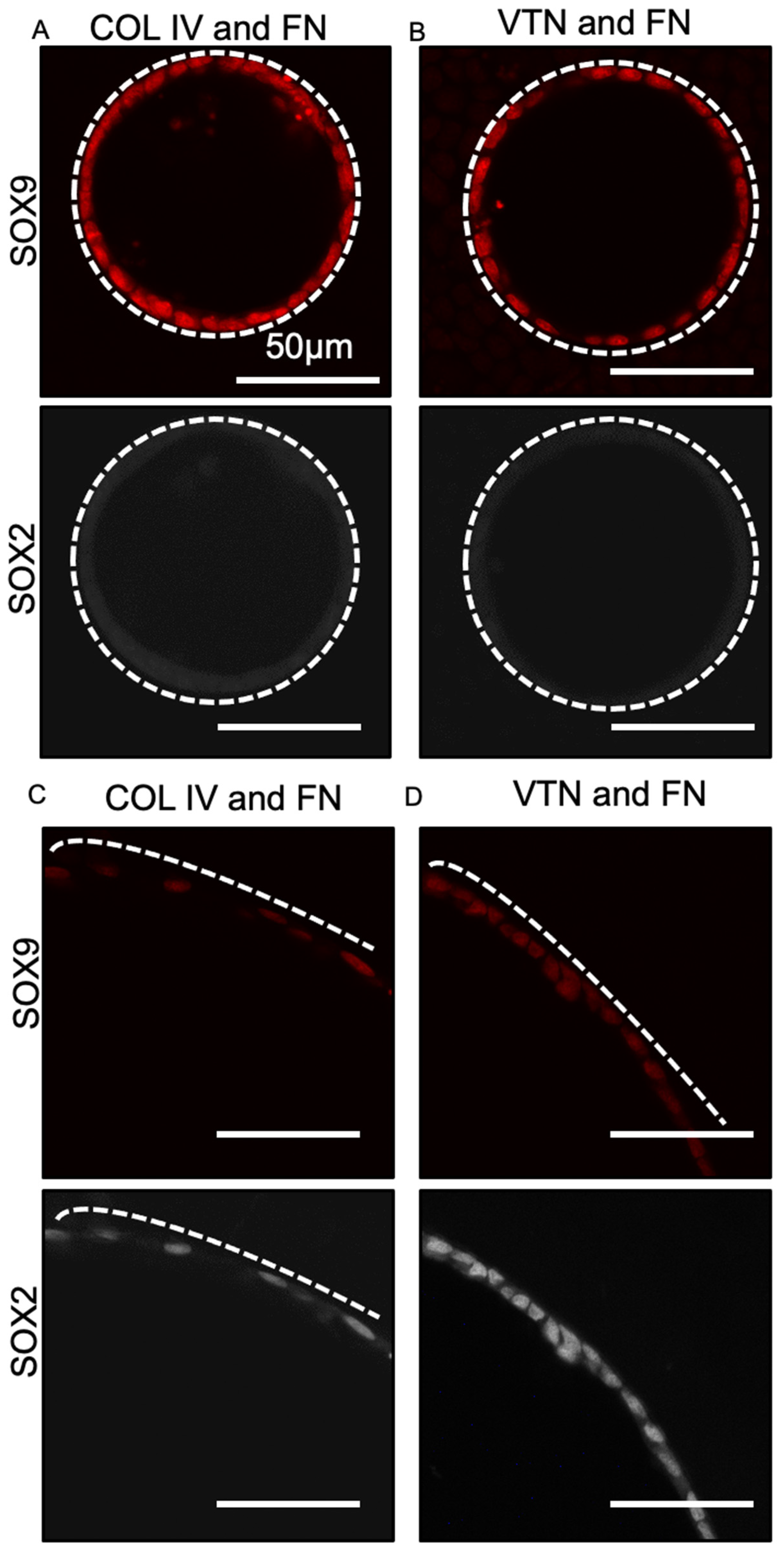
3.2. Epithelial Tube Formation Was Insensitive to Substrate Extracellular Matrix Coating Composition
3.3. Fate Changes in 100 µm Tubes Were Insensitive to Substrate Extracellular Matrix Coating Composition
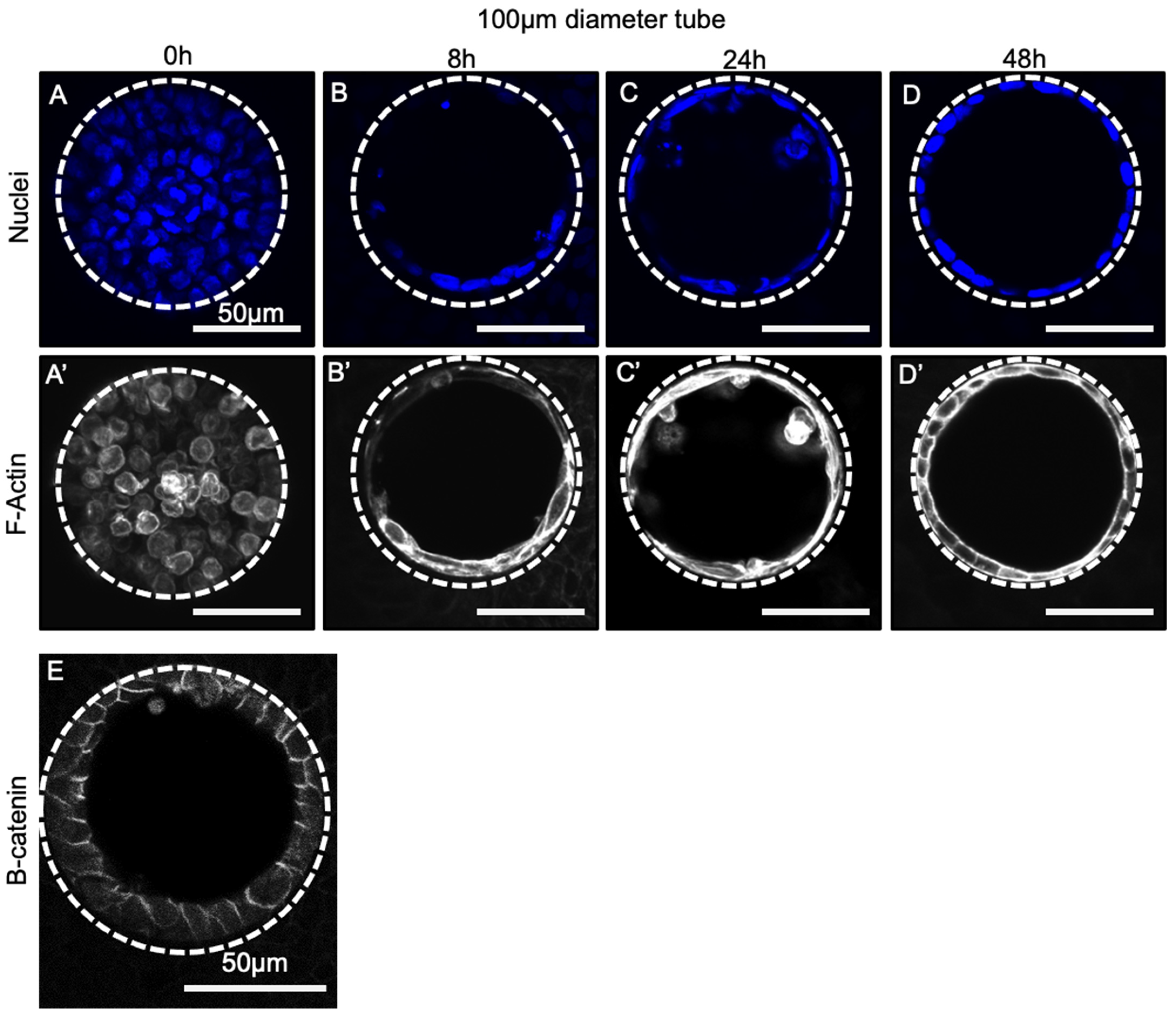
3.4. Progenitor Cells Self-Assemble into a Confluent Polarized Monolayer Lining the Tube Mould within 48 h of Seeding
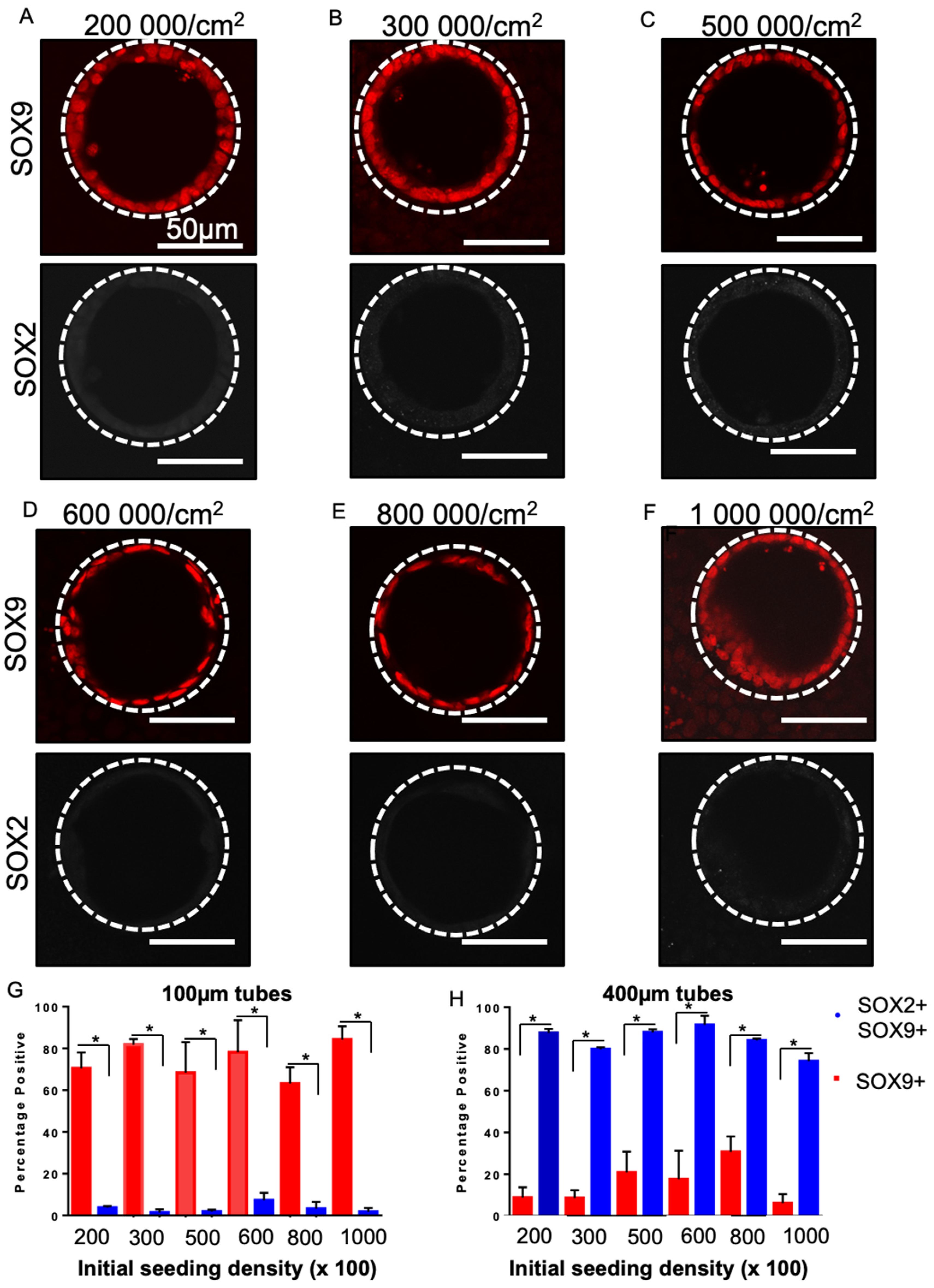
3.5. Cellular Morphology Is Sensitive to Tube Diameter but Not Seeding Density
3.6. SOX2 Status Is Sensitive to Tube Diameter but Not Seeding Density
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nostro, M.C.; Sarangi, F.; Ogawa, S.; Holtzinger, A.; Corneo, B.; Li, X.; Micallef, S.J.; Park, I.H.; Basford, C.; Wheeler, M.B.; et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development 2011, 138, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [Green Version]
- McCauley, K.B.; Hawkins, F.; Serra, M.; Thomas, D.C.; Jacob, A.; Kotton, D.N. Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling. Cell Stem Cell 2017, 20, 844–857.e6. [Google Scholar] [CrossRef] [Green Version]
- Idelson, M.; Alper, R.; Obolensky, A.; Ben-Shushan, E.; Hemo, I.; Yachimovich-Cohen, N.; Khaner, H.; Smith, Y.; Wiser, O.; Gropp, M.; et al. Directed dSifferentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 2009, 5, 396–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.W.; Huang, S.X.; de Carvalho, A.; Ho, S.H.; Islam, M.N.; Volpi, S.; Notarangelo, L.D.; Ciancanelli, M.; Casanova, J.L.; Bhattacharya, J.; et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol. 2017, 19, 542–549. [Google Scholar] [CrossRef]
- Huang, S.X.; Islam, M.N.; O’Neill, J.; Hu, Z.; Yang, Y.G.; Chen, Y.W.; Mumau, M.; Green, M.D.; Vunjak-Novakovic, G.; Bhattacharya, J.; et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 84–91. [Google Scholar] [CrossRef] [Green Version]
