Antioxidant Network Based on Sulfonated Polyhydroxyalkanoate and Tannic Acid Derivative
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
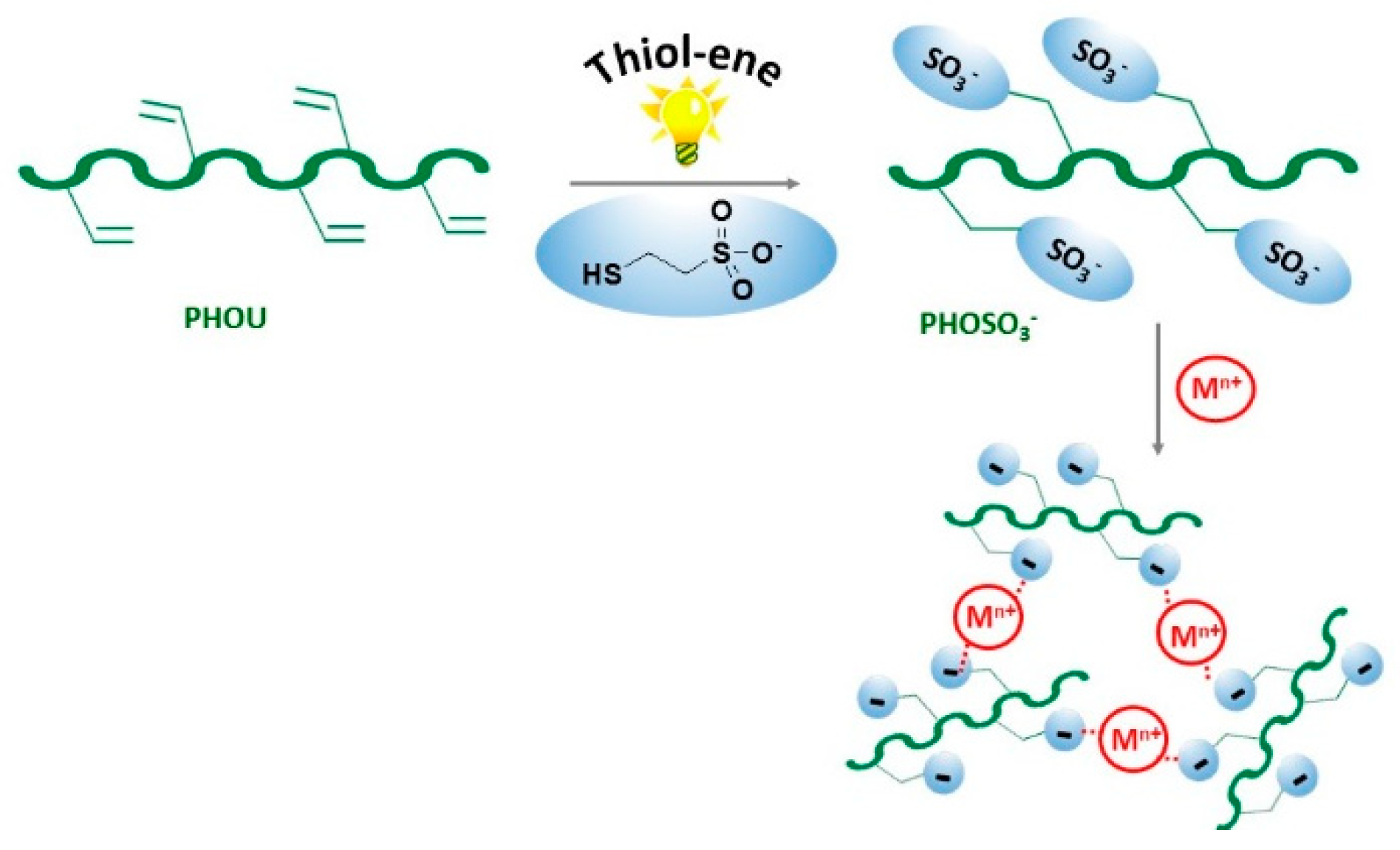
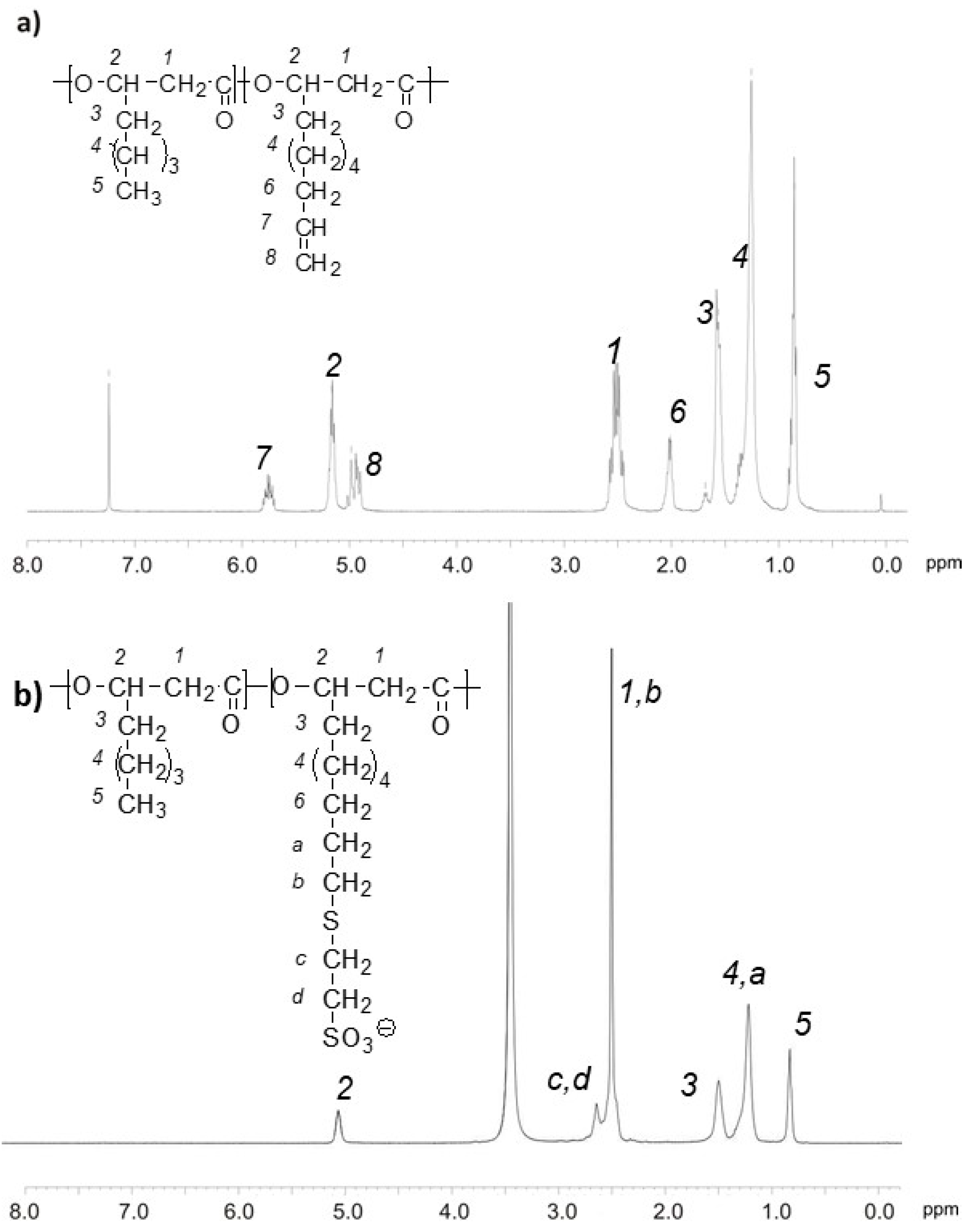
2.2. Synthesis of PHO Sulfonate, PHOSO3−
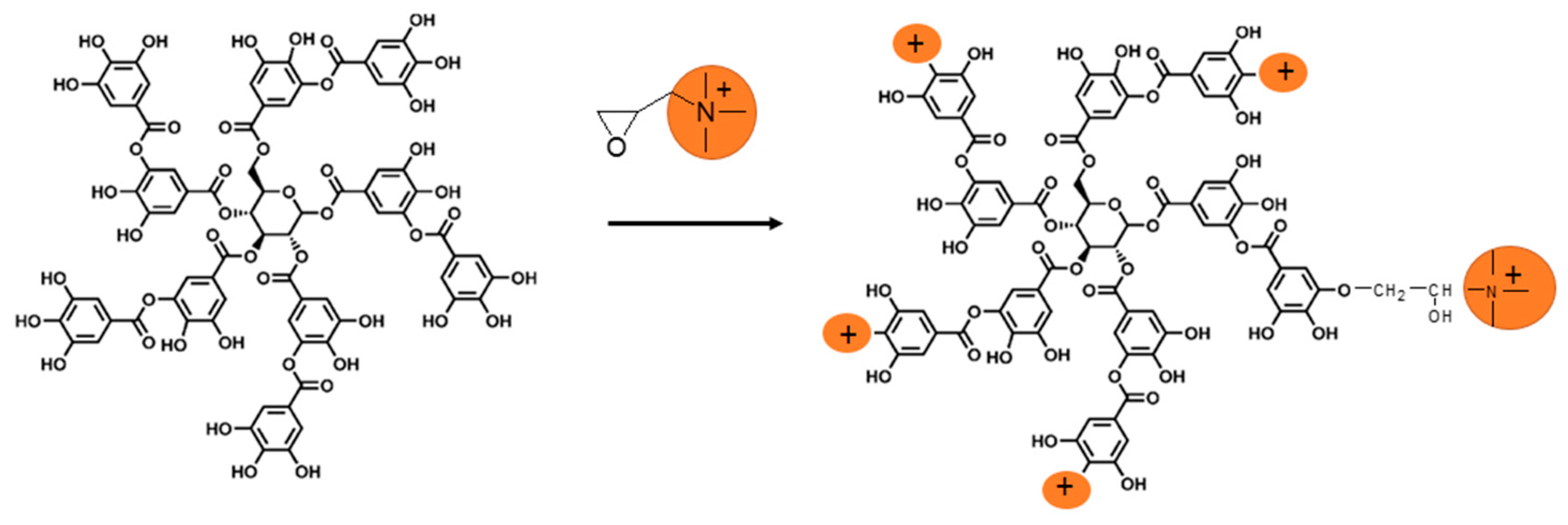
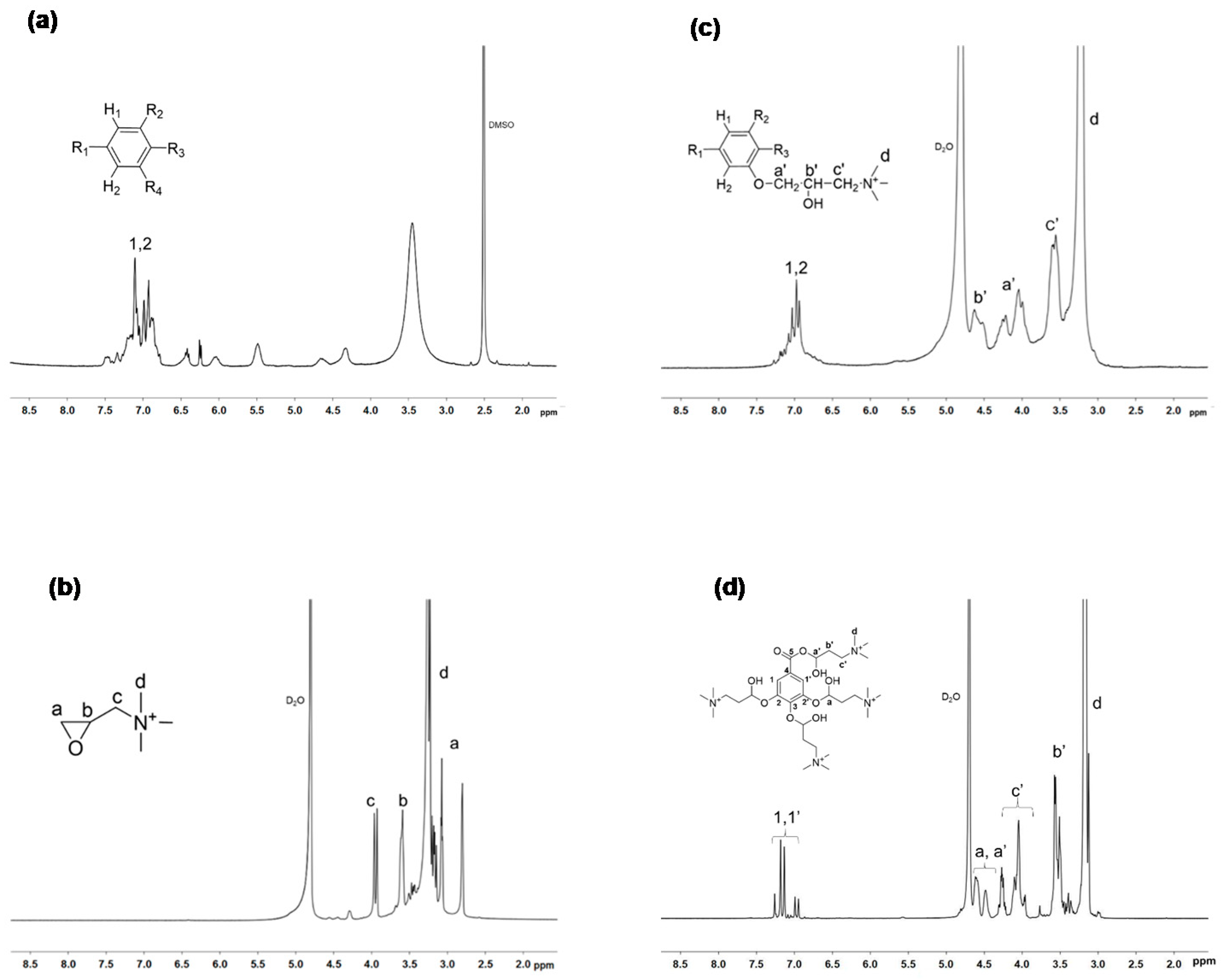
2.3. Synthesis of Trimethyl Ammonium Gallic Acid: GA-N(CH3)3+
2.4. Synthesis of Trimethyl Ammonium Tannic Acid: TA-N(CH3)3+
2.5. Elaboration of Network Based on PHOSO3−
2.6. DPPH Test
2.7. Characterization
3. Results and Discussion
3.1. Synthesis of Poly(3-Hydroxyalkanoate) Sulfonate, PHOSO3−, Ammonium Derivatives of Gallic Acid GA-N(CH3)3+ and Tannic Acid, TA-N(CH3)3+
3.2. Effects of the Nature of Cations on the Formation of Gels
3.3. Structure of Networks and Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinbüchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Lenz, R.W.; Marchessault, R.H. Bacterial Polyesters: Biosynthesis, Biodegradable Plastics and Biotechnology. Biomacromolecules 2005, 6, 1–8. [Google Scholar]
- Numata, K.; Abe, H.; Iwata, T. Biodegradability of Poly(hydroxyalkanoate) Materials. Materials 2009, 2, 1104–1126. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Vinogradova, O.N.; Syrvacheva, D.A.; Shishatskaya, E.I. Microbial Degradation of Polyhydroxyalkanoates with Different Chemical Compositions and Their Biodegradability. Microb. Ecol. 2017, 73, 353–367. [Google Scholar] [CrossRef]
- Zhang, J.; Shishatskaya, E.I.; Volova, T.G.; Silva, L.F.; Chen, G.-Q. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C 2018, 86, 144–150. [Google Scholar] [CrossRef]
