Computational Fluid Dynamics (CFD)-Based Optimization of Injection Process during Endoscopic Mucosal Therapy
Abstract
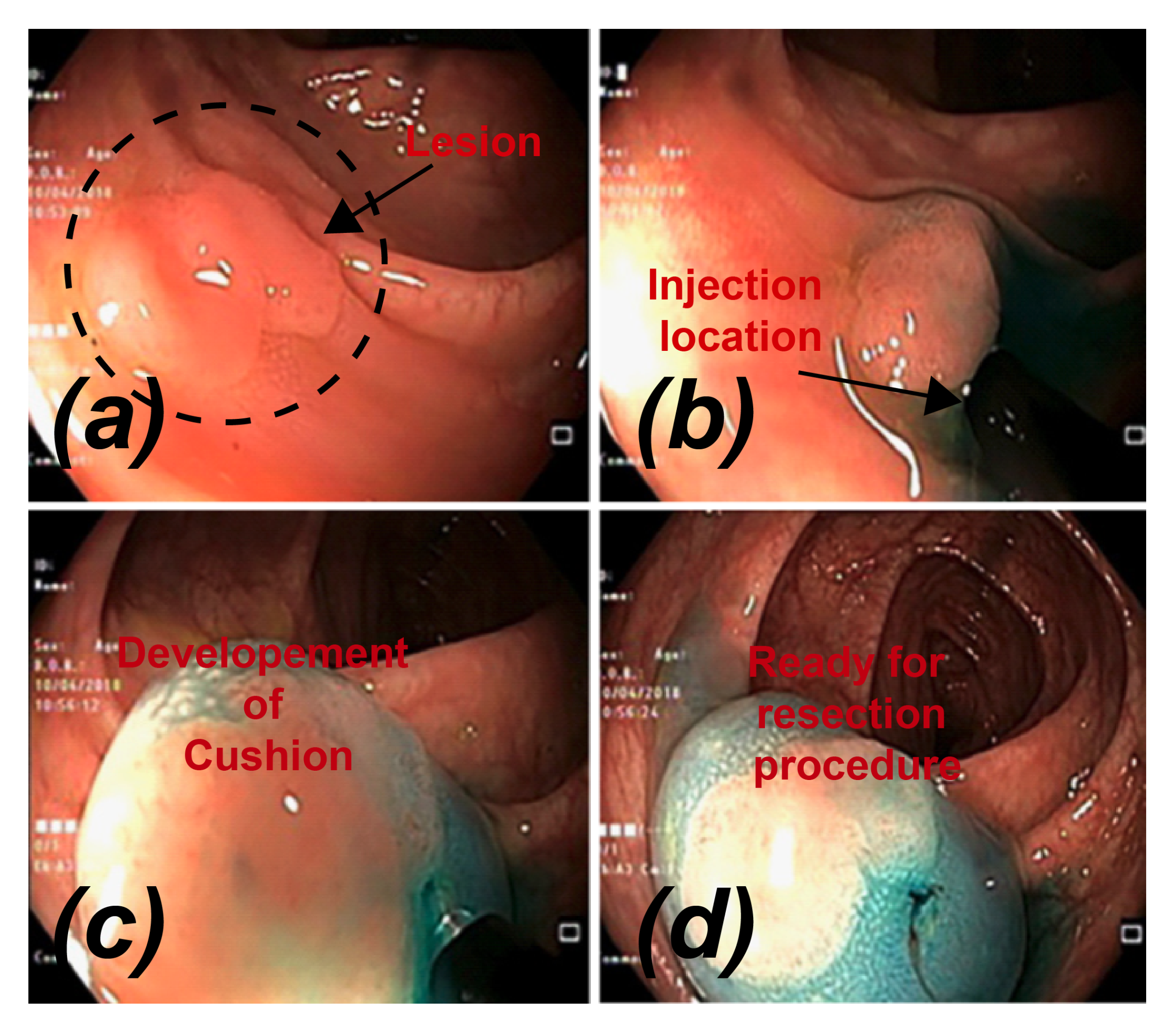
1. Introduction
2. Materials and Methods
3. Computational Modeling
3.1. Governing Equations
3.2. Lifting Agents
3.3. Flow Geometry and Boundary Conditions
4. Results
4.1. Static Injection
4.2. Dynamic Injection
4.3. Temperature
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Waye, J.D.; Schapiro, M.; Bond, J.H.; Panish, J.F.; et al. Prevention of colorectal cancer by colonoscopic polypectomy. N. Engl. J. Med. 1993, 329, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F.; et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Kakushima, N.; Fujishiro, M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J. Gastroenterol. WJG 2008, 14, 2962. [Google Scholar] [CrossRef]
- Hwang, J.H.; Konda, V.; Dayyeh, B.K.A.; Chauhan, S.S.; Enestvedt, B.K.; Fujii-Lau, L.L.; Komanduri, S.; Maple, J.T.; Murad, F.M.; Pannala, R.; et al. Endoscopic mucosal resection. Gastrointest. Endosc. 2015, 82, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Maple, J.T.; Dayyeh, B.K.A.; Chauhan, S.S.; Hwang, J.H.; Komanduri, S.; Manfredi, M.; Konda, V.; Murad, F.M.; Siddiqui, U.D.; Banerjee, S. Endoscopic submucosal dissection. Gastrointest. Endosc. 2015, 81, 1311–1325. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, S.; Dobosz, M.; Babicki, A.; Głowacki, J.; Nałęcz, A. Blood supply of colorectal polyps correlates with risk of bleeding after colonoscopic polypectomy. Gastrointest. Endosc. 2006, 63, 1004–1009. [Google Scholar] [CrossRef]
- Facciorusso, A.; Di Maso, M.; Antonino, M.; Del Prete, V.; Panella, C.; Barone, M.; Muscatiello, N. Polidocanol injection decreases the bleeding rate after colon polypectomy: A propensity score analysis. Gastrointest. Endosc. 2015, 82, 350–358. [Google Scholar] [CrossRef]
- Han, S.J.; Jung, Y.; Cho, Y.S.; Chung, I.K.; Kim, J.Y.; Eun, J.Y.; Lee, S.H.; Ko, G.B.; Lee, T.H.; Park, S.H.; et al. Clinical Effectiveness of Submucosal Injection with Indigo Carmine Mixed Solution for Colon Endoscopic Mucosal Resection. Dig. Dis. Sci. 2018, 63, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Libânio, D.; Pita, I.; Dinis-Ribeiro, M. Solutions for submucosal injection: What to choose and how to do it. World J. Gastroenterol. 2019, 25, 777. [Google Scholar] [CrossRef]
