Mechanisms Driving Microbial Community Composition in Anaerobic Co-Digestion of Waste-Activated Sewage Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Experimental Setup
2.2. Analytical Procedures
2.3. DNA Extraction and 16S rRNA Gene Amplicon Sequencing
2.4. Data Handling and Analysis
3. Results
3.1. Substrate Characterization, Biogas Production, and Reactor Performance
3.2. Microbial Community Composition
3.2.1. Archaea
3.2.2. Bacteria
4. Discussion
4.1. Substrates Characterization, Biogas Production, and Reactor Performance
4.2. Microbial Community Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Co-AD | Anaerobic co-digestion |
| AD | Anaerobic digestion/-er |
| CL | Canola lecithin |
| FW | Food waste |
| OLR | Organic loading rate |
| ACC | Archaeal community composition |
| BCC | Bacterial community composition |
| MCC | Microbial community composition |
| OTU | Operational taxonomic unit |
| OOI | OTU of interest |
| PWASS | Primary (PS) and waste-activated sludge (WAS) |
| HRT/SRT | Hydraulic retention time/Solids retention time |
| IE | Inhabitant equivalents |
| WWTP | Wastewater treatment plant |
| TS | Total solids |
| VS | Volatile solids |
Appendix A
| PWASS Experiment/Batch | TS | VS | Resulting OLR (kg VS m−3 d−1) | ||||
|---|---|---|---|---|---|---|---|
| (g L−1) | (g L−1) | ||||||
| Control/0% | 10% | 20% | 30% | ||||
| FW | 1 | 24.2 | 16.1 | 0.805 | |||
| 2 | 35.0 | 23.5 | 1.175 | 1.293 | |||
| 3 | 30.6 | 20.6 | 1.030 | 1.133 | 1.236 | ||
| 4 | 37.1 | 26.3 | 1.315 | 1.710 | |||
| CL | 5 | 37.5 | 27.6 | 1.380 | 1.518 | ||
| 6 | 33.4 | 22.6 | 1.130 | 1.243 | 1.356 | ||
| 7 | 36.8 | 22.3 | 1.115 | 1.338 | 1.450 | ||
| 8 | 26.5 | 17.5 | 0.875 | 1.138 | |||
References
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.; Siegrist, H.A.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, W.; Kang, S.; Kim, S.-H. Enhancement of Sewage Sludge Digestion by Co-digestion with Food Waste and Swine Waste. Waste Biomass-Valorization 2019, 11, 2421–2430. [Google Scholar] [CrossRef]
- Aichinger, P.; Wadhawan, T.; Kuprian, M.; Higgins, M.; Ebner, C.; Fimml, C.; Murthy, S.; Wett, B. Synergistic co-digestion of solid-organic-waste and municipal-sewage-sludge: 1 plus 1 equals more than 2 in terms of biogas production and solids reduction. Water Res. 2015, 87, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Björn, A.; Yekta, S.S.; Ziels, R.M.; Gustafsson, K.; Svensson, B.H.; Karlsson, A. Feasibility of OFMSW co-digestion with sewage sludge for increasing biogas production at wastewater treatment plants. Euro-Mediterr. J. Environ. Integr. 2017, 2, 21. [Google Scholar] [CrossRef]
