Systematical Engineering of Synthetic Yeast for Enhanced Production of Lycopene
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
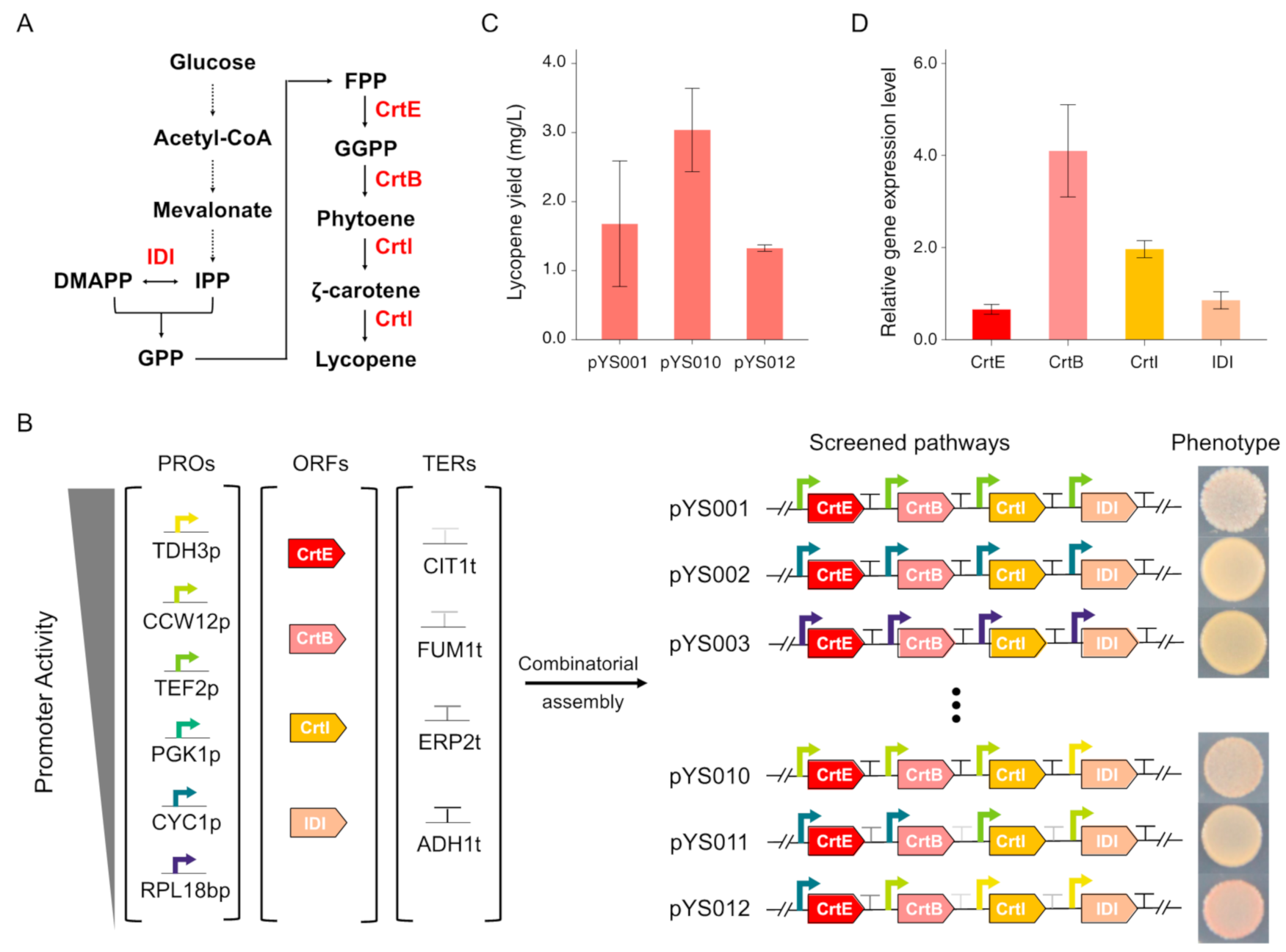
2.2. Plasmids and Strains Construction
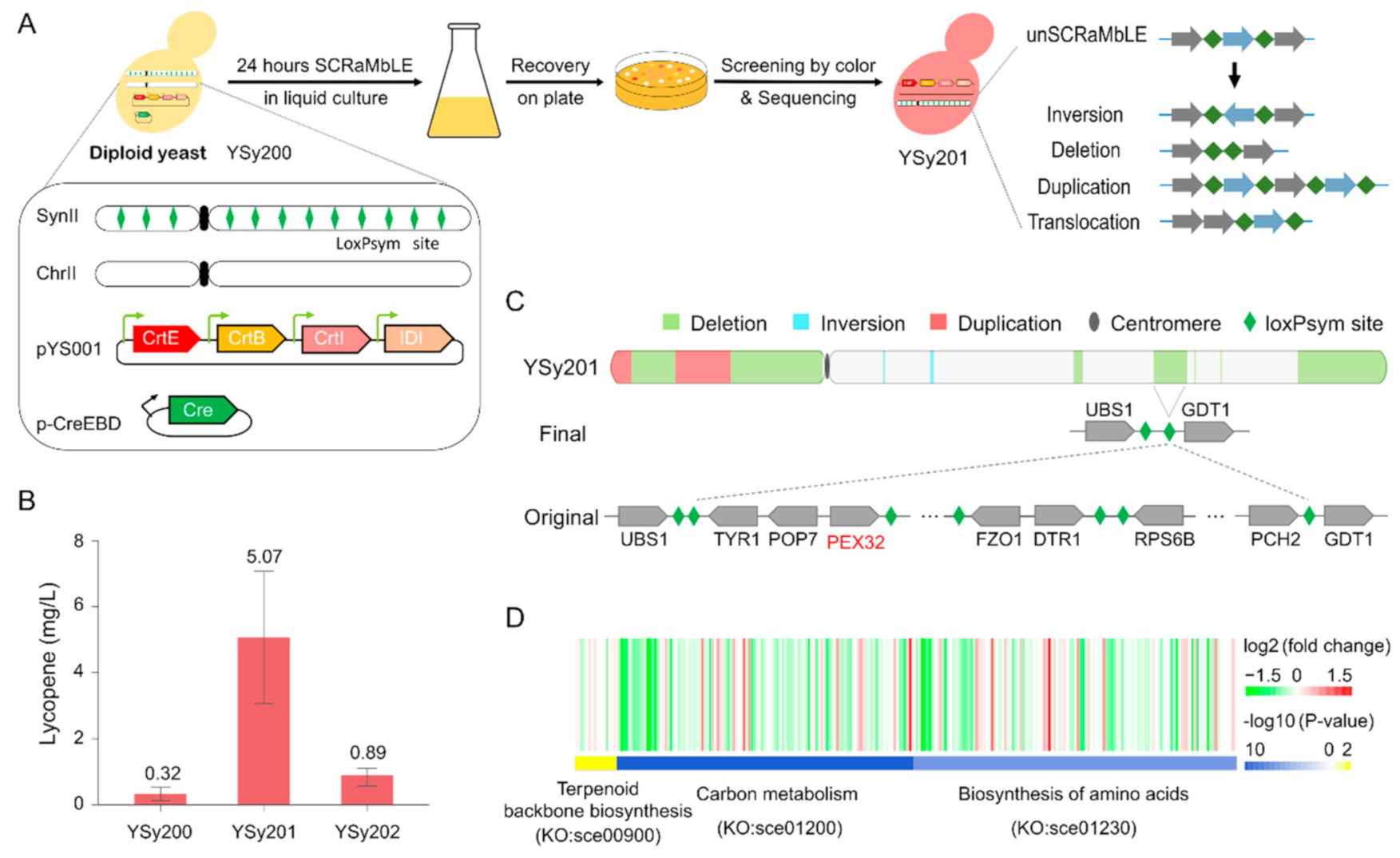
2.3. Lycopene High-Yield Strains SCRaMbLE and Screening
2.4. Lycopene Extraction and Quantification
2.5. Genomic DNA Extraction
2.6. Whole Genome Sequencing (WGS) and Data Processing
2.7. RNA Extraction and Transcriptome Analysis
2.8. Gene Differential Expression and Pathway Enrichment Analysis
2.9. Reverse Transcription and Quantitative PCR
2.10. Growth Assay
2.11. Pathway Stability Assay
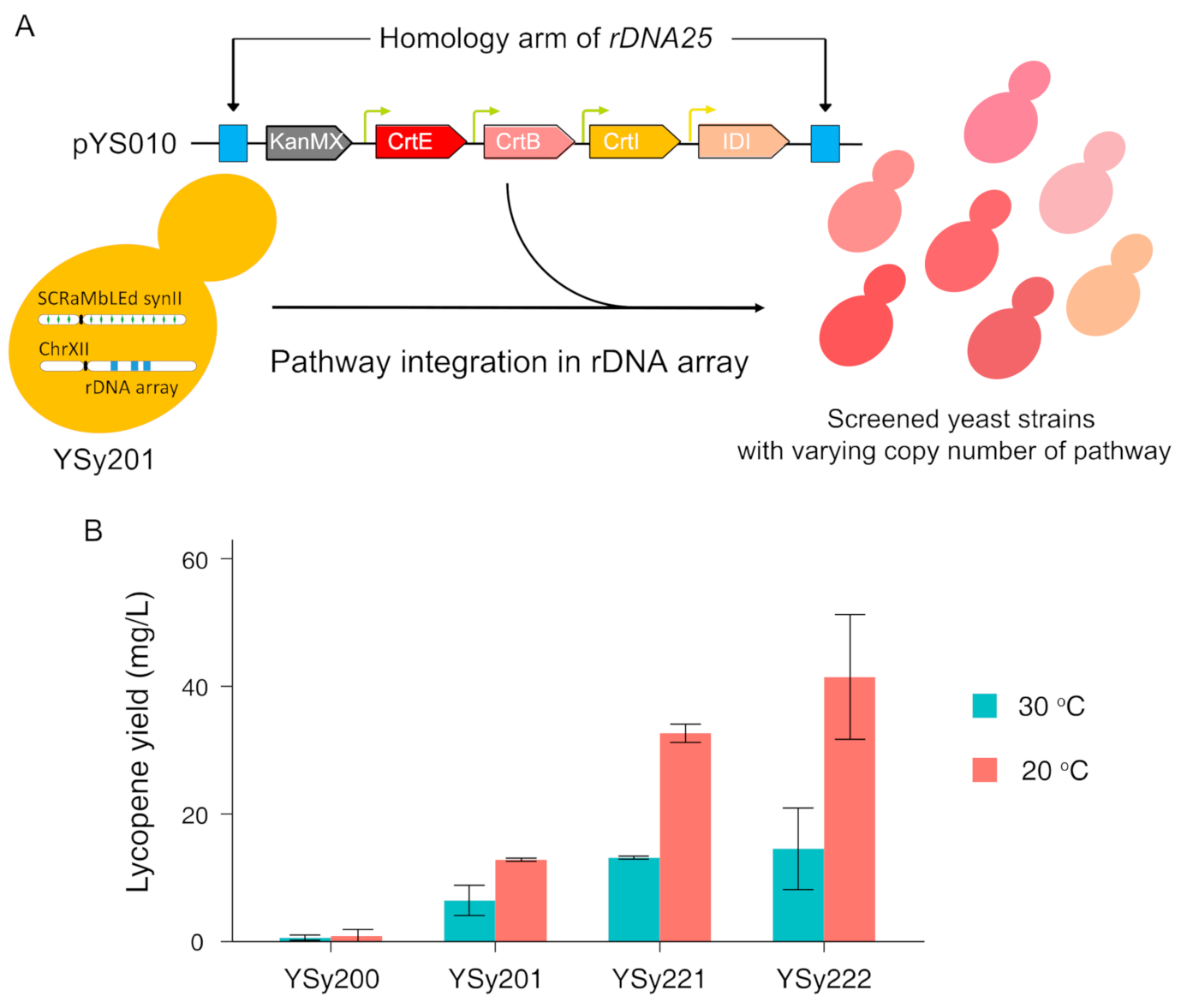
2.12. Copy Number Estimation of Integrated Pathway by Quantitative PCR
3. Results
3.1. SCRaMbLE to Generate a Yeast Strain with Increased Lycopene Production
3.2. Lycopene Biosynthesis Pathway Optimization by Combinatorial Assembly of Standard Biological Parts
3.3. Optimize Lycopene Production by Multicopy Integration of Constructed Pathway in SCRaMbLEd Host Strain
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, A.; Shen, H.; Agarwal, S.; Rao, A.V. Lycopene content of tomato products: Its stability, bioavailability and in vivo antioxidant properties. J. Med. Food 2001, 4, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Deng, Z.; Liu, T. Microbial production strategies and applications of lycopene and other terpenoids. World J. Microbiol. Biotechnol. 2016, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Y.; Fu, J.; Yang, Q.; Feng, L. High-throughput screening of lycopene-overproducing mutants of Blakeslea trispora by combining ARTP mutation with microtiter plate cultivation and transcriptional changes revealed by RNA-seq. Biochem. Eng. J. 2020, 161, 107664. [Google Scholar] [CrossRef]
- Sevgili, A.; Erkmen, O. Improved lycopene production from different substrates by mated fermentation of Blakeslea Trispora. Foods 2019, 8, 120. [Google Scholar] [CrossRef]
- Coussement, P.; Bauwens, D.; Maertens, J.; De Mey, M. Direct Combinatorial Pathway Optimization. ACS Synth Biol. 2017, 6, 224–232. [Google Scholar] [CrossRef]
