Skin Manifestations after Ionizing Radiation Exposure: A Systematic Review
Abstract
1. Introduction
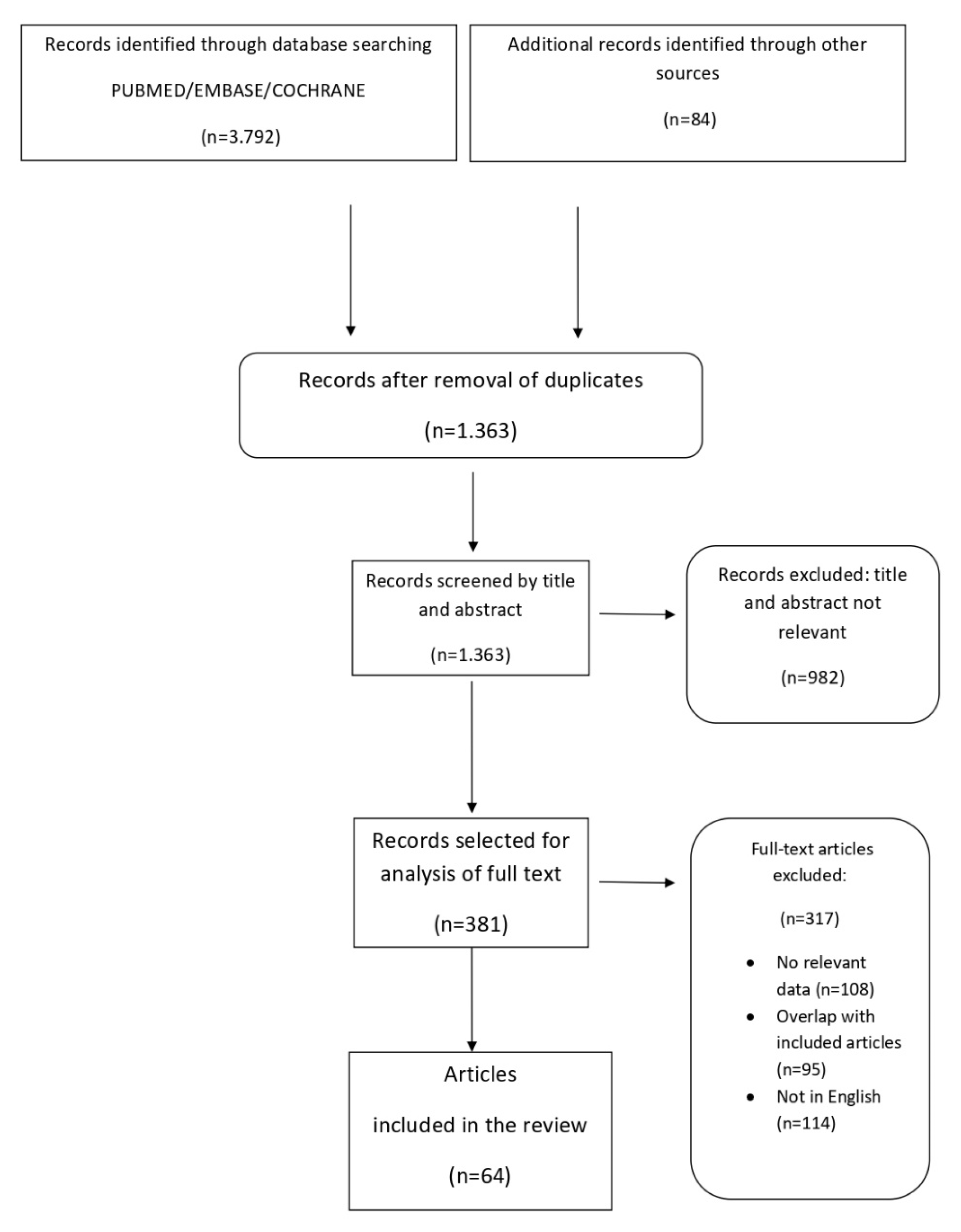
2. Materials and Methods
3. Results
4. Discussion
4.1. Radiation Dermatitis
4.2. Fibrosis
4.3. Radiation Recall Dermatitis
4.4. Radiation-Induced Skin Malignancies
4.5. Morphea
4.6. Bullous Pemphigoid
4.7. Lymphangioma Circumscriptum
4.8. Pseudosclerodermatous Panniculitis
4.9. Radiation Port Dermatophytosis
4.10. Others
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryan, J.L. Ionizing radiation: The good, the bad, and the ugly. J. Investig. Dermatol. 2012, 132 Pt 2, 985–993. [Google Scholar] [CrossRef]
- Frane, N.; Bitterman, A. Radiation Safety and Protection; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Bradley, J.A.; Mendenhall, N.P. Novel Radiotherapy Techniques for Breast Cancer. Annu Rev Med. 2018, 69, 277–288. [Google Scholar] [CrossRef]
- McQuestion, M. Evidence-based skin care management in radiation therapy: Clinical update. Semin. Oncol. Nurs. 2011, 27, e1–e17. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Guerrero, R.; Núñez, M.I.; del Moral, R.; Villalobos, M.; Martínez-Galán, J.; Valenzuela, M.T.; Muñoz-Gámez, J.A.; Oliver, F.J.; Martín-Oliva, D.; et al. Early and late skin reactions to radiotherapy for breast cancer and their correlation with radiation-induced DNA damage in lymphocytes. Breast Cancer Res. 2005, 7, R690. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Meineke, V. Radiation-induced alterations in cytokine production by skin cells. Exp. Hematol. 2007, 35, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, S.P.; Tolone, M.; Zingoni, T.; Tamburi, F.; Scali, E.; Bennardo, L.; Cannarozzo, G. A New 675 nm Laser Device in the Treatment of Melasma: Results of a Prospective Observational Study. Photobiomodul. Photomed. Laser Surg. 2020, 38, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.R.; Rzucidlo, E. Acute and chronic radiation injury. J. Vasc. Surg. 2011, 53, 15S–21S. [Google Scholar] [CrossRef] [PubMed]
- Hymes, S.R.; Strom, E.A.; Fife, C. Radiation dermatitis: Clinical presentation, pathophysiology, and treatment 2006. J. Am. Acad. Dermatol. 2006, 54, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Seité, S.; Bensadoun, R.J.; Mazer, J.M. Prevention and treatment of acute and chronic radiodermatitis. Breast Cancer Targets Ther. 2017, 9, 551–557. [Google Scholar] [CrossRef]
- Spałek, M. Chronic radiation-induced dermatitis: Challenges and solutions. Clin. Cosmet. Investig. Dermatol. 2016, 9, 473–482. [Google Scholar] [CrossRef]
- Cox, J.D.; Ang, K. Radiation Oncology: Rationale, Technique, Results, 9th ed.; Mosby Elsevier: Philadelphia, PA, USA, 2010. [Google Scholar]
- Denham, J.W.; Hauer-Jensen, M. The radiotherapeutic injury—A complex ‘wound’. Radiother. Oncol. 2002, 63, 129–145. [Google Scholar] [CrossRef]
- Lee, J.H.; Kay, C.S.; Maeng, L.S.; Oh, S.J.; Lee, A.H.; Lee, J.D.; Han, C.W.; Cho, S.H. The clinical features and pathophysiology of acute radiation dermatitis in patients receiving tomotherapy. Ann. Dermatol. 2009, 21, 358–363. [Google Scholar] [CrossRef]