- Wickham, R.; Galway, B.; Bustamante, H.; Nghiem, L.D. Biomethane potential evaluation of co-digestion of sewage sludge and organic wastes. Int. Biodeterior. Biodegrad. 2016, 113, 3–8. [Google Scholar] [CrossRef]
- Mallapaty, S. How China could be carbon neutral by mid-century. Nature 2020, 586, 482–483. [Google Scholar] [CrossRef]
- European Commission; Directorate General for Climate Action. State of the Union 2020: EU Climate Target Plan 2030: Building a Modern, Sustainable and Resilient Europe; European Commission Publications Office: Luxembourg, 2020. [Google Scholar]
- Long, J.H.; Aziz, T.N.; Reyes, F.L.D.L.; Ducoste, J. Anaerobic co-digestion of fat, oil, and grease (FOG): A review of gas production and process limitations. Process. Saf. Environ. Prot. 2012, 90, 231–245. [Google Scholar] [CrossRef]
- Keucken, A.; Habagil, M.; Batstone, D.; Jeppsson, U.; Arnell, M. Anaerobic co-digestion of sludge and organic food waste-performance, inhibition, and impact on the microbial community. Energies 2018, 11, 2325. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Harnisz, M.; Korzeniewska, E.; Koniuszewska, I. Inhibitors of the methane fermentation process with particular emphasis on the microbiological aspect: A review. Energy Sci. Eng. 2020, 8, 1880–1897. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Wang, D.; Chen, F.; Li, X.; Zeng, G.; Yang, Q. Potential impact of salinity on methane production from food waste anaerobic digestion. Waste Manag. 2017, 67, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Xiao, C.; Workie, E.; Zhang, J.; He, Y.; Tong, Y.W. Bioelectrochemical Enhancement of Methanogenic Metabolism in Anaerobic Digestion of Food Waste Under Salt Stress Conditions. ACS Sustain. Chem. Eng. 2021, 9, 13526–13535. [Google Scholar] [CrossRef]
- Misson, G.; Mainardis, M.; Marroni, F.; Peressotti, A.; Goi, D. Environmental methane emissions from seagrass wrack and evaluation of salinity effect on microbial community composition. J. Clean. Prod. 2020, 285, 125426. [Google Scholar] [CrossRef]
- De Vrieze, J.; Christiaens, M.E.; Walraedt, D.; Devooght, A.; Ijaz, U.Z.; Boon, N. Microbial community redundancy in anaerobic digestion drives process recovery after salinity exposure. Water Res. 2017, 111, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, R.H.; McIlroy, S.J.; Kristensen, J.M.; Nierychlo, M.; Karst, S.M.; Dueholm, M.S.; Albertsen, M.; Nielsen, P.H. Identifying the abundant and active microorganisms common to full-scale anaerobic digesters. Microbiology 2017. [Google Scholar] [CrossRef]
