Toward Spatial Identities in Human Brain Organoids-on-Chip Induced by Morphogen-Soaked Beads
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Human ESC Aggregates
2.3. Device Assembly and Hydrogel Embedment
2.4. Beads Soaking with Morphogens
2.5. qPCR
2.6. Immunostainings
2.7. Antibodies
2.8. Imaging and Analysis
3. Results
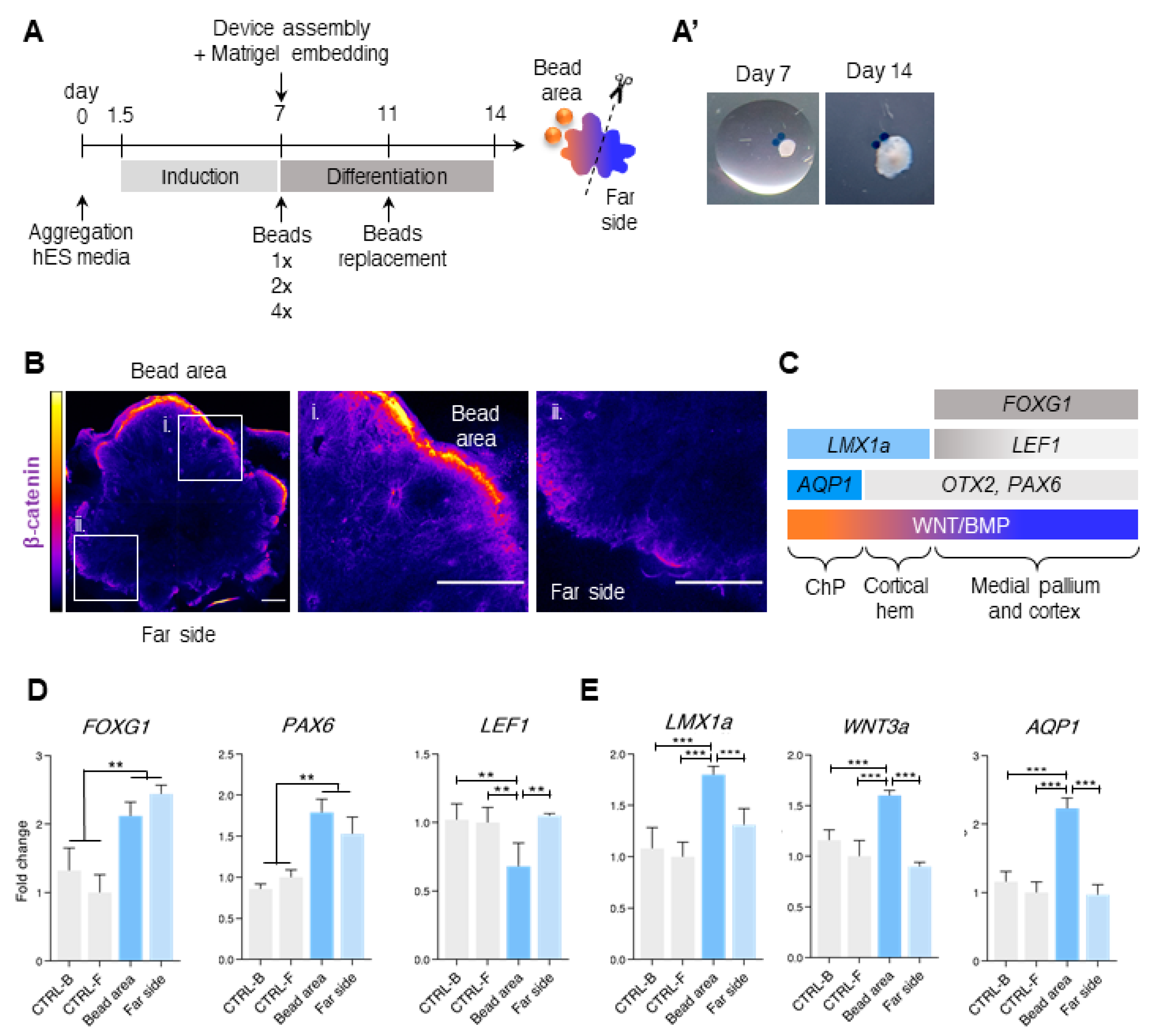
3.1. Efficient Live-Imaging of Neuroepithelium Tissues Derived from hESCs
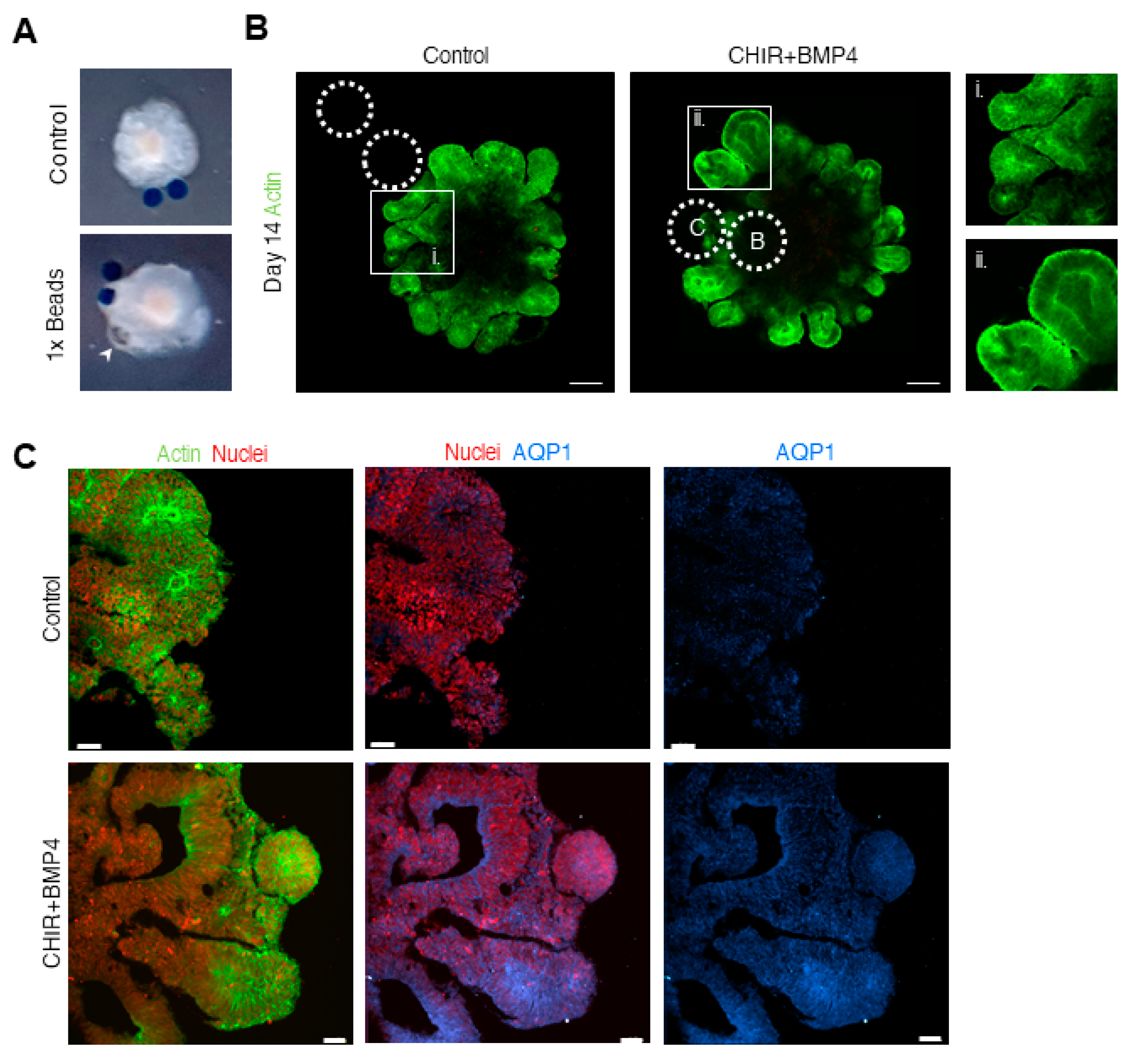
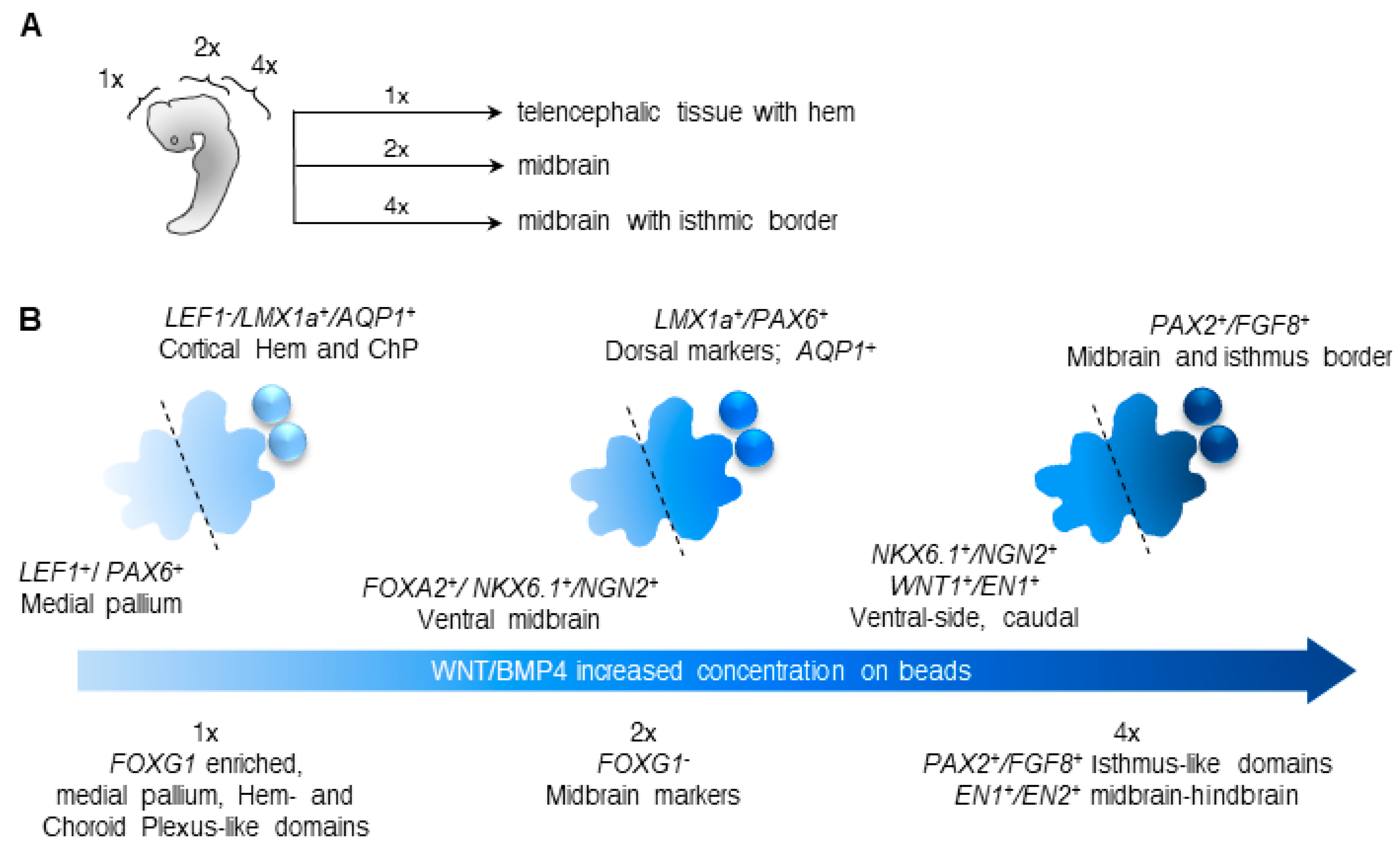
3.2. Different Concentrations of CHIR and BMP4 Polarize Human Brain Organoids
3.3. Liquid-Filled Cysts and Larger Ventricles Correspond to An AQP1 Elevation in the 1× Condition
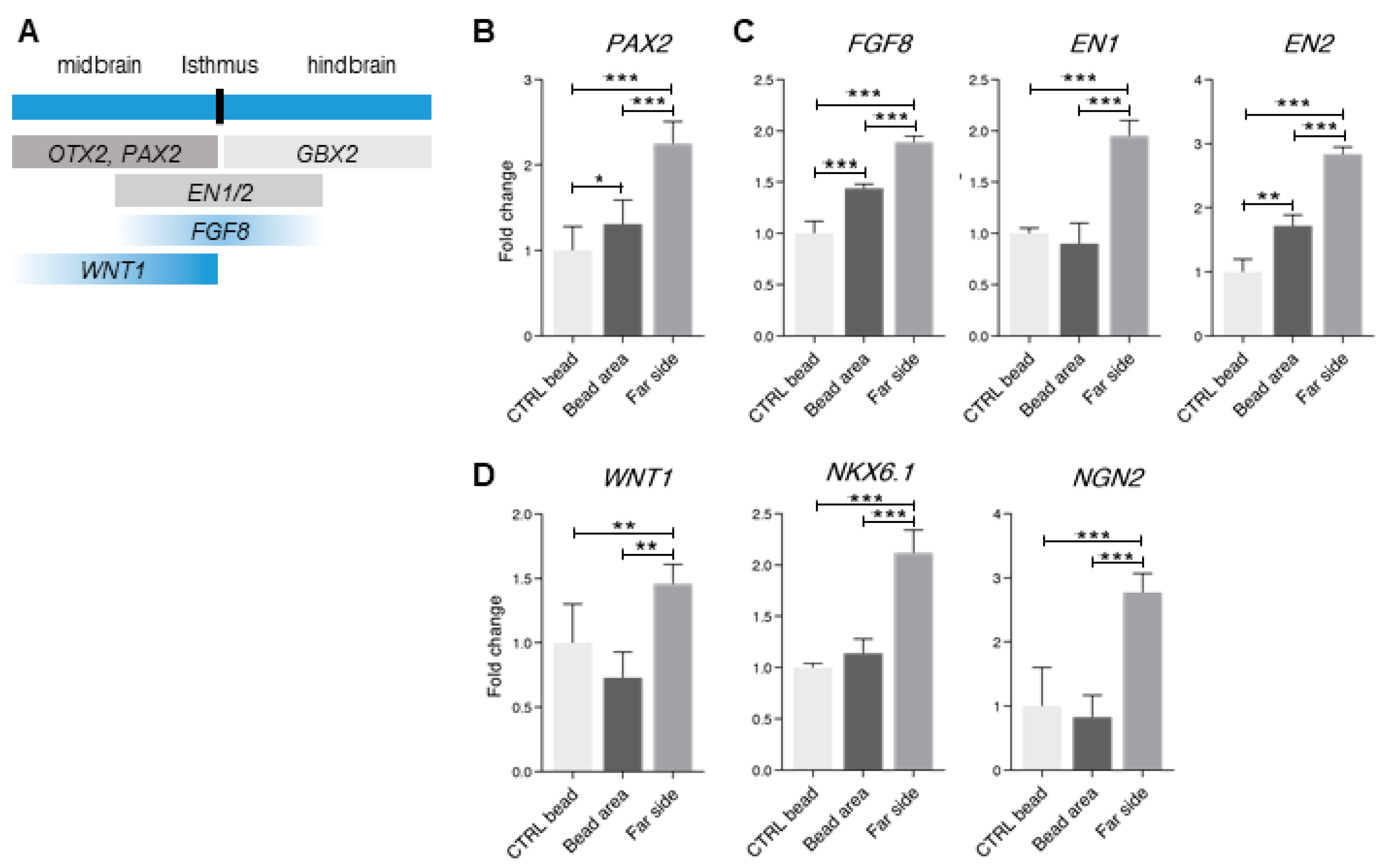
3.4. Dorsal and Ventral Midbrain-Related Markers Are Upregulated in the 2× Condition
3.5. Midbrain-Hindbrain Border Domains and Isthmus-Related Markers Are Observed in the 4× Condition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pasca, A.M.; Sloan, S.A.; Clarke, L.E.; Tian, Y.; Makinson, C.D.; Huber, N.; Kim, C.H.; Park, J.Y.; O’Rourke, N.A.; Nguyen, K.D.; et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 2015, 12, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, E.; Kriegstein, A.R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 2017, 18, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.G.; Badsha, F.; Florio, M.; Kanton, S.; Gerber, T.; Wilsch-Bräuninger, M.; Lewitus, E.; Sykes, A.; Hevers, W.; Lancaster, M.; et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl. Acad. Sci. USA 2015, 112, 15672–15677. [Google Scholar] [CrossRef] [PubMed]
- Karzbrun, E.; Kshirsagar, A.; Cohen, S.R.; Hanna, J.H.; Reiner, O. Human brain organoids on a chip reveal the physics of folding. Nat. Phys. 2018, 14, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, H.; Kadoshima, T.; Soen, M.; Narii, N.; Ishida, Y.; Ohgushi, M.; Takahashi, J.; Eiraku, M.; Sasai, Y. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Bonfio, C.; Chadwick, J.; Begum, F.; Skehel, M.; Lancaster, M.A. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 2020, 369. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Tabata, T.; Takei, Y. Morphogens, their identification and regulation. Development 2004, 131, 703–712. [Google Scholar] [CrossRef]
- Sagner, A.; Briscoe, J. Morphogen interpretation: Concentration, time, competence, and signaling dynamics. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e271. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Small, S. Morphogen rules: Design principles of gradient-mediated embryo patterning. Development 2015, 142, 3996–4009. [Google Scholar] [CrossRef] [PubMed]
- Shimogori, T.; Banuchi, V.; Ng, H.Y.; Strauss, J.B.; Grove, E.A. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development 2004, 131, 5639–5647. [Google Scholar] [CrossRef] [PubMed]
- Kiecker, C.; Lumsden, A. The Role of Organizers in Patterning the Nervous System. Annu. Rev. Neurosci. 2012, 35, 347–367. [Google Scholar] [CrossRef]
- Nordström, U.; Jessell, T.M.; Edlund, T. Progressive induction of caudal neural character by graded wnt signaling. Nat. Neurosci. 2002, 5, 525–532. [Google Scholar] [CrossRef]
- Green, D.; Whitener, A.E.; Mohanty, S.; Lekven, A.C. Vertebrate nervous system posteriorization: Grading the function of Wnt signaling. Dev. Dyn. 2015, 244, 507–512. [Google Scholar] [CrossRef]
- Ribes, V.; Briscoe, J. Establishing and interpreting graded Sonic Hedgehog signaling during vertebrate neural tube patterning: The role of negative feedback. Cold Spring Harb. Perspect. Biol. 2009, 1, a002014. [Google Scholar] [CrossRef]
- Noelanders, R.; Vleminckx, K. How Wnt Signaling Builds the Brain: Bridging Development and Disease. Neuroscientist 2017, 23, 314–329. [Google Scholar] [CrossRef]
- Braun, M.M.; Etheridge, A.; Bernard, A.; Robertson, C.P.; Roelink, H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development 2003, 130, 5579–5587. [Google Scholar] [CrossRef]