- Bray, F.N.; Simmons, B.J.; Wolfson, A.H.; Nouri, K. Acute and Chronic Cutaneous Reactions to Ionizing Radiation Therapy. Dermatol. Ther. 2016, 6, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Zenda, S.; Ota, Y.; Tachibana, H.; Ogawa, H.; Ishii, S.; Hashiguchi, C.; Akimoto, T.; Ohe, Y.; Uchitomi, Y. A prospective picture collection study for a grading atlas of radiation dermatitis for clinical trials in head-and-neck cancer patients. J. Radiat. Res. 2016, 57, 301–306. [Google Scholar] [CrossRef][Green Version]
- Leventhal, J.; Young, M.R. Radiation Dermatitis: Recognition, Prevention, and Management. Oncology 2017, 31, 885–899. [Google Scholar] [PubMed]
- Yarnold, J.; Brotons, M.C. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Negosanti, F.; Silvestri, M.; Bennardo, L.; Durastante, C.; Del Duca, E.; Cannarozzo, G.; Nisticò, S.P. Nd:YAG laser in association with pulsed dye laser for the treatment of PHACES syndrome. Dermatol. Rep. 2021, 13, 8751. [Google Scholar] [CrossRef]
- Schindl, A.; Schindl, M.; Schindl, L.; Jurecka, W.; Hönigsmann, H.; Breier, F. Increased dermal angiogenesis after low-intensity laser therapy for a chronic radiation ulcer determined by a video measuring system. J. Am. Acad. Dermatol. 1999, 40, 481–484. [Google Scholar] [CrossRef]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef]
- Berman, B.; Duncan, M. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br. J. Dermatol. 1990, 123, 339–346. [Google Scholar] [CrossRef]
- Silvestri, M.; Cristaudo, A.; Morrone, A.; Messina, C.; Bennardo, L.; Nisticò, S.P.; Mariano, M.; Cameli, N. Emerging Skin Toxicities in Patients with Breast Cancer Treated with New Cyclin-Dependent Kinase 4/6 Inhibitors: A Systematic Review. Drug Saf. 2021, 44, 725–732. [Google Scholar] [CrossRef]
- Burris, H.A., III; Hurtig, J. Radiation recall with anticancer agents. Oncologist 2010, 15, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Camidge, R.; Price, A. Characterizing the phenomenon of radiation recall dermatitis. Radiother. Oncol. 2001, 59, 237–245. [Google Scholar] [CrossRef]
- Jain, S.; Agarwal, J.; Laskar, S.; Gupta, T.; Shrivastava, S. Radiation recall dermatitis with gatifloxacin: A review of literature. J. Med. Imaging Radiat. Oncol. 2008, 52, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Zorzan, M.T.; de Mello Pereira, R.; Lima, L.F.; de Arruda Mattos, T.V.; Sá, R. Radiodermatitis as a consequence of radiation recall induced by acyclovir: Case report. Rep. Pract. Oncol. Radiother. 2021, 26, 475–480. [Google Scholar] [CrossRef]
- Cuperus, E.; Leguit, R.; Albregts, M.; Toonstra, J. Post radiation skin tumors: Basal cell carcinomas, squamous cell carcinomas and angiosarcomas. A review of this late effect of radiotherapy. Eur. J. Dermatol. 2013, 23, 749–757. [Google Scholar] [CrossRef]
- Mercuri, S.R.; Brianti, P.; Dattola, A.; Bennardo, L.; Silvestri, M.; Schipani, G.; Nisticò, S.P. CO2 laser and photodynamic therapy: Study of efficacy in periocular BCC. Dermatol. Ther. 2018, 31, e12616. [Google Scholar] [CrossRef]