- Wu, T.; Ye, L.; Zhao, D.; Li, S.; Li, Q.; Zhang, B.; Bi, C. Engineering membrane morphology and manipulating synthesis for increased lycopene accumulation in Escherichia coli cell factories. 3 Biotech 2018, 8, 269. [Google Scholar] [CrossRef]
- Sun, T.; Miao, L.; Li, Q.; Dai, G.; Lu, F.; Liu, T.; Zhang, X.; Ma, Y. Production of lycopene by metabolically-engineered Escherichia coli. Biotechnol. Lett. 2014, 36, 1515–1522. [Google Scholar] [CrossRef]
- Vadali, R.V.; Fu, Y.; Bennett, G.N.; San, K.Y. Enhanced lycopene productivity by manipulation of carbon flow to isopentenyl diphosphate in Escherichia coli. Biotechnol. Prog. 2005, 21, 1558–1561. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Yang, Q.; Yang, J. Metabolic Engineering Escherichia coli for the Production of Lycopene. Molecules 2020, 25, 3136. [Google Scholar] [CrossRef]
- Shi, B.; Ma, T.; Ye, Z.; Li, X.; Huang, Y.; Zhou, Z.; Ding, Y.; Deng, Z.; Liu, T. Systematic Metabolic Engineering of Saccharomyces cerevisiae for Lycopene Overproduction. J. Agric. Food Chem. 2019, 67, 11148–11157. [Google Scholar] [CrossRef]
- Redón, M.; Guillamón, J.M.; Mas, A.; Nicolas, R. Effect of lipid supplementation upon Saccharomyces cerevisiae lipid composition and fermentation performance at low temperature. Eur. Food Res. Technol. 2009, 228, 833–840. [Google Scholar] [CrossRef]
- Xie, W.; Lv, X.; Ye, L.; Zhou, P.; Yu, H. Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering. Metab. Eng. 2015, 30, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Shi, B.; Ye, Z.; Li, X.; Liu, M.; Chen, Y.; Xia, J.; Nielsen, J.; Deng, Z.; Liu, T. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab. Eng. 2019, 52, 134–142. [Google Scholar] [CrossRef]
- Hong, J.; Park, S.H.; Kim, S.; Kim, S.W.; Hahn, J.S. Efficient production of lycopene in Saccharomyces cerevisiae by enzyme engineering and increasing membrane flexibility and NAPDH production. Appl. Microbiol. Biotechnol. 2019, 103, 211–223. [Google Scholar] [CrossRef]
