Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 by a Recombinant Cupriavidusnecator
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of Plasmids and Strains
2.2. Culture Medium and Condition
2.3. Analyses
3. Result
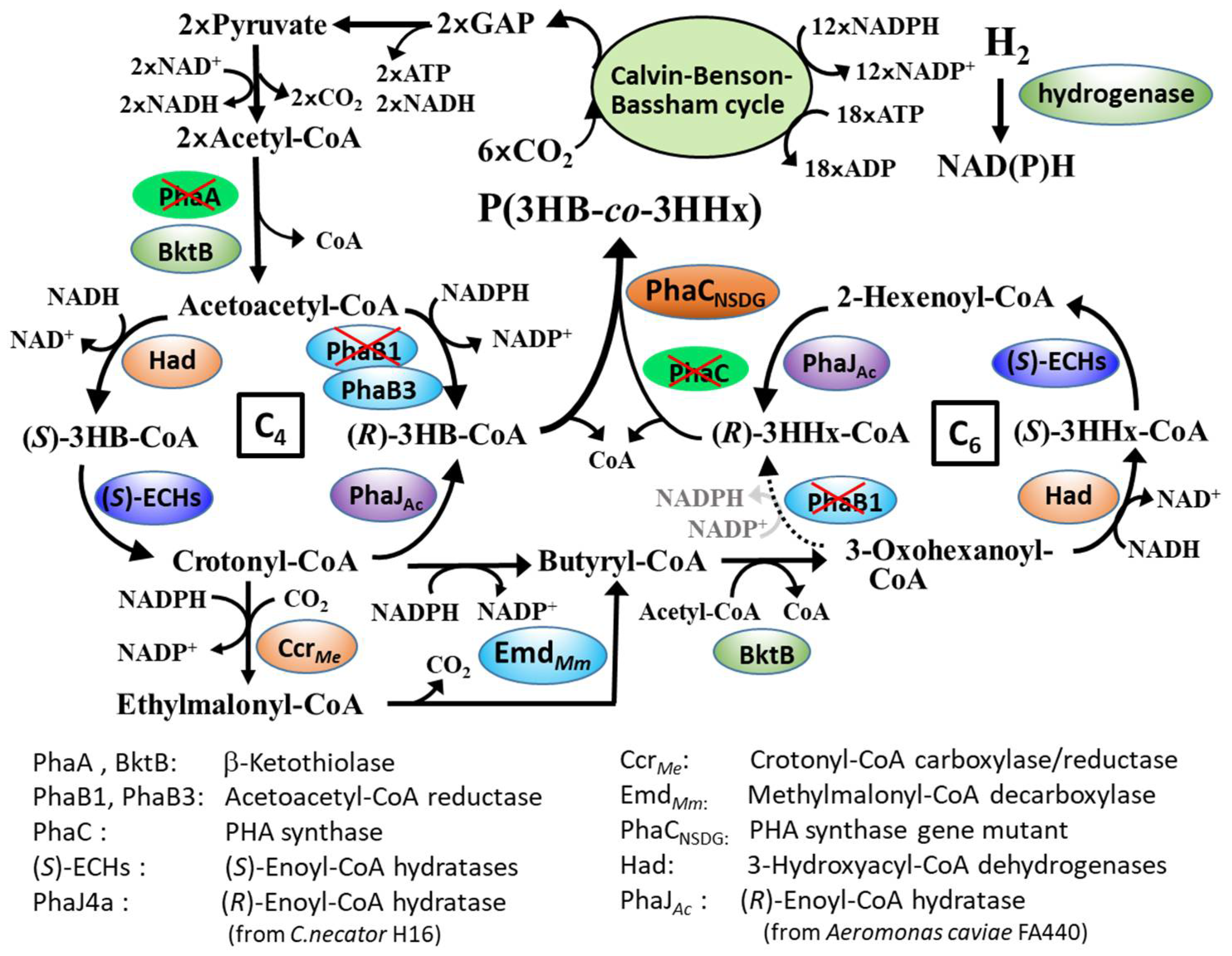
3.1. Autotrophic PHA Synthesis by C. necator MF01/pBPP-ccrMeJ4a-emd and MF01ΔB1/pBPP-ccrMeJ4a-emd
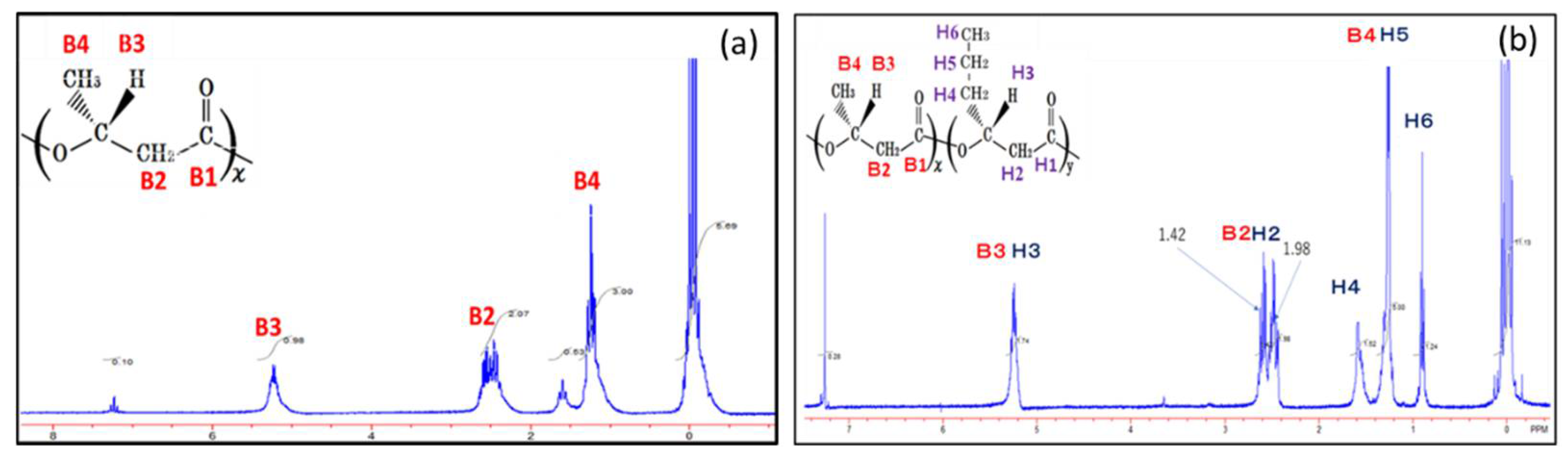
3.2. NMR Analysis of PHBHHx Synthesized by the Autotrophic Condition
3.3. Autotrophic PHA Synthesis by C. necator MF01/pBPP-ccrMeJAc-emd and MF01ΔB1/pBPP-ccrMeJAc-emd
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, K.Y.; Baidura, S. Renewable biomass feedstocks for production of sustainable biodegradable polymer. Curr. Opin. Green Sustain. Chem. 2021, 27, 100412. [Google Scholar] [CrossRef]
- Barron, A.; Taylor, D.S. Commercial Marine-Degradable Polymers for Flexible Packaging. Iscience 2020, 23, 101353. [Google Scholar] [CrossRef] [PubMed]