- Peces, M.; Astals, S.; Jensen, P.; Clarke, W. Deterministic mechanisms define the long-term anaerobic digestion microbiome and its functionality regardless of the initial microbial community. Water Res. 2018, 141, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, Y.; Chen, S.; Shi, Z.J.; Zhao, M.; Zhu, Z.; Yang, S.; Qu, Y.; Ma, Q.; He, Z.; et al. Microbial functional trait of rRNA operon copy numbers increases with organic levels in anaerobic digesters. ISME J. 2017, 11, 2874–2878. [Google Scholar] [CrossRef]
- Calusinska, M.; Goux, X.; Fossépré, M.; Muller, E.; Wilmes, P.; Delfosse, P. A year of monitoring 20 mesophilic full-scale bioreactors reveals the existence of stable but different core microbiomes in bio-waste and wastewater anaerobic digestion systems. Biotechnol. Biofuels 2018, 11, 1–19. [Google Scholar] [CrossRef]
- Wittebolle, L.; Marzorati, M.; Clement, L.; Balloi, A.; Daffonchio, D.; Heylen, K.; De Vos, P.; Verstraete, W.; Boon, N. Initial community evenness favours functionality under selective stress. Nature 2009, 458, 623–626. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, N.; Huber, J.A.; Vallino, J.J. Microbial Communities Are Well Adapted to Disturbances in Energy Input. mSystems 2016, 1, e00117-16. [Google Scholar] [CrossRef]
- Theuerl, S.; Klang, J.; Prochnow, A. Process Disturbances in Agricultural Biogas Production—Causes, Mechanisms and Effects on the Biogas Microbiome: A Review. Energies 2019, 12, 365. [Google Scholar] [CrossRef]
- Regueiro, L.; Veiga, P.; Figueroa, M.; Alonso-Gutierrez, J.; Stams, A.; Lema, J.; Carballa, M. Relationship between microbial activity and microbial community structure in six full-scale anaerobic digesters. Microbiol. Res. 2012, 167, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Langenheder, S.; Lindström, E.S.; Tranvik, L.J. Structure and Function of Bacterial Communities Emerging from Different Sources under Identical Conditions. Appl. Environ. Microbiol. 2006, 72, 212–220. [Google Scholar] [CrossRef]
- Peter, H.; Beier, S.; Bertilsson, S.; Lindström, E.; Langenheder, S.; Tranvik, L.J. Function-specific response to depletion of microbial diversity. ISME J. 2010, 5, 351–361. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Saunders, A.M.; He, Y.; Fang, J.; Nielsen, P.H.; Verstraete, W.; Boon, N. Ammonia and temperature determine potential clustering in the anaerobic digestion microbiome. Water Res. 2015, 75, 312–323. [Google Scholar] [CrossRef]