- Pegklidou, K.; Mantzouridou, F.; Tsimidou, M.Z. Lycopene production using Blakeslea trispora in the presence of 2-methyl imidazole: Yield, selectivity, and safety aspects. J. Agric. Food Chem. 2008, 56, 4482–4490. [Google Scholar] [CrossRef]
- Ray, B.L.; Raetz, C.R. The biosynthesis of gram-negative endotoxin. A novel kinase in Escherichia coli membranes that incorporates the 4′-phosphate of lipid A. J. Biol. Chem. 1987, 262, 1122–1128. [Google Scholar] [CrossRef]
- Alves, L.; Yuri, K.; Kanno, F.; Karp, S.G. Microbial production of carotenoids A review. Afr. J. Biotechnol. 2017, 16, 139–146. [Google Scholar]
- Shen, Y.; Wang, Y.; Chen, T.; Gao, F.; Gong, J.; Abramczyk, D.; Walker, R.; Zhao, H.; Chen, S.; Liu, W.; et al. Deep functional analysis of synII, a 770-kilobase synthetic yeast chromosome. Science 2017, 355, eaaf4791. [Google Scholar] [CrossRef]
- Xie, Z.X.; Li, B.Z.; Mitchell, L.A.; Wu, Y.; Qi, X.; Jin, Z.; Jia, B.; Wang, X.; Zeng, B.X.; Liu, H.M.; et al. “Perfect” designer chromosome V and behavior of a ring derivative. Science 2017, 355, eaaf4704. [Google Scholar] [CrossRef]
- Pretorius, I.S.; Boeke, J.D. Yeast 2.0-connecting the dots in the construction of the world’s first functional synthetic eukaryotic genome. FEMS Yeast Res. 2018, 18, foy032. [Google Scholar] [CrossRef]
- Wu, Y.; Li, B.Z.; Zhao, M.; Mitchell, L.A.; Xie, Z.X.; Lin, Q.H.; Wang, X.; Xiao, W.H.; Wang, Y.; Zhou, X.; et al. Bug mapping and fitness testing of chemically synthesized chromosome X. Science 2017, 355, eaaf4706. [Google Scholar] [CrossRef]
- Richardson, S.M.; Mitchell, L.A.; Stracquadanio, G.; Yang, K.; Dymond, J.S.; DiCarlo, J.E.; Lee, D.; Huang, C.L.; Chandrasegaran, S.; Cai, Y.; et al. Design of a synthetic yeast genome. Science 2017, 355, 1040–1044. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Wang, A.; Stracquadanio, G.; Kuang, Z.; Wang, X.; Yang, K.; Richardson, S.; Martin, J.A.; Zhao, Y.; Walker, R.; et al. Synthesis, debugging, and effects of synthetic chromosome consolidation: SynVI and beyond. Science 2017, 355, eaaf4831. [Google Scholar] [CrossRef]