- Hazer, B.; Steinbüchel, A. Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl. Microbiol. Biotechnol. 2007, 74, 1–12. Available online: https://link.springer.com/article/10.1007%2Fs00253-006-0732-8 (accessed on 12 June 2021). [CrossRef]
- Lu, J.; Tappel, R.C.; Nomura, C.T. Mini-Review: Biosynthesis of Poly(hydroxyalkanoates). Polym. Rev. 2009, 49, 226–248. [Google Scholar] [CrossRef]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; Serio, M.D. In Vivo and Post-Synthesis Strategies to Enhance the Properties of PHB-Based Materials: A Review. Front. Bioeng. Biotechnol. 2021, 8, 619266. [Google Scholar] [CrossRef] [PubMed]
- Sashiwa, H.; Fukuda, R.; Okura, T.; Sato, S.; Nakayama, A. Microbial Degradation Behavior in Seawater of Polyester Blends Containing Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx). Mar. Drugs 2018, 16, 34. [Google Scholar] [CrossRef]
- Kaneka Corporation News Release. Completion of Kaneka Biodegradable Polymer PHBH™ Plant with Annual Production of 5000 Tons. 19 December 2019. Available online: https://www.kaneka.co.jp/en/topics/news/nr20191219/ (accessed on 20 August 2021).