- Rai, R.; Keshavarz, T.; Roether, J.; Boccaccini, A.R.; Roy, I. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater. Sci. Eng. R Rep. 2011, 72, 29–47. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molcules 2018, 23, 362. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.F.; Martin, D.P. Applications of Polyhydroxyalkanoates (PHA). Med. Pharm. 2002, 10, 91–121. [Google Scholar]
- Bear, M.M.; Renard, E.; Randriamahefa, S.; Langlois, V.; Guérin, P. Preparation of a bacterial polyester with carboxy groups in side chains. C. R. l’Academie. Sci. Ser. IIC Univers. 2001, 4, 289–293. [Google Scholar]
- Chen, G.-Q.; Chen, X.-Y.; Wu, F.-Q.; Chen, J.-C. Polyhydroxyalkanoates (PHA) toward cost competitiveness and functionality. Adv. Ind. Eng. Polym. Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Renard, E.; Poux, A.; Timbart, L.; Langlois, A.V.; Guérin, P. Preparation of a Novel Artificial Bacterial Polyester Modified with Pendant Hydroxyl Groups. Biomacromolecules 2005, 6, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, M.S.; Hazer, B.; Ozturk, T.; Çaykara, T. Hydroxylation of pendant vinyl groups of poly(3-hydroxy undec-10-enoate) in high yield. J. Appl. Polym. Sci. 2005, 97, 2132–2139. [Google Scholar] [CrossRef]
- Stigers, J.D.; Tew, G.N. Poly(3-hydroxyalkanoate)s Functionalized with Carboxylic Acid Groups in the Side Chain. Biomacromolecules 2003, 4, 193–195. [Google Scholar] [CrossRef]
- Renard, E.; Timbart, L.; Vergnol, G.; Langlois, V. Role of carboxyl pendant groups of medium chain length poly(3-hydroxyalkanoate)s in biomedical temporary applications. J. Appl. Polym. Sci. 2010, 117, 1888–1896. [Google Scholar] [CrossRef]