- McCauley, K.B.; Alysandratos, K.D.; Jacob, A.; Hawkins, F.; Caballero, I.S.; Vedaie, M.; Yang, W.; Slovik, K.J.; Morley, M.; Carraro, G.; et al. Single-Cell Transcriptomic Profiling of Pluripotent Stem Cell-Derived SCGB3A2+ Airway Epithelium. Stem Cell Rep. 2018, 10, 1579–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.P.; Bear, C.E.; Chin, S.; Pasceri, P.; Thompson, T.O.; Huan, L.J.; Ratjen, F.; Ellis, J.; Rossant, J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat. Biotechnol. 2012, 30, 876–882. [Google Scholar] [CrossRef] [Green Version]
- Longmire, T.A.; Ikonomou, L.; Hawkins, F.; Christodoulou, C.; Cao, Y.; Jean, J.C.; Kwok, L.W.; Mou, H.; Rajagopal, J.; Shen, S.S.; et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 2012, 10, 398–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanagaki, S.; Ikeo, S.; Suezawa, T.; Yamamoto, Y.; Seki, M.; Hirai, T.; Hagiwara, M.; Suzuki, Y.; Gotoh, S. Directed induction of alveolar type I cells derived from pluripotent stem cells via Wnt signaling inhibition. Stem Cells 2021, 39, 156–169. [Google Scholar] [CrossRef]
- Hawkins, F.J.; Suzuki, S.; Beermann, M.L.; Barillà, C.; Wang, R.; Villacorta-Martin, C.; Berical, A.; Jean, J.C.; Le Suer, J.; Matte, T.; et al. Derivation of Airway Basal Stem Cells from Human Pluripotent Stem Cells. Cell Stem Cell 2021, 28, 79–95.e8. [Google Scholar] [CrossRef]
- Metzger, R.J.; Klein, O.D.; Martin, G.R.; Krasnow, M.A. The branching programme of mouse lung development. Nature 2008, 453, 745–750. [Google Scholar] [CrossRef] [Green Version]
- Kitterman, J.A. The effects of mechanical forces on fetal lung growth. Clin. Perinatol. 1996, 23, 727–740. [Google Scholar] [CrossRef]
- Harding, R. Fetal pulmonary development: The role of respiratory movements. Equine Vet. J. Suppl. 1997, 29, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Alanis, D.M.; Chang, D.R.; Akiyama, H.; Krasnow, M.A.; Chen, J. Two nested developmental waves demarcate a compartment boundary in the mouse lung. Nat. Commun. 2014, 5, 3923. [Google Scholar] [CrossRef] [Green Version]