- Shimamura, E.; Kasuya, K.; Kobayashi, G.; Shiotani, T.; Shima, Y.; Doi, Y. Physical Properties and Biodegradability of Microbial Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 1994, 27, 878–880. [Google Scholar] [CrossRef]
- Doi, Y.; Kitamura, S.; Abe, H. Microbial Synthesis and Characteriza-tion of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 1995, 28, 4822–4828. [Google Scholar] [CrossRef]
- Boey, J.Y.; Mohamad, L.; Khok, Y.S.; Tay, G.S.; Baidurah, S. A Review of the Applications and Biodegradation of Polyhydroxyalkanoates and Poly(lactic acid) and Its Composites. Polymers 2021, 13, 1544. [Google Scholar] [CrossRef]
- Insomphun, C.; Xie, H.; Mifune, J.; Kawashima, Y.; Orita, I.; Nakamura, S.; Fukui, T. Improved artificial pathway for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) with high C6-monomer composition from fructose in Ralstonia eutropha. Metab. Eng. 2015, 27, 38–45. [Google Scholar] [CrossRef]
- Matassa, S.; Boon, N.; Verstraete, W. Resource recovery from used water: The manufacturing abilities of hydrogen-oxidizing bacteria. Water Res. 2015, 68, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Barashkov, V.A. Characteristics of proteins synthesized by hydrogen-oxidizing microorganisms. Appl. Biochem. Microbiol. 2010, 46, 574–579. Available online: https://www.frontiersin.org/articles/10.3389/fbioe.2020.619266/full (accessed on 12 June 2021). [CrossRef]
- Ishizaki, A.; Tanaka, K. Production of poly-β-hydroxybutyric acid from carbon dioxide by Alcaligenes eutrophus ATCC 17697T. J. Ferment. Bioeng. 1991, 71, 254–257. [Google Scholar] [CrossRef]
- Tanaka, K.; Ishizaki, A.; Kanamaru, T.; Kawano, T. Production of poly(D-3-hydroxybutyrate) from CO2, H2, and CO2 by high cell density autotrophic cultivation of Alcaligenes eutrophus. Biotechnol. Bioeng. 1995, 45, 268–275. Available online: https://onlinelibrary.wiley.com/doi/10.1002/bit.260450312 (accessed on 12 June 2021). [CrossRef]
- Taga, N.; Tanaka, K.; Ishizaki, A. Effects of rheological change by addition of carboxymethylcellulose in culture media of an air-lift fermentor on poly-D-3-hydroxybutyric acid productivity in autotrophic culture of hydrogen-oxidizing bacterium, Alcaligenes eutrophus. Biotechnol. Bioeng. 1997, 53, 529–533. [Google Scholar] [CrossRef]