- McDaniel, B.; Badri, T.; Steele, R.B. Basal Cell Carcinoma; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Zargari, O. Radiation-induced basal cell carcinoma. Dermatol. Pract. Concept. 2015, 5, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Misago, N.; Ogusu, Y.; Narisawa, Y. Keloidal basal cell carcinoma after radiation therapy. Eur. J. Dermatol. 2004, 14, 182–185. [Google Scholar]
- Schena, D.; Rosina, P.; Chieregato, G. Onset of multiple basal cell carcinoma 60 years after X-ray treatment for tinea capitis. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Maalej, M.; Frikha, H.; Kochbati, L.; Bouaouina, N.; Sellami, D.; Benna, F.; Gargouri, W.; Dhraief, S.; Nasr, C.; Daoud, J.; et al. Radio-induced malignancies of the scalp about 98 patients with 150 lesions and literature review. Cancer Radiother. 2004, 8, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Pentangelo, G.; Nisticò, S.P.; Provenzano, E.; Cisale, G.Y.; Bennardo, L. Topical 5% Imiquimod Sequential to Surgery for HPV-Related Squamous Cell Carcinoma of the Lip. Medicina 2021, 57, 563. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, L.; Bennardo, F.; Giudice, A.; Passante, M.; Dastoli, S.; Morrone, P.; Provenzano, E.; Patruno, C.; Nisticò, S.P. Local Chemotherapy as an Adjuvant Treatment in Unresectable Squamous Cell Carcinoma: What Do We Know So Far? Curr. Oncol. 2021, 28, 2317–2325. [Google Scholar] [CrossRef]
- Ron, E.; Modan, B.; Preston, D.; Alfandary, E.; Stovall, M.; Boice, J.D., Jr. Radiation-induced skin carcinomas of the head and neck. Radiat. Res. 1991, 125, 318–325. [Google Scholar] [CrossRef]
- Edwards, M.J.; Hirsch, R.M.; Broadwater, J.R.; Netscher, D.T.; Ames, F.C. Squamous Cell Carcinoma Arising in Previously Burned or Irradiated Skin. Arch. Surg. 1989, 124, 115–117. [Google Scholar] [CrossRef]
- Fodor, J.; Orosz, Z.; Szabó, É.; Sulyok, Z.; Polgár, C.; Zaka, Z.; Major, T. Angiosarcoma after conservation treatment for breast carcinoma: Our experience and a review of the literature. J. Am. Acad. Dermatol. 2006, 54, 499–504. [Google Scholar] [CrossRef]
- Rao, J.; DeKoven, J.G.; Beatty, J.D.; Jones, G. Cutaneous angiosarcoma as a delayed complication of radiation therapy for carcinoma of the breast. J. Am. Acad. Dermatol. 2003, 49, 532–538. [Google Scholar] [CrossRef]
- Vorburger, S.A.; Xing, Y.; Hunt, K.K.; Bocian, J.J. Angiosarcoma of the breast. Cancer 2005, 104, 2682–2688. [Google Scholar] [CrossRef]
- Amajoud, Z.; Vertongen, A.S.; Weytens, R.; Hauspy, J. Radiation induced angiosarcoma of the breast: Case series and review of the literature. Facts Views Vis. Obgyn. 2018, 10, 215–220. [Google Scholar]
- Ronen, S.; Ivan, D.; Torres-Cabala, C.A.; Curry, J.L.; Tetzlaff, M.T.; Aung, P.P. Post-radiation vascular lesions of the breast. J. Cutan. Pathol. 2019, 46, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, J.Y.; Ferreira, M.N.; Leventhal, J.S. Dermatologic toxicities associated with radiation therapy in women with breast cancer. Int. J. Womens Dermatol. 2020, 6, 349–356. [Google Scholar] [CrossRef]