- Que, J.; Choi, M.; Ziel, J.W.; Klingensmith, J.; Hogan, B.L. Morphogenesis of the trachea and esophagus: Current players and new roles for noggin and Bmps. Differentiation 2006, 74, 422–437. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Chu, Q.; Jiang, K.; Li, J.; Tang, N. The Strength of Mechanical Forces Determines the Differentiation of Alveolar Epithelial Cells. Dev. Cell 2018, 44, 297–312.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzou, D.; Spurlin, J.W., III; Pavlovich, A.L.; Stewart, C.R.; Gleghorn, J.P.; Nelson, C.M. Morphogenesis and morphometric scaling of lung airway development follows phylogeny in chicken, quail, and duck embryos. Evodevo 2016, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Przybyla, L.; Lakins, J.N.; Weaver, V.M. Tissue Mechanics Orchestrate Wnt-Dependent Human Embryonic Stem Cell Differentiation. Cell Stem Cell 2016, 19, 462–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaedi, M.; Mendez, J.J.; Bove, P.F.; Sivarapatna, A.; Raredon, M.S.; Niklason, L.E. Alveolar epithelial differentiation of human induced pluripotent stem cells in a rotating bioreactor. Biomaterials 2014, 35, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Wang, Y.; Nayak, P.S.; Dammann, C.E.; Sanchez-Esteban, J. Stretch-induced fetal type II cell differentiation is mediated via ErbB1-ErbB4 interactions. J. Biol. Chem. 2012, 287, 18091–18102. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Huang, Z.; Nayak, P.S.; Matthews, B.D.; Warburton, D.; Shi, W.; Sanchez-Esteban, J. Strain-induced differentiation of fetal type II epithelial cells is mediated via the integrin alpha6beta1-ADAM17/tumor necrosis factor-alpha-converting enzyme (TACE) signaling pathway. J. Biol. Chem. 2013, 288, 25646–25657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, H.; Gjorevski, N.; Inman, J.L.; Bissell, M.J.; Nelson, C.M. Self-organization of engineered epithelial tubules by differential cellular motility. Proc. Natl. Acad. Sci. USA 2009, 106, 