- Mercy, G.; Mozziconacci, J.; Scolari, V.F.; Yang, K.; Zhao, G.; Thierry, A.; Luo, Y.; Mitchell, L.A.; Shen, M.; Shen, Y.; et al. 3D organization of synthetic and scrambled chromosomes. Science 2017, 355, eaaf4597. [Google Scholar] [CrossRef]
- Shen, Y.; Stracquadanio, G.; Wang, Y.; Yang, K.; Mitchell, L.A.; Xue, Y.; Cai, Y.; Chen, T.; Dymond, J.S.; Kang, K.; et al. SCRaMbLE generates designed combinatorial stochastic diversity in synthetic chromosomes. Genome Res. 2016, 26, 36–49. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, L.; Wang, Y.; Zhang, W.; Guo, Y.; Shen, Y.; Jiang, L.; Wu, Q.; Zhang, C.; Cai, Y.; et al. Identifying and characterizing SCRaMbLEd synthetic yeast using ReSCuES. Nat. Commun. 2018, 9, 1930. [Google Scholar] [CrossRef]
- Jia, B.; Wu, Y.; Li, B.Z.; Mitchell, L.A.; Liu, H.; Pan, S.; Wang, J.; Zhang, H.R.; Jia, N.; Li, B.; et al. Precise control of SCRaMbLE in synthetic haploid and diploid yeast. Nat. Commun. 2018, 9, 1933. [Google Scholar] [CrossRef]
- Liu, W.; Luo, Z.; Wang, Y.; Pham, N.T.; Tuck, L.; Pérez-Pi, I.; Liu, L.; Shen, Y.; French, C.; Auer, M.; et al. Rapid pathway prototyping and engineering using in vitro and in vivo synthetic genome SCRaMbLE-in methods. Nat. Commun. 2018, 9, 1936. [Google Scholar] [CrossRef]
- Gowers, G.F.; Chee, S.M.; Bell, D.; Suckling, L.; Kern, M.; Tew, D.; McClymont, D.W.; Ellis, T. Improved betulinic acid biosynthesis using synthetic yeast chromosome recombination and semi-automated rapid LC-MS screening. Nat. Commun. 2020, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Dong, J.; Zhou, T.; Auxillos, J.; Li, T.; Zhang, W.; Wang, L.; Shen, Y.; Luo, Y.; Zheng, Y.; et al. YeastFab: The design and construction of standard biological parts for metabolic engineering in Saccharomyces cerevisiae. Nucleic. Acids Res. 2015, 43, e88. [Google Scholar] [CrossRef] [PubMed]
- Ganley, A.R.; Ide, S.; Saka, K.; Kobayashi, T. The effect of replication initiation on gene amplification in the rDNA and its relationship to aging. Mol. Cell. 2009, 35, 683–693. [Google Scholar] [CrossRef]