- Kunz, M.; Herrmann, M.; Wedlich, D.; Gradl, D. Autoregulation of canonical Wnt signaling controls midbrain development. Dev. Biol. 2004, 273, 390–401. [Google Scholar] [CrossRef][Green Version]
- Abu-Khalil, A.; Fu, L.; Grove, E.A.; Zecevic, N.; Geschwind, D.H. Wnt genes define distinct boundaries in the developing human brain: Implications for human forebrain patterning. J. Comp. Neurol. 2004, 474, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Rifes, P.; Isaksson, M.; Rathore, G.S.; Aldrin-Kirk, P.; Møller, O.K.; Barzaghi, G.; Lee, J.; Egerod, K.L.; Rausch, D.M.; Parmar, M.; et al. Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat. Biotechnol. 2020, 38, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Guo, Y.; Zhu, Y.; Qin, J. Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv. 2018, 8, 1677–1685. [Google Scholar] [CrossRef]
- Demers, C.J.; Soundararajan, P.; Chennampally, P.; Cox, G.A.; Briscoe, J.; Collins, S.D.; Smith, R.L. Development-on-chip: In vitro neural tube patterning with a microfluidic device. Development 2016, 143, 1884–1892. [Google Scholar] [CrossRef]
- Cederquist, G.Y.; Asciolla, J.J.; Tchieu, J.; Walsh, R.M.; Cornacchia, D.; Resh, M.D.; Studer, L. Specification of positional identity in forebrain organoids. Nat. Biotechnol. 2019, 37, 436–444. [Google Scholar] [CrossRef]
- Bagley, J.A.; Reumann, D.; Bian, S.; Lévi-Strauss, J.; Knoblich, J.A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 2017, 14, 743–751. [Google Scholar] [CrossRef]
- Birey, F.; Andersen, J.; Makinson, C.D.; Islam, S.; Wei, W.; Huber, N.; Fan, H.C.; Metzler, K.R.C.; Panagiotakos, G.; Thom, N.; et al. Assembly of functionally integrated human forebrain spheroids. Nature 2017, 545, 54–59. [Google Scholar] [CrossRef]
- Xiang, Y.; Tanaka, Y.; Patterson, B.; Kang, Y.J.; Govindaiah, G.; Roselaar, N.; Cakir, B.; Kim, K.Y.; Lombroso, A.P.; Hwang, S.M.; et al. Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell Stem Cell 2017, 21, 383–398.e7. [Google Scholar] [CrossRef]