14890–14895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjorevski, N.; Nelson, C.M. Endogenous patterns of mechanical stress are required for branching morphogenesis. Integr. Biol. 2010, 2, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Soleas, J.P.; D’Arcangelo, E.; Huang, L.; Karoubi, G.; Nostro, M.C.; McGuigan, A.P.; Waddell, T.K. Assembly of lung progenitors into developmentally-inspired geometry drives differentiation via cellular tension. Biomaterials 2020, 254, 120128. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Qi, J.; Kam, L.C. Microcontact printing of proteins for cell biology. J. Vis. Exp. 2008, 22, 1065. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.X.; Green, M.D.; de Carvalho, A.T.; Mumau, M.; Chen, Y.W.; D’Souza, S.L.; Snoeck, H.W. The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat. Protoc. 2015, 10, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Campiche, M.A.; Gautier, A.; Hernandez, E.I.; Reymond, A. An Electron Microscope Study of the Fetal Development of Human Lung. Pediatrics 1963, 32, 976–994. [Google Scholar] [CrossRef]
- Miller, A.J.; Hill, D.R.; Nagy, M.S.; Aoki, Y.; Dye, B.R.; Chin, A.M.; Huang, S.; Zhu, F.; White, E.S.; Lama, V.; et al. In Vitro Induction and In Vivo Engraftment of Lung Bud Tip Progenitor Cells Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 101–119. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, M.Z.; Caritg, O.; Jeng, Q.; Johnson, J.A.; Sun, D.; Howell, K.J.; Brady, J.L.; Laresgoiti, U.; Allen, G.; Butler, R.; et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife 2017, 6, e26575. [Google Scholar] [CrossRef]
- Nelson, C.M.; Vanduijn, M.M.; Inman, J.L.; Fletcher, D.A.; Bissell, M.J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 2006, 314, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.M.; Inman, J.L.; Bissell, M.J. Three-dimensional lithographically defined organotypic tissue arrays for quantitative analysis of morphogenesis and neoplastic progression. Nat. Protoc. 