- Patton, K.T.; Deyrup, A.T.; Weiss, S.W. Atypical vascular lesions after surgery and radiation of the breast: A clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am. J. Surg. Pathol. 2008, 32, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, S.J.; Pinnock, N.; Giblin, V.; Fisher, C.; Thway, K.; Thomas, J.M.; Hayes, A.J. Treatment and outcome of radiation-induced soft-tissue sarcomas at a specialist institution. Eur. J. Surg. Oncol. 2009, 35, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.O.; Bayoumi, Y.; Balbaid, A.; Orz, Y.; AlShakweer, W.; Eltawel, M.H.; Tunio, M. Radiation Induced Multiple Skin Neoplasms Following Craniospinal Irradiation for Medulloblastoma. A Case Report. Am. J. Case Rep. 2020, 21, e917694. [Google Scholar] [CrossRef]
- Antley, C.A.; Carney, M.; Smoller, B.R. Microcystic adnexal carcinoma arising in the setting of previous radiation therapy. J. Cutan. Pathol. 1999, 26, 48–50. [Google Scholar] [CrossRef]
- Kose, R.; Coban, Y.K.; Ciralik, H. Eccrine porocarcinoma arising from preexisting eccrine poroma of the scalp after radiotherapy for cervical cancer. Dermatol. Online J. 2006, 12, 18. [Google Scholar] [CrossRef]
- Schaffer, J.V.; Carroll, C.; Dvoretsky, I.; Huether, M.J.; Girardi, M. Postirradiation morphea of the breast presentation of two cases and review of the literature. Dermatology 2000, 200, 67–71. [Google Scholar] [CrossRef]
- Trivedi, A.; DeWitt, C.M.; McGevna, L. Radiation-induced circumscribed superficial morphea after brachytherapy for endometrial adenocarcinoma. Int. J. Womens Dermatol. 2017, 3, 234–236. [Google Scholar] [CrossRef]
- Spalek, M.; Jonska-Gmyrek, J.; Gałecki, J. Radiation-induced morphea—A literature review. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Fett, N.; Werth, V.P. Update on morphea: Part II. Outcome measures and treatment. J. Am. Acad. Dermatol. 2011, 64, 231–242, quiz 43–44. [Google Scholar] [CrossRef]
- Friedman, O.; Barnea, Y.; Hafner, A. Underdiagnosed and disfiguring: Radiation-induced morphea following breast cancer treatment. Breast 2018, 39, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Palleria, C.; Bennardo, L.; Dastoli, S.; Iannone, L.F.; Silvestri, M.; Manti, A.; Nisticò, S.P.; Russo, E.; De Sarro, G. Angiotensin-converting-enzyme inhibitors and angiotensin II receptor blockers induced pemphigus: A case series and literature review. Dermatol. Ther. 2019, 32, e12748. [Google Scholar] [CrossRef] [PubMed]
- Moro, F.; Fania, L.; Sinagra, J.L.M.; Salemme, A.; Di Zenzo, G. Bullous Pemphigoid: Trigger and Predisposing Factors. Biomolecules 2020, 10, 1432. [Google Scholar] [CrossRef] [PubMed]
- Ashack, K.A.; Kuritza, V.; Visconti, M.J.; Ashack, L. Dermatologic Sequelae Associated with Radiation Therapy. Am. J. Clin. Dermatol. 2020, 21, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, T.; Kim, K.; Hong, S.; Baek, S.; Moon, J. Radiation induced pemphigoid disease. Obstet. Gynecol. Sci. 2020, 63, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, M. Bullous pemphigoid after radiation therapy. J. Am. Acad. Dermatol. 1989, 20, 14. [Google Scholar] [CrossRef]