- Libby, A.R.G.; Joy, D.A.; Elder, N.H.; Bulger, E.A.; Krakora, M.Z.; Gaylord, E.A.; Mendoza-Camacho, F.; Butts, J.C.; McDevitt, T.C. Elongation of Caudalized Human Organoids Mimics Neural Tube Development. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gurdon, J.B.; Mitchell, A.; Mahony, D. Direct and continuous assessment by cells of their position in a morphogen gradient. Nature 1995, 376, 520–521. [Google Scholar] [CrossRef]
- Pagán-Westphal, S.M.; Tabin, C.J. The transfer of left-right positional information during chick embryogenesis. Cell 1998, 93, 25–35. [Google Scholar] [CrossRef]
- Habib, S.J.; Chen, B.C.; Tsai, F.C.; Anastassiadis, K.; Meyer, T.; Betzig, E.; Nusse, R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science 2013, 339, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Gafni, O.; Weinberger, L.; Mansour, A.A.; Manor, Y.S.; Chomsky, E.; Ben-Yosef, D.; Kalma, Y.; Viukov, S.; Maza, I.; Zviran, A.; et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013, 504, 282–286. [Google Scholar] [CrossRef]
- Karzbrun, E.; Tshuva, R.Y.; Reiner, O. An On-Chip Method for Long-Term Growth and Real-Time Imaging of Brain Organoids. Curr. Protoc. Cell Biol. 2018, 81, e62. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.-Y.; Meir, Y.-J.J.; Coates, C.J.; Handler, A.M.; Pelczar, P.; Moisyadi, S.; Kaminski, J.M. piggyBac is a flexible and highly active transposon as compared to sleeping beauty, Tol2, and Mos1 in mammalian cells. Proc. Natl. Acad. Sci. USA 2006, 103, 15008–15013. [Google Scholar] [CrossRef]
- Sakaue-Sawano, A.; Kurokawa, H.; Morimura, T.; Hanyu, A.; Hama, H.; Osawa, H.; Kashiwagi, S.; Fukami, K.; Miyata, T.; Miyoshi, H.; et al. Visualizing Spatiotemporal Dynamics of Multicellular Cell-Cycle Progression. Cell 2008, 132, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Sapir, T.; Levy, T.; Kozer, N.; Shin, I.; Zamor, V.; Haffner-Krausz, R.; McGlade, J.C.; Reiner, O. Notch activation by shootin1 opposing activities on 2 ubiquitin ligases. Cereb. Cortex 2018, 28, 3115–3128. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Hu, Y.; St Amand, T.; Zhang, M.; Ramamurthy, R.; Qiu, M.; Chen, Y.P. Msx1 is required for the induction of Patched by Sonic hedgehog in the mammalian tooth germ. Dev. Dyn. 1999, 215, 45–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Zhao, X.; Yu, X.; Hu, Y.; Geronimo, B.; Fromm, S.H.; Chen, Y.P. A new function of BMP4: Dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development 2000, 127, 1431–1443. [Google Scholar]
- Ogura, T.; Sakaguchi, H.; Miyamoto, S.; Takahashi, J. Three-dimensional induction of dorsal, intermediate and ventral spinal cord tissues from human pluripotent stem cells. Development 2018, 145. [Google Scholar] [CrossRef]
- Takahashi, T.; Nowakowski, R.S.; Caviness, V.S. The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J. Neurosci. 1995, 15, 6046–6057. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Okamoto, M.; Shinoda, T.; Kawaguchi, A. Interkinetic nuclear migration generates and opposes ventricular-zone crowding: Insight into tissue mechanics. Front. Cell. Neurosci. 2014, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Liem, K.F.; Tremml, G.; Roelink, H.; Jessell, T.M. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 1995, 82, 969–979. [Google Scholar] [CrossRef]
- Echelard, Y.; Epstein, D.J.; St-Jacques, B.; Shen, L.; Mohler, J.; McMahon, J.A.; McMahon, A.P. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 1993, 75, 1417–1430. [Google Scholar] [CrossRef]
- Lee, K.J.; Jessell, T.M. The specification of dorsal cell fates in the vertebrate central nervous system. Annu. Rev. Neurosci. 1999, 22, 261–294. [Google Scholar] [CrossRef]
- Wilson, L.; Maden, M. The mechanisms of dorsoventral patterning in the vertebrate neural tube. Dev. Biol. 2005, 282, 1–13. [Google Scholar] [CrossRef]