- Sugimoto, T.; Tsuge, T.; Tanaka, K.; Ishizaki, A. Control of acetic acid concentration by pH-stat continuous substrate feeding in heterotrophic culture phase of two-stage cultivation of Alcaligenes eutrophus for production of P(3HB) from CO2, H2 and O2 under non-explosive condition. Biotechnol. Bioeng. 1999, 62, 625–631. Available online: https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1097-0290(19990320)62:6%3C625::AID-BIT1%3E3.0.CO;2-D (accessed on 15 June 2021). [CrossRef]
- Ishizaki, A.; Tanaka, K.; Taga, N. Microbial production of poly-D-3-hydroxybutyrate from CO2. Appl. Microbiol. Biotechnol. 2001, 57, 6–12. Available online: https://link.springer.com/article/10.1007%2Fs002530100775 (accessed on 15 June 2021). [PubMed]
- Mifune, J.; Nakamura, S.; Fukui, T. Engineering of pha operon on Cupriavidus necator chromosome for efficient biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from vegetable oil. Polym. Degrad. Stab. 2010, 95, 1305–1312. [Google Scholar] [CrossRef]
- Zhang, M.; Kurita, S.; Orita, I.; Nakamura, S.; Fukui, T. Modification of acetoacetyl-CoA reduction step in Ralstonia eutropha for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from structurally unrelated compounds. Microb. Cell Fact. 2019, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, T.; Watanabe, S.; Shimada, D.; Abe, H.; Doi, Y.; Taguchi, S. Combination of N149S and D171G mutations in Aeromonas caviae polyhydroxyalkanoate synthase and impact on polyhydroxyalkanoate biosynthesis. FEMS Microbiol. Lett. 2007, 277, 217–222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawashima, Y.; Cheng, W.; Mifune, J.; Orita, I.; Nakamura, S.; Fukui, T. Characterization and functional analyses of R-specific enoyl coenzyme A hydratases in polyhydroxyalkanoate-producing Ralstonia eutropha. Appl. Environ. Microbiol. 2012, 78, 493–502. Available online: http://refhub.elsevier.com/S1096-7176(14)00128-1/sbref20 (accessed on 15 June 2021). [CrossRef] [PubMed]