- Mul, V.E.; van Geest, A.J.; Pijls-Johannesma, M.C.; Theys, J.; Verschueren, T.A.; Jager, J.J.; Lambin, P.; Baumert, B.G. Radiation-induced bullous pemphigoid: A systematic review of an unusual radiation side effect. Radiother. Oncol. 2007, 82, 5–9. [Google Scholar] [CrossRef]
- Calikoglu, E.; Anadolu, R.; Erdem, C.; Calikoglu, T. Localized bullous pemphigoid as an unusual complication of radiation therapy. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 646–647. [Google Scholar] [CrossRef]
- Massa, A.F.; Menezes, N.; Baptista, A.; Moreira, A.I.; Ferreira, E.O. Cutaneous Lymphangioma circumscriptum—Dermoscopic features. An. Bras. Dermatol. 2015, 90, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, K.; Nishigori, C. Acquired lymphangiectasia associated with treatment for preceding malignant neoplasm: A retrospective series of 73 Japanese patients. Arch. Dermatol. 2009, 145, 841–842. [Google Scholar] [CrossRef][Green Version]
- Schulz, D.; Lein, A.; Nicula, A.P.; Schierle, K.; Solovan, C. Lymphangioma Circumscriptum Post Radiotherapy for Penile Cancer Treated with CO2 Laser. Acta Dermatovenerol. Croat. 2018, 26, 53–57. [Google Scholar] [PubMed]
- Winkelmann, R.K.; Grado, G.L.; Quimby, S.R.; Connolly, S.M. Pseudosclerodermatous panniculitis after irradiation: An unusual com- plication of megavoltage treatment of breast carcinoma. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 1993; Volume 68, pp. 122–127. [Google Scholar]
- Sandoval, M.; Giesen, L.; Cataldo, K.; Gonzalez, S. Postirradiation Pseudosclerodermatous Panniculitis of the Leg: Report of a Case and Review of the Literature. Am. J. Dermatopathol. 2015, 37, 587–589. [Google Scholar] [CrossRef]
- Shirsat, H.S.; Walsh, N.M.; McDonald, L.J.; Rutledge, R.; Porter, G.; Barnes, P.J. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: A dramatic example of a rare entity and a pitfall in diagnosis. J. Cutan. Pathol. 2016, 43, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Pielasinski, Ú.; Machan, S.; Camacho, D.; Juárez, Á.; Cedeño, M.; Maciá, J.A.R.; Requena, L. Postirradiation pseudosclerodermatous panniculitis: Three new cases with additional histopathologic features supporting the radiotherapy etiology. Am. J. Dermatopathol. 2013, 35, 129–134. [Google Scholar] [CrossRef]
- Maor, M.H. Dermatophytosis confined to irradiated skin: A case report. Int. J. Radiat. Oncol. Biol. Phys. 1988, 14, 825–826. [Google Scholar] [CrossRef]
- Casamiquela, K.M.; Cohen, P.R. Radiation port dermatophytosis: Tinea corporis occurring at the site of irradiated skin. Dermatol. Online J. 2012, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, V.; Brunetti, G.; Puca, R.V.; Ruocco, E. The immunocompromised district: A unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Krivda, S.J. Lichen planus confined to a radiation therapy site. J. Am. Acad. Dermatol. 2002, 46, 604–605. [Google Scholar] [CrossRef]
- Pan, Z.; Bland, K.I.; Wei, S. Composite cutaneous atypical vascular lesion and Langerhans cell histiocytosis after radiation for breast carcinoma: Can radiation induce Langerhans cell histiocytosis? Dermatol. Online J. 2011, 17, 6. [Google Scholar] [CrossRef]