2008, 3, 674–678. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Richer, E.J.; Huang, T.; Brody, S.L. Growth and differentiation of mouse tracheal epithelial cells: Selection of a proliferative population. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L1315–L1321. [Google Scholar] [CrossRef]
- Soleas, J.P.; Waddell, T.K.; McGuigan, A.P. Topographically grooved gel inserts for aligning epithelial cells during air-liquid-interface culture. Biomater. Sci. 2015, 3, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, M.; Mitrofanova, O.; Broguiere, N.; Geraldo, S.; Dutta, D.; Tabata, Y.; Elci, B.; Brandenberg, N.; Kolotuev, I.; Gjorevski, N.; et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 2020, 585, 574–578. [Google Scholar] [CrossRef]
- Paz, A.C.; Soleas, J.; Poon, J.C.; Trieu, D.; Waddell, T.K.; McGuigan, A.P. Challenges and opportunities for tissue-engineering polarized epithelium. Tissue Eng. Part. B Rev. 2014, 20, 56–72. [Google Scholar] [CrossRef] [PubMed]
- McCracken, K.W.; Cata, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Takasato, M.; Er, P.X.; Becroft, M.; Vanslambrouck, J.M.; Stanley, E.G.; Elefanty, A.G.; Little, M.H. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 2014, 16, 118–126. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Corsini, N.S.; Wolfinger, S.; Gustafson, E.H.; Phillips, A.W.; Burkard, T.R.; Otani, T.; Livesey, F.J.; Knoblich, J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017, 35, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Abdeen, A.A.; Wycislo, K.L.; Fan, T.M.; Kilian, K.A. Interfacial geometry dictates cancer cell tumorigenicity. Nat. Mater. 2016, 15, 856–862. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Loskill, P.; Huebsch, N.; Koo, S.; Svedlund, F.L.; Marks, N.C.; Hua, E.W.; Grigoropoulos, C.P.; Conklin, B.R.; et al. Self-organizing human cardiac microchambers mediated by geometric confinement. Nat. Commun. 2015, 6, 7413. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Si, G.; Huang, J.; Samuel, A.D.T.; Perrimon, N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature 2018, 555, 103–106. [Google Scholar] [CrossRef]