- Gietz, R.D.; Schiestl, R.H.; Willems, A.R.; Woods, R.A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 1995, 11, 355–360. [Google Scholar] [CrossRef]
- Annaluru, N.; Muller, H.; Mitchell, L.A.; Ramalingam, S.; Stracquadanio, G.; Richardson, S.M.; Dymond, J.S.; Kuang, Z.; Scheifele, L.Z.; Cooper, E.M.; et al. Total synthesis of a functional designer eukaryotic chromosome. Science 2014, 344, 55–58. [Google Scholar] [CrossRef]
- Li, R.; Yu, C.; Li, Y.; Lam, T.W.; Yiu, S.M.; Kristiansen, K.; Wang, J. SOAP2: An improved ultrafast tool for short read alignment. Bioinformatics 2009, 25, 1966–1967. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Teste, M.A.; Duquenne, M.; François, J.M.; Parrou, J.L. Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol. Biol. 2009, 10, 99. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Blount, B.A.; Gowers, G.F.; Ho, J.C.H.; Ledesma-Amaro, R.; Jovicevic, D.; McKiernan, R.M.; Xie, Z.X.; Li, B.Z.; Yuan, Y.J.; Ellis, T. Rapid host strain improvement by in vivo rearrangement of a synthetic yeast chromosome. Nat. Commun. 2018, 9, 1932. [Google Scholar] [CrossRef]
- Shen, M.J.; Wu, Y.; Yang, K.; Li, Y.; Xu, H.; Zhang, H.; Li, B.Z.; Li, X.; Xiao, W.H.; Zhou, X.; et al. Heterozygous diploid and interspecies SCRaMbLEing. Nat. Commun. 2018, 9, 1934. [Google Scholar] [CrossRef] [PubMed]
- Birchler, J.A.; Yao, H.; Chudalayandi, S. Unraveling the genetic basis of hybrid vigor. Proc. Natl. Acad. Sci. USA 2006, 103, 12957–12958. [Google Scholar] [CrossRef] [PubMed]
- Vizeacoumar, F.J.; Torres-Guzman, J.C.; Bouard, D.; Aitchison, J.D.; Rachubinski, R.A. Pex30p, Pex31p, and Pex32p form a family of peroxisomal integral membrane proteins regulating peroxisome size and number in Saccharomyces cerevisiae. Mol. Biol. Cell. 2004, 15, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Buijs, N.A.; Zhu, Z.; Gómez, D.O.; Boonsombuti, A.; Siewers, V.; Nielsen, J. Harnessing Yeast Peroxisomes for Biosynthesis of Fatty-Acid-Derived Biofuels and Chemicals with Relieved Side-Pathway Competition. J. Am. Chem. Soc. 2016, 138, 15368–15377. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Lopez, E.; Halper, S.M.; Cetnar, D.P.; Reis, A.C.; Strickland, D.; Klavins, E.; Salis, H.M. Automated design of thousands of nonrepetitive parts for engineering stable genetic systems. Nat. Biotechnol. 2020, 38, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Jack, B.R.; Leonard, S.P.; Mishler, D.M.; Renda, B.A.; Leon, D.; Suárez, G.A.; Barrick, J.E. Predicting the Genetic Stability of Engineered DNA Sequences with the EFM Calculator. ACS Synth. Biol. 2015, 4, 939–943. [Google Scholar] [CrossRef]
- Lee, M.E.; Aswani, A.; Han, A.S.; Tomlin, C.J.; Dueber, J.E. Expression-level optimization of a multi-enzyme pathway in the absence of a high-throughput assay. Nucleic. Acids Res. 2013, 41, 10668–10678. [Google Scholar] [CrossRef]
- Partow, S.; Siewers, V.; Bjørn, S.; Nielsen, J.; Maury, J. Characterization of different promoters for designing a new expression vector in Saccharomyces cerevisiae. Yeast 2010, 27, 955–964. [Google Scholar] [CrossRef]
- Ko, Y.S.; Kim, J.W.; Lee, J.A.; Han, T.; Kim, G.B.; Park, J.E.; Lee, S.Y. Tools and strategies of systems metabolic engineering for the development of microbial cell factories for chemical production. Chem. Soc. Rev. 2020, 49, 4615–4636. [Google Scholar] [CrossRef]
- Philipps, G.; De Vries, S.; Jennewein, S. Development of a metabolic pathway transfer and genomic integration system for the syngas-fermenting bacterium Clostridium ljungdahlii. Biotechnol. Biofuels 2019, 12, 112. [Google Scholar] [CrossRef]
- Wery, J.; Gutker, D.; Renniers, A.C.; Verdoes, J.C.; Van Ooyen, A.J. High copy number integration into the ribosomal DNA of the yeast Phaffia rhodozyma. Gene 1997, 184, 89–97. [Google Scholar] [CrossRef]
- Klabunde, J.; Diesel, A.; Waschk, D.; Gellissen, G.; Hollenberg, C.P.; Suckow, M. Single-step co-integration of multiple expressible heterologous genes into the ribosomal DNA of the methylotrophic yeast Hansenula polymorpha. Appl. Microbiol. Biotechnol. 2002, 58, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Murata, N. Regulation of the desaturation of fatty acids and its role in tolerance to cold and salt stress. Curr. Opin. Microbiol. 2002, 5, 208–210. [Google Scholar] [CrossRef]