- Easwaralingam, N.; Wu, Y.; Cheung, D.; Tiver, K.; Fuller, S.; Edirimanne, S. Radiotherapy for breast cancer associated with a cutaneous presentation of systemic mastocytosis a case report and literature review. J. Surg. Case Rep. 2018, 2018, rjy317. [Google Scholar] [CrossRef] [PubMed]
- Chodkiewicz, H.M.; Cohen, P.R. Radiation port erythema multiforme: Erythema multiforme localized to the radiation port in a patient with non-small cell lung cancer. Skinmed 2012, 10, 390–392. [Google Scholar] [PubMed]
- Cohen, P.R.; Prieto, V.G. Radiation port xanthogranuloma: Solitary xanthogranuloma occurring within the irradiated skin of a breast cancer patient-report and review of cutaneous neoplasms developing at the site of radiotherapy. J. Cutan. Pathol. 2010, 37, 891–894. [Google Scholar] [CrossRef] [PubMed]
| Radiation-Induced Dermatologic Condition | Dose of Eposure | Pathogenesis | Clinical Manifestation | Treatment |
|---|---|---|---|---|
| Acute radiation dermatitis | ≥2–40 Gy [11] | Damage to DNA, proteins, lipids and carbohydrates induced by free radicals; recruitment of inflammatory cells, apoptosis, and necrosis of epidermal cells [13,14]. | Erythema to desquamation and ulceration [15] | Emollients, moisturizers, topical steroids, hydrogel and hydrocolloid dressing [15]. |
| Chronic radiation dermatitis | >50 Gy [11] | Secretion of pro-inflammatory and profibrotic cytokines by inflammatory cells [18]. | Teleangectasias, hyperkeratosis, atrophy, hyper or hypopigmentation, alopecia, ulceration, wounds, necrosis [9]. | Lasertherapy, antibacterial agents, debridement, surgical intervention [10,15]. |
| Fibrosis | >50 Gy [22] | Radiation induced DNA damage and reactive oxygen and nitrogen species production, stimulation of inflammatory and fibrotic processes [22]. | Cutaneous induration and retraction, lymph-edema, restricted movements, necrosis and ulceration [45]. | Mechanical massage, oral antioxidants, pentoxifylline, hyperbaric oxygen therapy, superoxide dismutase (SOD), IFNγ, laser therapy with epidermal grafting [15,23]. |
| Radiation Recall Dermatitis | ≥20 Gy in some cases below 20 Gy [25] | Changes in stem cells in the irradiated area, cellular DNA damage and oxidative stress, idiosyncratic drug hypersensitivity reactions [25]. | Rash, dry desquamation, pruritus, swelling, edema, blistering, maculopapular rashes, ulceration and skin necrosis [26]. | Topical or systemic steroids, non-steroidal anti-inflammatory drugs and antihistamines [25,26]. |
| Basal cell carcinoma (BCC) | >30 Gy [29] | Direct DNA damage by ionizing radiation, and indirect damage caused by fee radicals; alteration in DNA repair processes, cell deasth, inflammation, angiogenesis [1]. | The same as the BCCs of different etiology | Surgical excision, Mohs micrographic surgery, radiation, electrodesiccation and curettage, photodynamic therapy, cryosurgery, topical therapies, and systemic medication [29,31]. |
| Squamous cell carcinoma (SCC) | >30 Gy [29] | = | The same as the SCCs of different etiology | Surgical excision [29]. |