- von Erlach, T.C.; Bertazzo, S.; Wozniak, M.A.; Horejs, C.M.; Maynard, S.A.; Attwood, S.; Robinson, B.K.; Autefage, H.; Kallepitis, C.; Del Rio Hernandez, A.; et al. Cell-geometry-dependent changes in plasma membrane order direct stem cell signalling and fate. Nat. Mater. 2018, 17, 237–242. [Google Scholar] [CrossRef]
- Luca, V.C.; Kim, B.C.; Ge, C.; Kakuda, S.; Wu, D.; Roein-Peikar, M.; Haltiwanger, R.S.; Zhu, C.; Ha, T.; Garcia, K.C. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science 2017, 355, 1320–1324. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleas, J.P.; Huang, L.; D’Arcangelo, E.; Nostro, M.C.; Waddell, T.K.; McGuigan, A.P.; Karoubi, G. Guided Self-Assembly of ES-Derived Lung Progenitors into Biomimetic Tube Structures That Impact Cell Differentiation. Bioengineering 2021, 8, 209. https://doi.org/10.3390/bioengineering8120209
Soleas JP, Huang L, D’Arcangelo E, Nostro MC, Waddell TK, McGuigan AP, Karoubi G. Guided Self-Assembly of ES-Derived Lung Progenitors into Biomimetic Tube Structures That Impact Cell Differentiation. Bioengineering. 2021; 8(12):209. https://doi.org/10.3390/bioengineering8120209
Chicago/Turabian StyleSoleas, John P., Linwen Huang, Elisa D’Arcangelo, Maria Cristina Nostro, Thomas K. Waddell, Alison P. McGuigan, and Golnaz Karoubi. 2021. "Guided Self-Assembly of ES-Derived Lung Progenitors into Biomimetic Tube Structures That Impact Cell Differentiation" Bioengineering 8, no. 12: 209. https://doi.org/10.3390/bioengineering8120209
APA StyleSoleas, J. P., Huang, L., D’Arcangelo, E., Nostro, M. C., Waddell, T. K., McGuigan, A. P., & Karoubi, G. (2021). Guided Self-Assembly of ES-Derived Lung Progenitors into Biomimetic Tube Structures That Impact Cell Differentiation. Bioengineering, 8(12), 209. https://doi.org/10.3390/bioengineering8120209