- Fukui, T.; Ohsawa, K.; Mifune, J.; Orita, I.; Nakamura, S. Evaluation of promoters for gene expression in polyhydroxyalkanoate-producing Cupriavidus necator H16. Appl. Microbiol. Biotechnol. 2011, 89, 1527–1536. Available online: http://refhub.elsevier.com/S1096-7176(14)00128-1/sbref13 (accessed on 15 June 2021). [CrossRef] [PubMed]
- Kato, M.; Bao, H.J.; Kang, C.-K.; Fukui, T.; Doi, Y. Production of a novel copolyester of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids by Pseudomonas sp. 61-3 from sugars. Appl. Microbiol. Biotechnol. 1996, 45, 363–370. Available online: https://link.springer.com/article/10.1007/s002530050697 (accessed on 10 August 2021). [CrossRef]
- Volova, T.G.; Kiselev, E.G.; Shishatskaya, E.I.; Zhila, E.I.; Boyandin, A.N.; Syrvacheva, D.A.; Vinogradova, O.N.; Kalacheva, G.S.; Vasiliev, A.D.; Peterson, I.V. Cell growth and accumulation of polyhydroxyalkanoates from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 2013, 146, 215–222. [Google Scholar] [CrossRef]
- Heinrich, D.; Raberg, M.; Steinbüchel, A. Synthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from unrelated carbon sources in engineered Rhodospirillum rubrum. FEMS Microbiol. Lett. 2015, 362, fnv038. [Google Scholar] [CrossRef]
- Nangle, S.N.; Ziesack, M.; Buckley, S.; Trivedi, D.; Loh, D.M.; Nocera, D.G.; Silvera, P.A. Valorization of CO2 through lithoautotrophic production of sustainable chemicals in Cupriavidus necator. Metab. Eng. 2020, 62, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, Y.; Yamamoto, M.; Thorbecke, R.; Mizuno, S.; Tsuge, T. Autotrophic biosynthesis of polyhydroxyalkanoate by Ralstonia eutropha from non-combustible gas mixture with low hydrogen content. Biotechnol. Lett. 2020, 42, 1655–1662. Available online: https://link.springer.com/article/10.1007/s10529-020-02876-3 (accessed on 10 August 2021). [CrossRef]
- Tarawat, S.; Incharoensakdi, A.; Monshupanee, T. Cyanobacterial production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from carbon dioxide or a single organic substrate: Improved polymer elongation with an extremely high 3-hydroxyvalerate mole proportion. J. Appl. Phycol. 2020, 32, 1095–1102. [Google Scholar] [CrossRef]