- Ju, F.; Lau, F.; Zhang, T. Linking Microbial Community, Environmental Variables, and Methanogenesis in Anaerobic Biogas Digesters of Chemically Enhanced Primary Treatment Sludge. Environ. Sci. Technol. 2017, 51, 3982–3992. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen-Grubb, M.; Dechesne, A.; Proctor, C.; Hammes, F.; Johnson, D.; Quintela-Baluja, M.; Graham, D.; Daffonchio, D.; Fodelianakis, S.; Hahn, N.; et al. A conceptual framework for invasion in microbial communities. ISME J. 2016, 10, 2773–2779. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, N.; Braz, G.H.R.; Regueiro, L.; Lema, J.M.; Carballa, M. Microbial invasions in sludge anaerobic digesters. Appl. Microbiol. Biotechnol. 2020, 105, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.L.; Barney, J.N. Rethinking Biological Invasions as a Metacommunity Problem. Front. Ecol. Evol. 2021, 8, 584701. [Google Scholar] [CrossRef]
- Uhlenhut, F.; Schlüter, K.; Gallert, C. Wet biowaste digestion: ADM1 model improvement by implementation of known genera and activity of propionate oxidizing bacteria. Water Res. 2018, 129, 384–393. [Google Scholar] [CrossRef]
- Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2018.
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Dyksma, S.; Jansen, L.; Gallert, C. Syntrophic acetate oxidation replaces acetoclastic methanogenesis during thermophilic digestion of biowaste. Microbiome 2020, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Vereinigung für Wasserwirtschaft A und A. Merkblatt DWA-Co-Vergärung in Kommunalen Klärschlammfaulbehältern, Abfallvergärungsanlagen und Landwirtschaftlichen Biogasanlagen; DWA-Bundesgeschäftsstelle: Hennef, Germany, 2020. [Google Scholar]
- Dyksma, S.; Gallert, C. Candidatus Syntrophosphaera thermopropionivorans: A novel player in syntrophic propionate oxidation during anaerobic digestion. Environ. Microbiol. Rep. 2019, 11, 558–570. [Google Scholar] [CrossRef]
- Astals, S.; Esteban-Gutiérrez, M.; Arevalo, T.F.; Aymerich, E.; García-Heras, J.; Mata-Alvarez, J. Anaerobic digestion of seven different sewage sludges: A biodegradability and modelling study. Water Res. 2013, 47, 6033–6043. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, L.; Duan, Q.; Hu, G.; Zhang, G. Semi-continuous anaerobic co-digestion of dairy manure with three crop residues for biogas production. Bioresour. Technol. 2014, 156, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, H.; Wang, Z.; Tan, T.; Qin, P. Biogas by Semi-Continuous Anaerobic Digestion of Food Waste. Appl. Biochem. Biotechnol. 2015, 175, 3901–3914. [Google Scholar] [CrossRef]