- Park, W.H.; Lenz, R.W.; Goodwin, S. Epoxidation of Bacterial Polyesters with Unsaturated Side Chains. I. Production and Epoxidation of Polyesters from 10-Undecenoic Acid. Macromolecules 1998, 31, 1480–1486. [Google Scholar] [CrossRef]
- Hazer, B.; Steinbüchel, A. Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl. Microbiol. Biotechnol. 2007, 74, 1–12. [Google Scholar] [CrossRef]
- Domenek, S.; Langlois, V.; Renard, E. Bacterial polyesters grafted with poly(ethylene glycol): Behaviour in aqueous media. Polym. Degrad. Stab. 2007, 92, 1384–1392. [Google Scholar] [CrossRef]
- Renard, E.; Tanguy, P.-Y.; Samain, E.; Guérin, P. Synthesis of novel graft polyhydroxyalkanoates. Macromol. Symp. 2003, 197, 11–18. [Google Scholar] [CrossRef]
- Renard, E.; Ternat, C.; Langlois, V.; Guérin, P. Synthesis of Graft Bacterial Polyesters for Nanoparticles Preparation. Macromol. Biosci. 2003, 3, 248–252. [Google Scholar] [CrossRef]
- Babinot, J.; Renard, E.; Langlois, V. Preparation of Clickable Poly(3-hydroxyalkanoate) (PHA): Application to Poly(ethylene glycol) (PEG) Graft Copolymers Synthesis. Macromol. Rapid Commun. 2010, 31, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Babinot, J.; Renard, E.; Le Droumaguet, B.; Guigner, J.-M.; Mura, S.; Nicolas, J.; Couvreur, P.; Langlois, V.; Patrick, C. Facile Synthesis of Multicompartment Micelles Based on Biocompatible Poly(3-hydroxyalkanoate). Macromol. Rapid Commun. 2012, 34, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Babinot, J.; Renard, E.; Langlois, V. Controlled Synthesis of Well Defined Poly(3-hydroxyalkanoate)s-based Amphiphilic Diblock Copolymers Using Click Chemistry. Macromol. Chem. Phys. 2010, 212, 278–285. [Google Scholar] [CrossRef]