- Taepucharoen, K.; Tarawat, S.; Puangcharoen, M.; Incharoensakdi, A.; Monshupanee, T. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) under photoautotrophy and heterotrophy by non-heterocystous N2-fixing cyanobacterium. Bioresour. Technol. 2017, 239, 523–527. [Google Scholar] [CrossRef]
- Bowien, B.; Kusian, B. Genetics and control of CO2 assimilation in the chemoautotroph Ralstonia eutropha. Arch. Microbiol. 2002, 178, 85–93. [Google Scholar] [CrossRef]
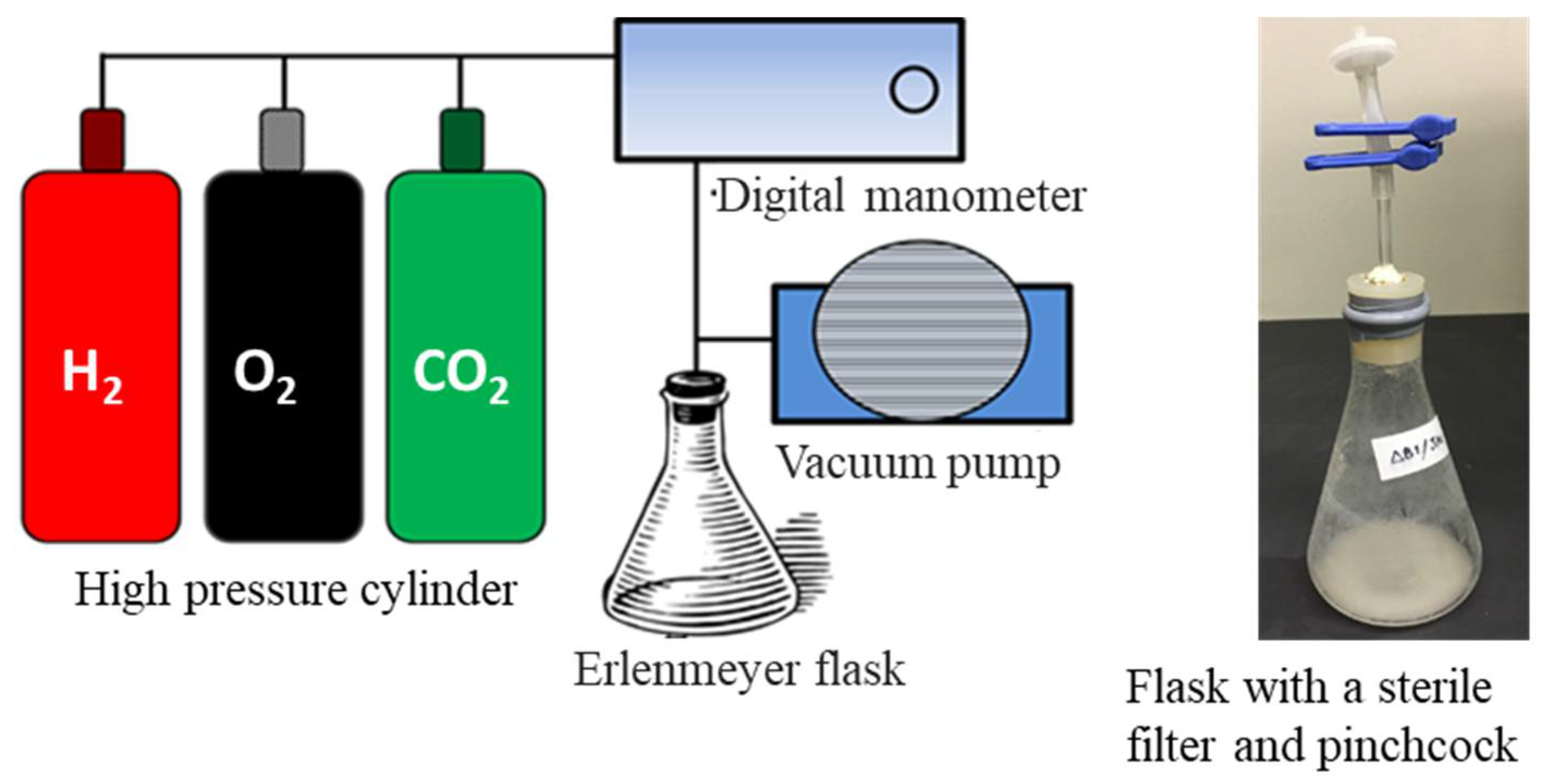
| Bacterial Strain or Plasmids | Genotype/Characteristic | References or Sources |
|---|---|---|
| C. necator | ||
| H16 | Wild type | DSM 428 |
| MF01 | H16 derivative, ∆phaC::phaCNSDG, ∆phaA::bktB | [19] |
| MF01ΔB1 | MF01 derivative; ΔphaB1 | [11] |
| Plasmids | ||
| pBPP | pBBR1-MCS2 derivative, PphaP1, TrrnB, | [23] |
| pBPP-ccrMeJ4a-emd | pBPP derivative, ccrMe, phaJ4a, emdMm | [11] |
| pBPP-ccrMeJAc-emd | pBPP derivative, ccrMe, phaJAc, emdMm | [20] |
| Recombinant (Strains/Plasmid Vector) | (NH4)2SO4 (g/L) | DCM (g/L) | PHBHHx Content (wt%) | Monomer Composition (mol%) | |
|---|---|---|---|---|---|
| 3HB | 3HHx | ||||
| MF01/pBPP-ccrMeJ4a-emd | 0.5 | 8.52 ± 1.92 | 85.8 ± 13.2 | 96.7 ± 1.4 | 3.3 ± 1.4 |
| MF01/pBPP-ccrMeJ4a-emd | 1.0 | 12.18 ± 0.40 | 64.0 ± 3.4 | 94.8 ± 1.1 | 5.3 ± 1.1 |
| MF01/pBPP-ccrMeJ4a-emd | 2.0 | 8.01 ± 1.65 | 37.4 ± 2.4 | 97.7 ± 0.8 | 2.4 ± 0.8 |
| MF01ΔB1/pBPP-ccrMeJ4a-emd | 0.5 | 4.35 ± 0.78 | 59.0 ± 16.2 | 56.5 ± 1.7 | 43.5 ± 1.7 |
| MF01ΔB1/pBPP-ccrMeJ4a-emd | 1.0 | 10.65 ± 1.35 | 61.7 ± 4.6 | 52.3 ± 6.2 | 47.7 ± 6.2 |
| MF01ΔB1/pBPP-ccrMeJ4a-emd | 2.0 | 6.99 ± 1.14 | 21.1 ± 0.5 | 71.6 ± 1.9 | 28.5 ± 1.9 |
| Recombinant (Strains/Plasmid) | DCM (g/L) | PHBHHx Content (wt%) | 3HHx (mol%) |
|---|---|---|---|
| MF01/pBPP-ccrMeJ4a-emd a | 1.76 ± 0.02 | 48.5 ± 0.4 | 6.4 ± 0.13 |
| MF01/pBPP-ccrMeJAc-emd | 1.81 ± 0.03 | 54.0 ± 3.1 | 10.4 ± 0.2 |
| MF01ΔB1/pBPP-ccrMeJ4a-emd a | 1.57 ± 0.02 | 47.9 ± 2.0 | 22.2 ± 1.2 |
| MF01ΔB1/pBPP-ccrMeJAc-emd | 1.72 ± 0.01 | 54.1 ± 2.1 | 14.0 ± 0.6 |
| Recombinant (Strains/Plasmid Vector) | (NH4)2 SO4 (g/L) | DCM (g/L) | PHBHHx Content (wt%) | Monomer (mol%) | |