- Weiterbildender Studiengang Wasser und Umwelt; Deutsche Vereinigung für Wasserwirtschaft, Abwasser und Abfall (Eds.) Abwasserbehandlung: Gewässerbelastung, Bemessungsgrundlagen, Mechanische Verfahren, Biologische Verfahren, Reststoffe aus der Abwasserbehandlung, Kleinkläranlagen; Univ.-Verl.: Weimar, Germany, 2009. [Google Scholar]
- Nasir, I.M.; Ghazi, T.I.; Omar, R.; Idris, A. Batch and semi-continuous biogas production from cattle manure. Int. J. Eng. Technol. 2013, 10, 16–21. [Google Scholar]
- Lovley, D.R.; Phillips, E.J. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl. Environ. Microbiol. 1987, 53, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Cywinska, A. Application of selected chemical compounds to limit the growth of filamentous bacteria in activated sludge. Environ. Prot. Eng. 2007, 33, 221. [Google Scholar]
- Adams, H.; Crump, B.; Kling, G. Metacommunity dynamics of bacteria in an arctic lake: The impact of species sorting and mass effects on bacterial production and biogeography. Front. Microbiol. 2014, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Q.; Ma, Y.; Wang, X.; Peng, X. Dynamics of microbial community in a mesophilic anaerobic digester treating food waste: Relationship between community structure and process stability. Bioresour. Technol. 2015, 189, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Kuchenbuch, A.; Fetzer, I.; Harms, H.; Kleinsteuber, S. Long-term monitoring reveals stable and remarkably similar microbial communities in parallel full-scale biogas reactors digesting energy crops. FEMS Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef] [PubMed]
- Orellana, E.; Davies-Sala, C.; Guerrero, L.D.; Vardé, I.; Altina, M.; Lorenzo, M.C.; Figuerola, E.L.M.; Pontiggia, R.M.; Erijman, L. Microbiome network analysis of co-occurrence patterns in anaerobic co-digestion of sewage sludge and food waste. Water Sci. Technol. 2019, 79, 1956–1965. [Google Scholar] [CrossRef]
- Sundberg, C.; Abu Al-Soud, W.; Larsson, M.; Alm, E.; Yekta, S.S.; Svensson, B.H.; Sørensen, S.; Karlsson, A. 454 pyrosequencing analyses of bacterial and archaeal richness in 21 full-scale biogas digesters. FEMS Microbiol. Ecol. 2013, 85, 612–626. [Google Scholar] [CrossRef]
- Abendroth, C.; Vilanova, C.; Günther, T.; Luschnig, O.; Porcar, M. Eubacteria and archaea communities in seven mesophile anaerobic digester plants in Germany. Biotechnol. Biofuels 2015, 8, 87. [Google Scholar] [CrossRef]