- Jia, S.H.; Doan, L.T.; Currle, D.S.; Paff, M.; Rheem, J.Y.; Schreyer, R.; Robert, B.; Monuki, E.S. Border formation in a Bmp gradient reduced to single dissociated cells. Proc. Natl. Acad. Sci. USA 2008, 105, 3398–3403. [Google Scholar]
- Eiraku, M.; Watanabe, K.; Matsuo-Takasaki, M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell 2008, 3, 519–532. [Google Scholar] [CrossRef]
- Grove, E.A.; Tole, S.; Limon, J.; Yip, L.; Ragsdale, C.W. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development 1998, 125, 2315–2325. [Google Scholar] [PubMed]
- Lee, S.M.; Tole, S.; Grove, E.; McMahon, A.P. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development 2000, 127, 457–467. [Google Scholar] [PubMed]
- Furuta, Y.; Piston, D.W.; Hogan, B.L. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 1997, 124, 2203–2212. [Google Scholar] [PubMed]
- Hébert, J.M.; Mishina, Y.; McConnell, S.K. BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron 2002, 35, 1029–1041. [Google Scholar] [CrossRef]
- Dou, C.L.; Li, S.; Lai, E. Dual role of brain factor-1 in regulating growth and patterning of the cerebral hemispheres. Cereb. Cortex 1999, 9, 543–550. [Google Scholar] [CrossRef]
- Hanashima, C.; Fernandes, M.; Hebert, J.M.; Fishell, G. The role of Foxg1 and dorsal midline signaling in the generation of Cajal-Retzius subtypes. J. Neurosci. 2007, 27, 11103–11111. [Google Scholar] [CrossRef] [PubMed]
- Galceran, J.; Miyashita-Lin, E.M.; Devaney, E.; Rubenstein, J.L.; Grosschedl, R. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development 2000, 127, 469–482. [Google Scholar] [PubMed]
- Stoykova, A.; Gruss, P. Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J. Neurosci. 1994, 14, 1395–1412. [Google Scholar] [CrossRef]
- Mallamaci, A.; Stoykova, A. Gene networks controlling early cerebral cortex arealization. Eur. J. Neurosci. 2006, 23, 847–856. [Google Scholar] [CrossRef]