|---|---|---|---|---|---|
| 3HB | 3HHx | ||||
| MF01/pBPP-ccrMeJAc-emd | 0.5 | 7.25 ± 0.57 | 76.2 ± 0.0 | 94.0 ± 1.3 | 6.0 ± 1.3 |
| MF01/pBPP-ccrMeJAc-emd | 1.0 | 11.22 ± 2.67 | 64.6 ± 8.1 | 88.7 ± 6.4 | 11.3 ± 6.4 |
| MF01/pBPP-ccrMeJAc-emd | 2.0 | 8.46 ± 0.42 | 19.9 ± 1.7 | 88.6 ± 0.9 | 11.5 ± 0.9 |
| MF01ΔB1/pBPP-ccrMeJAc-emd | 0.5 | 6.93 ± 0.36 | 74.6 ± 2.2 | 86.6 ± 1.0 | 14.0 ± 1.3 |
| MF01ΔB1/pBPP-ccrMeJAc-emd | 1.0 | 8.52 ± 1.00 | 67.8 ± 1.8 | 87.1 ± 2.3 | 11.1 ± 1.3 |
| MF01ΔB1/pBPP-ccrMeJAc-emd | 2.0 | 6.03 ± 0.78 | 39.1 ± 1.3 | 90.4 ± 0.7 | 9.6 ± 0.7 |
| Scheme. | Nutritional Condition | PHA Production | PHA Composition (mol%) | Ref. |
|---|---|---|---|---|
| C. necator H 16 a | H2/CO2/air = 30:15:balance | - | Various type of PHAs with C4-C14 monomers | [27] |
| C. necator H 16 b | H2/O2/CO2/N2 = 3.6: 7.6: 12.3: 76.5 (non-combustible gas) | DCM0.14 ± 0.05 g/L (PHA content 57 ± 10 wt%) | 3HB-based copolymer with 1.2 % 3HV c and 1.2% 3H4MV d | [28] |
| Anabaena spiroides TISTR 8075 | CO2 + light | PHBV productivity 0.5 mg gdw−1day−1 | PHBV (3HV 43.2%) | [29] |
| Oscillatoria okeni TISTR 8549 | CO2 + light | PHBV concentration 108 mg/L | PHBV (3HV 5.5%) | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, K.; Yoshida, K.; Orita, I.; Fukui, T. Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 by a Recombinant Cupriavidusnecator. Bioengineering 2021, 8, 179. https://doi.org/10.3390/bioengineering8110179
Tanaka K, Yoshida K, Orita I, Fukui T. Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 by a Recombinant Cupriavidusnecator. Bioengineering. 2021; 8(11):179. https://doi.org/10.3390/bioengineering8110179
Chicago/Turabian StyleTanaka, Kenji, Kazumasa Yoshida, Izumi Orita, and Toshiaki Fukui. 2021. "Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 by a Recombinant Cupriavidusnecator" Bioengineering 8, no. 11: 179. https://doi.org/10.3390/bioengineering8110179
APA StyleTanaka, K., Yoshida, K., Orita, I., & Fukui, T. (2021). Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 by a Recombinant Cupriavidusnecator. Bioengineering, 8(11), 179. https://doi.org/10.3390/bioengineering8110179