- Shade, A.; Peter, H.; Allison, S.D.; Baho, D.L.; Berga, M.; Buergmann, H.; Huber, D.H.; Langenheder, S.; Lennon, J.T.; Martiny, J.B.H.; et al. Fundamentals of Microbial Community Resistance and Resilience. Front. Microbiol. 2012, 3, 417. [Google Scholar] [CrossRef]
- Lam, T.Y.; Mei, R.; Wu, Z.; Lee, P.K.; Liu, W.-T.; Lee, P.-H. Superior resolution characterisation of microbial diversity in anaerobic digesters using full-length 16S rRNA gene amplicon sequencing. Water Res. 2020, 178, 115815. [Google Scholar] [CrossRef]
- Fenchel, T.; King, G.M.; Blackburn, T.H. Bacterial Metabolism. In Bacterial Biogeochemistry; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–34. [Google Scholar]
- Wormald, R.; Humphreys, P. Hydrogenotrophic methanogenesis dominates at high pH. Access Microbiol. 2019, 1, 169. [Google Scholar] [CrossRef]
- Hunik, J.H.; Hamelers, H.V.M.; Koster, I.W. Growth-rate inhibition of acetoclastic methanogens by ammonia and pH in poultry manure digestion. Biol. Wastes 1990, 32, 285–297. [Google Scholar] [CrossRef]
- Wormald, R.M.; Rout, S.P.; Mayes, W.; Gomes, H.; Humphreys, P.N. Hydrogenotrophic Methanogenesis Under Alkaline Conditions. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Kotsyurbenko, O.R.; Friedrich, M.W.; Simankova, M.V.; Nozhevnikova, A.N.; Golyshin, P.N.; Timmis, K.N.; Conrad, R. Shift from Acetoclastic to H 2 -Dependent Methanogenesis in a West Siberian Peat Bog at Low pH Values and Isolation of an Acidophilic Methanobacterium Strain. Appl. Environ. Microbiol. 2007, 73, 2344–2348. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, Z.S. Species Sorting and Neutral Theory Analyses Reveal Archaeal and Bacterial Communities are Assembled Differently in Hot Springs. Front. Bioeng. Biotechnol. 2020, 8, 464. [Google Scholar] [CrossRef]
- Berga, M.; Székely, A.J.; Langenheder, S. Effects of disturbance intensity and frequency on bacterial community composition and function. PLoS ONE 2012, 7, e36959. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Buckley, H.L.; Etienne, R.S.; Lear, G. Both species sorting and neutral processes drive assembly of bacterial communities in aquatic microcosms. FEMS Microbiol. Ecol. 2013, 86, 288–302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leng, L.; Yang, P.; Singh, S.; Zhuang, H.; Xu, L.; Chen, W.-H.; Dolfing, J.; Li, D.; Zhang, Y.; Zeng, H.; et al. A review on the bioenergetics of anaerobic microbial metabolism close to the thermodynamic limits and its implications for digestion applications. Bioresour. Technol. 2018, 247, 1095–1106. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Dong, B.; Dai, X. Characterizing the sludge moisture distribution during anaerobic digestion process through various approaches. Sci. Total. Environ. 2019, 675, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Puig-Castellví, F.; Cardona, L.; Bouveresse, D.J.-R.; Cordella, C.B.Y.; Mazéas, L.; Rutledge, D.N.; Chapleur, O. Assessment of the microbial interplay during anaerobic co-digestion of wastewater sludge using common components analysis. PLoS ONE 2020, 15, e0232324. [Google Scholar] [CrossRef]
- Björnsson, L.; Hugenholtz, P.; Tyson, G.W.; Blackall, L.L. Filamentous Chloroflexi (green non-sulfur bacteria) are abundant in wastewater treatment processes with biological nutrient removalccThe EMBL accession numbers for the sequences reported in this paper are X84472 (strain SBR1029 16S rDNA), X84474 (strain SBR1031 16S rDNA), X84498 (strain SBR1064 16S rDNA), X84565 (strain SBR2022 16S rDNA), X84576 (strain SBR2037 16S rDNA) and X84607 (strain SBR2076 16S rDNA). Microbiology 2002, 148, 2309–2318. [Google Scholar]
- Holmes, D.E.; Nevin, K.P.; Woodard, T.L.; Peacock, A.D.; Lovley, D.R. Prolixibacter bellariivorans gen. nov., sp. nov., a sugar-fermenting, psychrotolerant anaerobe of the phylum Bacteroidetes, isolated from a marine-sediment fuel cell. Int. J. Syst. Evol. Microbiol. 2007, 57, 701–707. [Google Scholar] [CrossRef]
- Iino, T.; Sakamoto, M.; Ohkuma, M. Prolixibacter denitrificans sp. nov., an iron-corroding, facultatively aerobic, nitrate-reducing bacterium isolated from crude oil, and emended descriptions of the genus Prolixibacter and Prolixibacter bellariivorans. Int. J. Syst. Evol. Microbiol. 2015, 65, 2865–2869. [Google Scholar] [CrossRef]
- Iino, T.; Mori, K.; Itoh, T.; Kudo, T.; Suzuki, K.-I.; Ohkuma, M. Description of Mariniphaga anaerophila gen. nov., sp. nov., a facultatively aerobic marine bacterium isolated from tidal flat sediment, reclassification of the Draconibacteriaceae as a later heterotypic synonym of the Prolixibacteraceae and description of the family Marinifilaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 3660–3667. [Google Scholar] [CrossRef]
- Chen, S.; Dong, X. Proteiniphilum acetatigenes gen. nov., sp. nov., from a UASB reactor treating brewery wastewater. Int. J. Syst. Evol. Microbiol. 2005, 55, 2257–2261. [Google Scholar] [CrossRef]