- Castelo-Branco, G.; Wagner, J.; Rodriguez, F.J.; Kele, J.; Sousa, K.; Rawal, N.; Pasolli, H.A.; Fuchs, E.; Kitajewski, J.; Arenas, E. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc. Natl. Acad. Sci. USA 2003, 100, 12747–12752. [Google Scholar] [CrossRef]
- Rawal, N.; Castelo-Branco, G.; Sousa, K.M.; Kele, J.; Kobayashi, K.; Okano, H.; Arenas, E. Dynamic temporal and cell type-specific expression of Wnt signaling components in the developing midbrain. Exp. Cell Res. 2006, 312, 1626–1636. [Google Scholar] [CrossRef]
- Kittappa, R.; Chang, W.W.; Awatramani, R.B.; McKay, R.D.G. The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol. 2007, 5, 2875–2884. [Google Scholar] [CrossRef]
- Gale, E.; Li, M. Midbrain dopaminergic neuron fate specification: Of mice and embryonic stem cells. Mol. Brain 2008, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Joksimovic, M.; Awatramani, R. Wnt/β-catenin signaling in midbrain dopaminergic neuron specification and neurogenesis. J. Mol. Cell Biol. 2014, 6, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Katahira, T.; Matsunaga, E.; Sato, T. Isthmus organizer for midbrain and hindbrain development. Brain Res. Brain Res. Rev. 2005, 49, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Joyner, A.L.; Liu, A.; Millet, S. Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr. Opin. Cell Biol. 2000, 12, 736–741. [Google Scholar] [CrossRef]
- Gibbs, H.C.; Chang-Gonzalez, A.; Hwang, W.; Yeh, A.T.; Lekven, A.C. Midbrain-hindbrain boundary morphogenesis: At the intersection of wnt and Fgf signaling. Front. Neuroanat. 2017, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, M.; Lun, K.; Luz, M.; Werner, M.; Brand, M. Positioning of the midbrain-hindbrain boundary organizer through global posteriorization of the neuroectoderm mediated by Wnt8 signaling. Development 2005, 132, 1261–1272. [Google Scholar] [CrossRef]