- Babinot, J.; Guigner, J.-M.; Renard, E.; Langlois, V. Poly(3-hydroxyalkanoate)-derived amphiphilic graft copolymers for the design of polymersomes. Chem. Commun. 2012, 48, 5364–5366. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.; Scholz, C. Synthesis and Characterization of a Cationic Poly(β-hydroxyalkanoate). Biomacromolecules 2008, 9, 2091–2096. [Google Scholar] [CrossRef]
- Modjinou, T.; Lemechko, P.; Babinot, J.; Versace, D.L.; Langlois, V.; Renard, E. Poly(3-hydroxyalkanoate) sulfonate: From nanopartices toward water soluble polyesters. Eur. Polym. J. 2015, 68, 471–479. [Google Scholar]
- Jain-Beuguel, C.; Li, X.; Renault, L.H.; Modjinou, T.; Colin, C.S.; Gref, R.; Renard, E.; Langlois, V. Water-Soluble Poly(3-hydroxyalkanoate) Sulfonate: Versatile Biomaterials Used as Coatings for Highly Porous Nano-Metal Organic Framework. Biomacromolecules 2019, 20, 3324–3332. [Google Scholar] [CrossRef]
- Chung, C.W.; Kim, H.W.; Kim, Y.B.; Rhee, Y.H. Poly(ethylene glycol)-grafted poly(3-hydroxyundecenoate) networks for enhanced blood compatibility. Int. J. Biol. Macromol. 2003, 32, 17–22. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, Z.; Loh, X.J.; Wu, Y.-L.; Li, Z. PHA-Based Thermogel as a Controlled Zero-Order Chemotherapeutic Delivery System for the Effective Treatment of Melanoma. ACS Appl. Bio Mater. 2019, 2, 3591–3600. [Google Scholar] [CrossRef]