| Cutaneous angiosarcomas | >30 Gy [44] | = | Red/purple plaques or nodules with ecchimotic appearance [41]. | Surgical excission [43]. |
| Atypical vascular lesions (AVL) | 40–60 Gy [44] | = | Red or bluish papules or vesicles. | Surgical excision [45]. |
| Morphea | no relationship with the radiation parameters [51] | Abnormal secretion of cytokines causes activation of fibroblasts and an extensive fibrosis; development of neoantigens with T cells activation and release of TGF-β [51,52]. | Eplaques which subsequently become purplish and indurated, associated with pain and retraction [53]. | Phototherapy (NB-UVB, UVA, UVA1), tacrolimus, corticosteroids, calcipotriol, imiquimod, systemic corticosteroids [54]. |
| Bullous pemphigoid | >20 Gy [58] | Alterations of the antigenic properties of the cell surface induced by radiation, trigger the anti-gen-antibody reaction [59]; radiation-induced tissue damage favors the deposition of these antibodies in the basement membrane [60]. | Subepidermal blisters [59]. | Topical and systemic glucocorticoids, immunosuppressive drugs, systemic antibiotics, anti-CD20 agents [58]. |
| Lymphangioma circumscriptum | 40–60 Gy [64] | Radiation-induced dilation and sequestration of lymphatic channels [63]. | Clusters of translucent vesicles [63]. | Lasertherapy, sclerotherapy, surgical excision, cryotherapy, radiotherapy and electrocautery [64]. |
| Pseudosclerodermatous Panniculitis. | >50 Gy [67] | Release of profibrotic cytokines, delayed im-mune response to radiation-induced neoantigens, gene polymorphisms related to TGF-β1 and retinoic acid [68]. | Indurated erythematous plaques [67]. | Systemic steroids [69]. |
| Radiation port dermatophytosis | 9–70.9 Gy [70] | Alteration of the function of precursor T-lymphocytes and Langerhans cells; lymphedema and impaired lymph drainage [72]. | Tinea corporis in the field of irradiation [70]. | Topical or systemic antifungals [71]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennardo, L.; Passante, M.; Cameli, N.; Cristaudo, A.; Patruno, C.; Nisticò, S.P.; Silvestri, M. Skin Manifestations after Ionizing Radiation Exposure: A Systematic Review. Bioengineering 2021, 8, 153. https://doi.org/10.3390/bioengineering8110153
Bennardo L, Passante M, Cameli N, Cristaudo A, Patruno C, Nisticò SP, Silvestri M. Skin Manifestations after Ionizing Radiation Exposure: A Systematic Review. Bioengineering. 2021; 8(11):153. https://doi.org/10.3390/bioengineering8110153
Chicago/Turabian StyleBennardo, Luigi, Maria Passante, Norma Cameli, Antonio Cristaudo, Cataldo Patruno, Steven Paul Nisticò, and Martina Silvestri. 2021. "Skin Manifestations after Ionizing Radiation Exposure: A Systematic Review" Bioengineering 8, no. 11: 153. https://doi.org/10.3390/bioengineering8110153
APA StyleBennardo, L., Passante, M., Cameli, N., Cristaudo, A., Patruno, C., Nisticò, S. P., & Silvestri, M. (2021). Skin Manifestations after Ionizing Radiation Exposure: A Systematic Review. Bioengineering, 8(11), 153. https://doi.org/10.3390/bioengineering8110153