- Waters, S.T.; Lewandoski, M. A threshold requirement for Gbx2 levels in hindbrain development. Development 2006, 133, 1991–2000. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.S.; Hwang, H.S.; Lee, D.R.; Park, C.Y.; Jung, S.J.; You, Y.R.; Kim, D.S.; Kim, D.W. Wnt signal activation induces midbrain specification through direct binding of the beta-catenin/TCF4 complex to the EN1 promoter in human pluripotent stem cells. Exp. Mol. Med. 2018, 50, 24. [Google Scholar] [CrossRef]
- Kiecker, C.; Niehrs, C. A morphogen gradient of Wnt/β-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 2001, 128, 4189–4201. [Google Scholar]
- Toda, S.; McKeithan, W.L.; Hakkinen, T.J.; Lopez, P.; Klein, O.D.; Lim, W.A. Engineering synthetic morphogen systems that can program multicellular patterning. Science 2020, 370, 327–331. [Google Scholar] [CrossRef]
- Stapornwongkul, K.S.; de Gennes, M.; Cocconi, L.; Salbreux, G.; Vincent, J.P. Patterning and growth control in vivo by an engineered GFP gradient. Science 2020, 370, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Wurst, W.; Bally-Cuif, L.; Bally-Cuif, L. Neural plate patterning: Upstream and downstream of the isthmic organizer. Nat. Rev. Neurosci. 2001, 2, 99–108. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Reuven, L.; Reiner, O. Toward Spatial Identities in Human Brain Organoids-on-Chip Induced by Morphogen-Soaked Beads. Bioengineering 2020, 7, 164. https://doi.org/10.3390/bioengineering7040164
Ben-Reuven L, Reiner O. Toward Spatial Identities in Human Brain Organoids-on-Chip Induced by Morphogen-Soaked Beads. Bioengineering. 2020; 7(4):164. https://doi.org/10.3390/bioengineering7040164
Chicago/Turabian StyleBen-Reuven, Lihi, and Orly Reiner. 2020. "Toward Spatial Identities in Human Brain Organoids-on-Chip Induced by Morphogen-Soaked Beads" Bioengineering 7, no. 4: 164. https://doi.org/10.3390/bioengineering7040164
APA StyleBen-Reuven, L., & Reiner, O. (2020). Toward Spatial Identities in Human Brain Organoids-on-Chip Induced by Morphogen-Soaked Beads. Bioengineering, 7(4), 164. https://doi.org/10.3390/bioengineering7040164