- Mutalik, V.K.; Guimaraes, J.C.; Cambray, G.; Lam, C.; Christoffersen, M.J.; Mai, Q.A.; Tran, A.B.; Paull, M.; Keasling, J.D.; Arkin, A.P.; et al. Precise and reliable gene expression via standard transcription and translation initiation elements. Nat. Methods 2013, 10, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Zucca, S.; Pasotti, L.; Politi, N.; Cusella De Angelis, M.G.; Magni, P. A standard vector for the chromosomal integration and characterization of BioBrick™ parts in Escherichia coli. J. Biol. Eng. 2013, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Røkke, G.; Korvald, E.; Pahr, J.; Oyås, O.; Lale, R. BioBrick assembly standards and techniques and associated software tools. Methods Mol. Biol. 2014, 1116, 1–24. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chiu, T.-Y.; Zhang, J.-T.; Wang, S.-J.; Wang, S.-W.; Liu, L.-Y.; Ping, Z.; Wang, Y.; Chen, A.; Zhang, W.-W.; et al. Systematical Engineering of Synthetic Yeast for Enhanced Production of Lycopene. Bioengineering 2021, 8, 14. https://doi.org/10.3390/bioengineering8010014
Zhang Y, Chiu T-Y, Zhang J-T, Wang S-J, Wang S-W, Liu L-Y, Ping Z, Wang Y, Chen A, Zhang W-W, et al. Systematical Engineering of Synthetic Yeast for Enhanced Production of Lycopene. Bioengineering. 2021; 8(1):14. https://doi.org/10.3390/bioengineering8010014
Chicago/Turabian StyleZhang, Yu, Tsan-Yu Chiu, Jin-Tao Zhang, Shu-Jie Wang, Shu-Wen Wang, Long-Ying Liu, Zhi Ping, Yong Wang, Ao Chen, Wen-Wei Zhang, and et al. 2021. "Systematical Engineering of Synthetic Yeast for Enhanced Production of Lycopene" Bioengineering 8, no. 1: 14. https://doi.org/10.3390/bioengineering8010014
APA StyleZhang, Y., Chiu, T.-Y., Zhang, J.-T., Wang, S.-J., Wang, S.-W., Liu, L.-Y., Ping, Z., Wang, Y., Chen, A., Zhang, W.-W., Chen, T., Wang, Y., & Shen, Y. (2021). Systematical Engineering of Synthetic Yeast for Enhanced Production of Lycopene. Bioengineering, 8(1), 14. https://doi.org/10.3390/bioengineering8010014