- Le Fer, G.; Babinot, J.; Versace, D.-L.; Langlois, V.; Renard, E. An Efficient Thiol-Ene Chemistry for the Preparation of Amphiphilic PHA-Based Graft Copolymers. Macromol. Rapid Commun. 2012, 33, 2041–2045. [Google Scholar] [CrossRef]
- Hao, J.; Deng, X. Semi-interpenetrating networks of bacterial poly(3-hydroxybutyrate) with net-poly(ethylene glycol). Polymer 2001, 42, 4091–4097. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Che, X.; Yu, L.; Jia, W.; Shen, R.; Chen, J.; Ma, Y.; Chen, G.Q. Synthesis and Characterization of Polyhydroxyalkanoate Organo/Hydrogels. Biomacromolecules 2019, 20, 3303–3312. [Google Scholar] [CrossRef] [PubMed]
- Pavon-Djavid, G.; Gamble, L.J.; Ciobanu, M.; Gueguen, V.; Castner, D.G.; Migonney, V. Bioactive Poly(ethylene terephthalate) Fibers and Fabrics: Grafting, Chemical Characterization, and Biological Assessment. Biomacromolecules 2007, 8, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.H.; Hart, A.P.; Williams, E.C.; Cooper, S.L.; Charef, S.; Labarre, D.; Jozefowicz, M. Anticoagulant effects of sulphonated polyurethanes. Biomaterials 1992, 13, 339–344. [Google Scholar] [CrossRef]
- Palarasah, Y.; Skjoedt, M.O.; Vitved, L.; Koch, C. Sodium Polyanethole Sulfonate as an Inhibitor of Activation of Complement Function in Blood Culture Systems. J. Clin. Microbiol. 2010, 48, 908–914. [Google Scholar] [CrossRef]
- Meder, F.; Brandes, C.; Treccani, L.; Rezwan, K. Controlling protein–particle adsorption by surface tailoring colloidal alumina particles with sulfonate groups. Acta Biomater. 2013, 9, 5780–5787. [Google Scholar] [CrossRef]