- Hahnke, S.; Langer, T.; Koeck, D.E.; Klocke, M. Description of Proteiniphilum saccharofermentans sp. nov., Petrimonas mucosa sp. nov. and Fermentimonas caenicola gen. nov., sp. nov., isolated from mesophilic laboratory-scale biogas reactors, and emended description of the genus Proteiniphilum. Int. J. Syst. Evol. Microbiol. 2016, 66, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Whitman, W.B.; Parte, A.C. Bergey’s Manual of Systematic Bacteriology; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Scheff, G.; Salcher, O.; Lingens, F. Trichococcus flocculiformis gen. nov. sp. nov. A new gram-positive filamentous bacterium isolated from bulking sludge. Appl. Microbiol. Biotechnol. 1984, 19, 114–119. [Google Scholar] [CrossRef]
- Strepis, N.; Naranjo, H.D.; Meier-Kolthoff, J.; Göker, M.; Shapiro, N.; Kyrpides, N.; Klenk, H.-P.; Schaap, P.J.; Stams, A.J.M.; Sousa, D.Z. Genome-guided analysis allows the identification of novel physiological traits in Trichococcus species. BMC Genom. 2020, 21, 24. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Zielińska, M. Bacterial communities in full-scale wastewater treatment systems. World J. Microbiol. Biotechnol. 2016, 32, 66. [Google Scholar] [CrossRef] [PubMed]

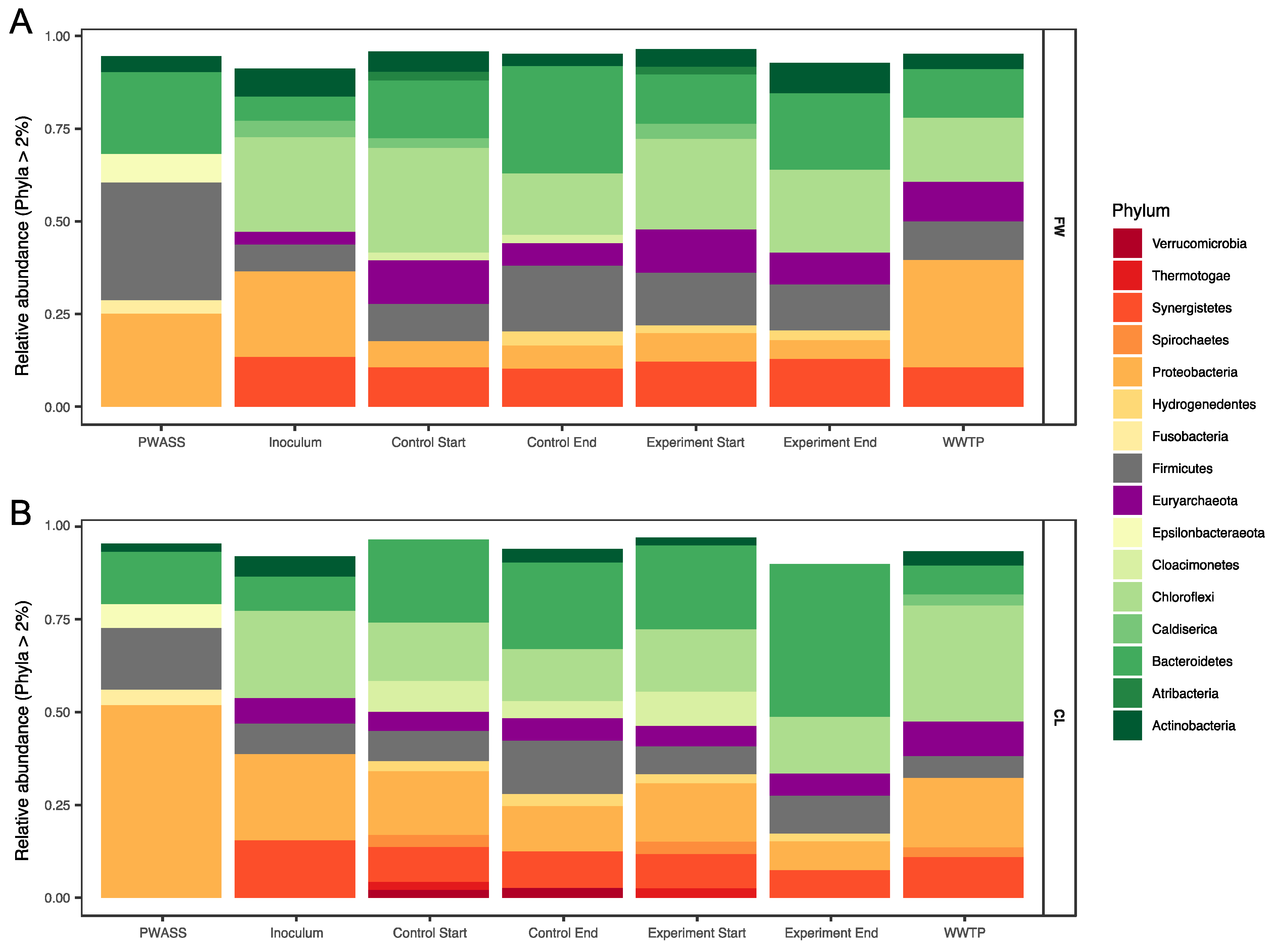

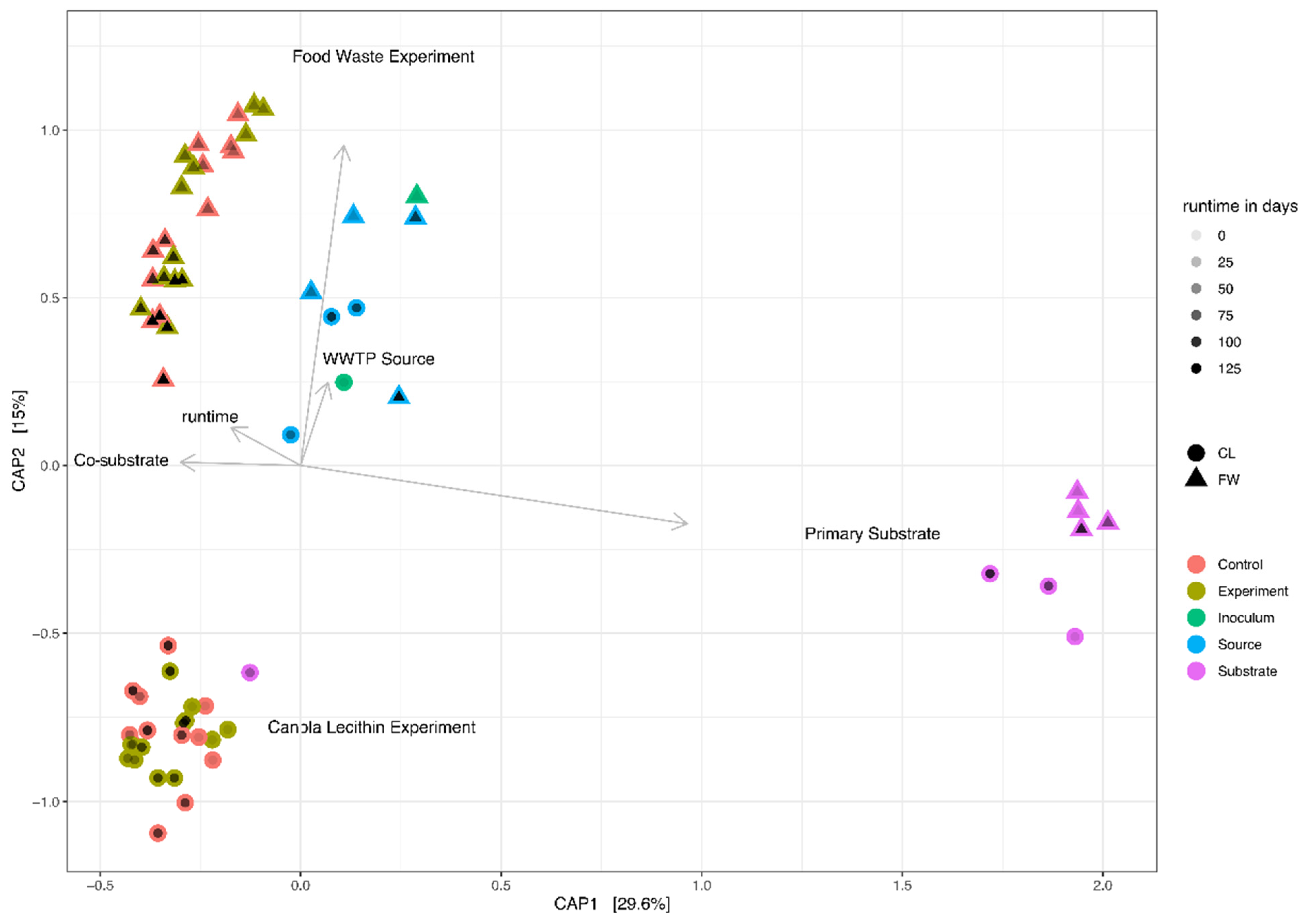
| PWASS a | Food Waste (FW) | Canola Lecithin (CL) | WWTP b | |
|---|---|---|---|---|
| Total solids (g L−1) | 33.3 ± 0.5 | 221.3 | 464.1 | 41.3 ± 10.9 |
| Volatile solids (g L−1) | 21.6 ± 0.4 | 197.7 | 463.4 | 23.8 ± 5.6 |
| Raw protein c | 40.00% | 15.68% | <0.65% | |
| Raw fat A/B c | 11.43% | 18.21% | 1.29% d,e | |
| Raw fiber c | 5.71% | 1.52% | 1.08% | |
| ADL c | 5.71% | 2.02% | <1.9% | |
| Carbohydrates f | 37.15% | 62.57% | n.a. | |
| Total-N (mg L−1) | 584.67 | 4210.0 | 271.0 | |
| COD (g L−1) | 31.23 | 256.75 | 529.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeske, J.T.; Gallert, C. Mechanisms Driving Microbial Community Composition in Anaerobic Co-Digestion of Waste-Activated Sewage Sludge. Bioengineering 2021, 8, 197. https://doi.org/10.3390/bioengineering8120197
Jeske JT, Gallert C. Mechanisms Driving Microbial Community Composition in Anaerobic Co-Digestion of Waste-Activated Sewage Sludge. Bioengineering. 2021; 8(12):197. https://doi.org/10.3390/bioengineering8120197
Chicago/Turabian StyleJeske, Jan Torsten, and Claudia Gallert. 2021. "Mechanisms Driving Microbial Community Composition in Anaerobic Co-Digestion of Waste-Activated Sewage Sludge" Bioengineering 8, no. 12: 197. https://doi.org/10.3390/bioengineering8120197
APA StyleJeske, J. T., & Gallert, C. (2021). Mechanisms Driving Microbial Community Composition in Anaerobic Co-Digestion of Waste-Activated Sewage Sludge. Bioengineering, 8(12), 197. https://doi.org/10.3390/bioengineering8120197