- Li, G.; Zhang, G.; Sun, R.; Wong, C.-P. Mechanical strengthened alginate/polyacrylamide hydrogel crosslinked by barium and ferric dual ions. J. Mater. Sci. 2017, 52, 8538–8545. [Google Scholar] [CrossRef]
- Larsen, B.E.; Bjørnstad, J.; Pettersen, E.O.; Tønnesen, H.H.; Melvik, J.E. Rheological characterization of an injectable alginate gel system. BMC Biotechnol. 2015, 15, 29. [Google Scholar] [CrossRef]
- Espona-Noguera, A.; Ciriza, J.; Cañibano-Hernández, A.; Fernandez, L.; Ochoa, I.; Saenz del Burgo, L.; Pedraz, J.L. Tunable injectable alginate-based hydrogel for cell therapy in Type 1 Diabetes Mellitus. Int. J. Biol. Macromol. 2018, 107, 1261–1269. [Google Scholar]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Zahrani, N.A.A.L.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review European. J. Med. Chem. 2020, 204, 112609–112646. [Google Scholar] [CrossRef] [PubMed]
- Glaive, A.-S.; Modjinou, T.; Versace, D.-L.; Abbad-Andaloussi, S.; Dubot, P.; Langlois, V.; Renard, E. Design of Antibacterial and Sustainable Antioxidant Networks Based on Plant Phenolic Derivatives Used As Delivery System of Carvacrol or Tannic Acid. ACS Sustain. Chem. Eng. 2017, 5, 2320–2329. [Google Scholar] [CrossRef]
- Bhone, K.; Lim, M.; Sing, C.; Wei, Z. Tannic Acid as Phytochemical Potentiator for Antibiotic Resistance Adaptation. APCBEE Procedia 2013, 7, 175–181. [Google Scholar]
- Xie, Y.; Chen, S.; Zhang, X.; Shi, Z.; Wei, Z.; Bao, J.; Zhao, W.; Zhao, C. Engineering of Tannic Acid Inspired Antifouling and Antibacterial Membranes through Co-deposition of Zwitterionic Polymers and Ag Nanoparticles. Ind. Eng. Chem. Res. 2019, 58, 11689–11697. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Feng, X.; Bu, Y.; Wu, D.; Jin, Z. Supramolecular Hydrogel Formation Based on Tannic Acid. Macromolecules 2017, 50, 666–676. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Zhang, Q.; Jin, Z. Tannic Acid-Based Multifunctional Hydrogels with Facile Adjustable Adhesion and Cohesion Contributed by Polyphenol Supramolecular Chemistry. ACS Omega 2017, 2, 6668–6676. [Google Scholar] [CrossRef]
- Zheng, L.-Y.; Shi, J.-M.; Chi, Y.-H. Tannic Acid Physically Cross-Linked Responsive Hydrogel. Macromol. Chem. Phys. 2018, 219. [Google Scholar] [CrossRef]
- Guo, J.; Sun, W.; Kim, J.P.; Lu, X.; Li, Q.; Lin, M.; Mrowczynski, O.; Rizk, E.B.; Cheng, J.; Qian, G.-Y.; et al. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018, 72, 35–44. [Google Scholar] [CrossRef]
- Park, K.; Jeong, H.; Tanum, J.; Yoo, J.-C.; Hong, J. Developing regulatory property of gelatin-tannic acid multilayer films for coating-based nitric oxide gas delivery system. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, J.; Guo, R.; Luo, J.; Yuan, Y.; Liu, X. Synthesis of New Biobased Antibacterial Methacrylates Derived from Tannic Acid and Their Application in UV-Cured Coatings. Ind. Eng. Chem. Res. 2014, 53, 10835–10840. [Google Scholar] [CrossRef]
- Assifaoui, A.; Lerbret, A.; Huynh, U.T.D.; Neiers, F.; Chambin, O.; Loupiac, C.; Cousin, F.; Uyen, H.T.D. Structural behaviour differences in low methoxy pectin solutions in the presence of divalent cations (Ca 2+ and Zn 2+): A process driven by the binding mechanism of the cation with the galacturonate unit. Soft Matter 2015, 11, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Huynh, U.T.D.; Lerbret, A.; Neiers, F.; Chambin, O.; Assifaoui, A. Binding of Divalent Cations to Polygalacturonate: A mechanism Drien by the Hydration Water. J. Phys. Chem. 2016, 120, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
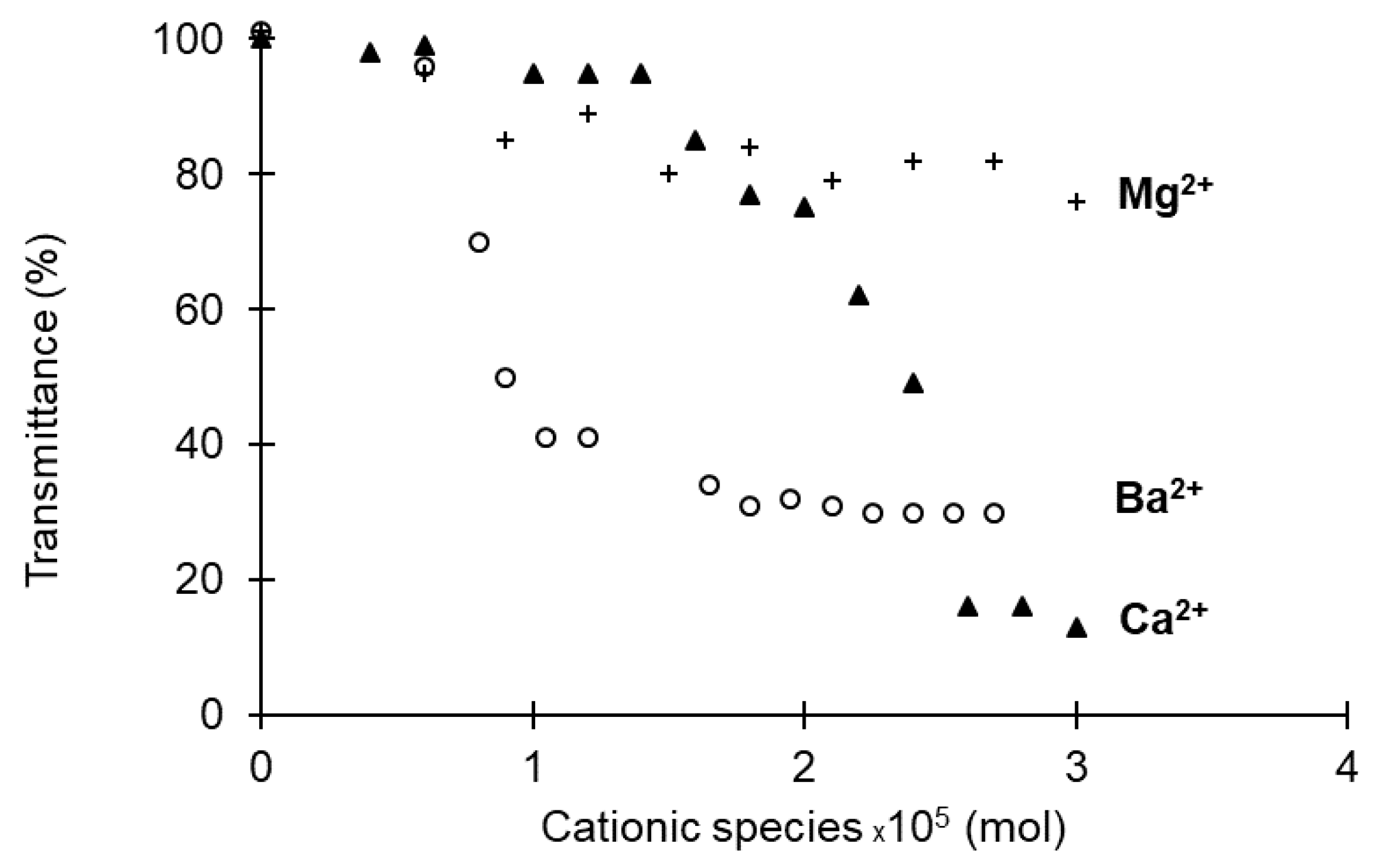
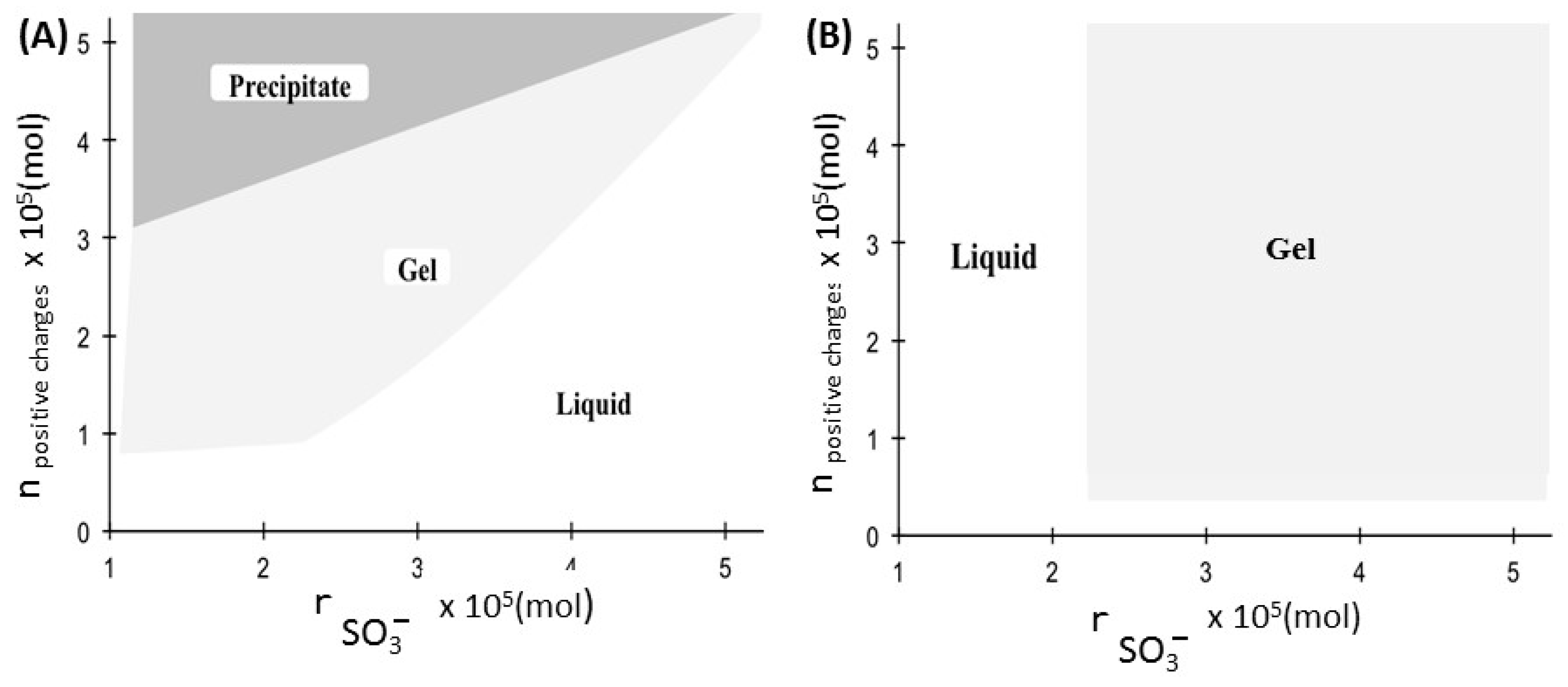
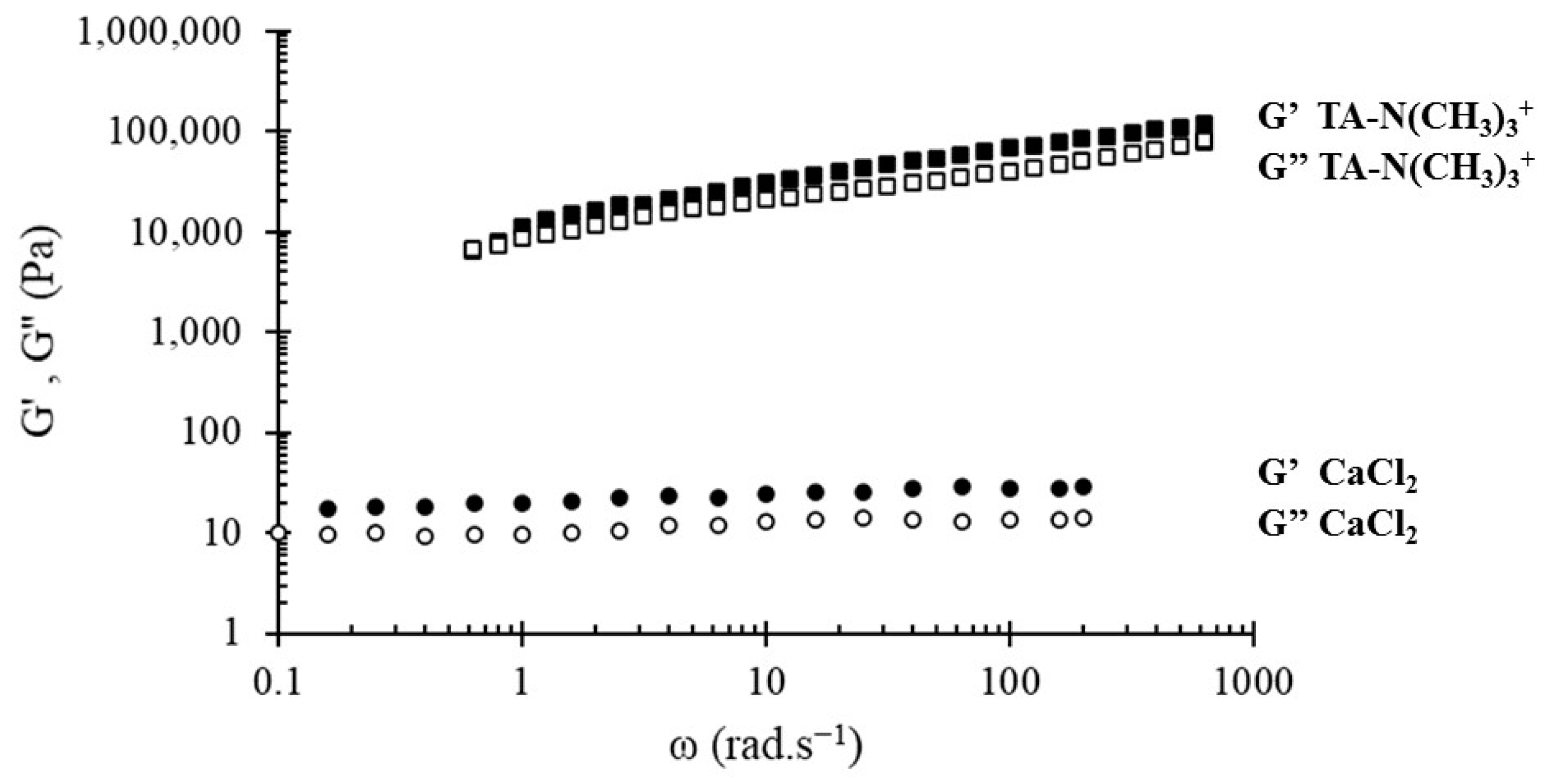

| [PHO-SO3−] (g/L) | nSO3− ×105 | nCa2+ ×105 | n Positive Charges ×105 | G’ (Pa) | G” (Pa) |
|---|---|---|---|---|---|
| 47 | 1.93 | 1.4 | 2.8 | 4.01 | 3.93 |
| 59 | 2.42 | 1.5 | 3.0 | 14.62 | 11.26 |
| 68 | 2.79 | 1.4 | 2.8 | 24.20 | 12.78 |
| 71 | 2.91 | 1.8 | 3.6 | 38.38 | 24.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brelle, L.; Renard, E.; Langlois, V. Antioxidant Network Based on Sulfonated Polyhydroxyalkanoate and Tannic Acid Derivative. Bioengineering 2021, 8, 9. https://doi.org/10.3390/bioengineering8010009
Brelle L, Renard E, Langlois V. Antioxidant Network Based on Sulfonated Polyhydroxyalkanoate and Tannic Acid Derivative. Bioengineering. 2021; 8(1):9. https://doi.org/10.3390/bioengineering8010009
Chicago/Turabian StyleBrelle, Laura, Estelle Renard, and Valerie Langlois. 2021. "Antioxidant Network Based on Sulfonated Polyhydroxyalkanoate and Tannic Acid Derivative" Bioengineering 8, no. 1: 9. https://doi.org/10.3390/bioengineering8010009
APA StyleBrelle, L., Renard, E., & Langlois, V. (2021). Antioxidant Network Based on Sulfonated Polyhydroxyalkanoate and Tannic Acid Derivative. Bioengineering, 8(1), 9. https://doi.org/10.3390/bioengineering8010009