- Repici, A.; Wallace, M.; Sharma, P.; Bhandari, P.; Lollo, G.; Maselli, R.; Hassan, C.; Rex, D.K. A novel submucosal injection solution for endoscopic resection of large colorectal lesions: A randomized, double-blind trial. Gastrointest. Endosc. 2018, 88, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Huai, Z.Y.; Xian, W.F.; Jiang, L.C.; Chen, W.X. Submucosal injection solution for endoscopic resection in gastrointestinal tract: A traditional and network meta-analysis. Gastroenterol. Res. Pract. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Yandrapu, H.; Desai, M.; Siddique, S.; Vennalganti, P.; Vennalaganti, S.; Parasa, S.; Rai, T.; Kanakadandi, V.; Bansal, A.; Titi, M.; et al. Normal saline solution versus other viscous solutions for submucosal injection during endoscopic mucosal resection: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Bluestein, D. Utilizing Computational Fluid Dynamics in cardiovascular engineering and medicine—What you need to know. Its translation to the clinic/bedside. Artif. Organs 2017, 41, 117. [Google Scholar] [CrossRef]
- Keeler, J.A.; Patki, A.; Woodard, C.R.; Frank-Ito, D.O. A computational study of nasal spray deposition pattern in four ethnic groups. J. Aerosol. Med. Pulm. Drug Deliv. 2016, 29, 153–166. [Google Scholar] [CrossRef]
- Tian, G.; Hindle, M.; Lee, S.; Longest, P.W. Validating CFD predictions of pharmaceutical aerosol deposition with in vivo data. Pharm. Res. 2015, 32, 3170–3187. [Google Scholar] [CrossRef]
- Fluent, A. ANSYS Fluent Theory Guide 15.0; ANSYS: Canonsburg, PA, USA, 2013. [Google Scholar]
- Uraoka, T.; Fujii, T.; Saito, Y.; Sumiyoshi, T.; Emura, F.; Bhandari, P.; Matsuda, T.; Fu, K.I.; Saito, D. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest. Endosc. 2005, 61, 736–740. [Google Scholar] [CrossRef]
- Sumiyoshi, T.; Fujii, T.; Sumiyoshi, Y.; Matsuda, T.; Kozu, T.; Saito, D.; Akasu, T.; Fu, K. Injected substances to the submucosa in endoscopic mucosal resection-glycerin solution versus normal saline solution. In Gastrointestinal Endoscopy; Mosby, Inc.: St. Louis, MO, USA, 2002; Volume 55, p. AB110. [Google Scholar]
- Ferreira, A.O.; Moleiro, J.; Torres, J.; Dinis-Ribeiro, M. Solutions for submucosal injection in endoscopic resection: A systematic review and meta-analysis. Endosc. Int. Open 2016, 4, E1–E16. [Google Scholar] [CrossRef]
- Isomoto, H.; Shikuwa, S.; Yamaguchi, N.; Fukuda, E.; Ikeda, K.; Nishiyama, H.; Ohnita, K.; Mizuta, Y.; Shiozawa, J.; Kohno, S. Endoscopic submucosal dissection for early gastric cancer: A large-scale feasibility study. Gut 2009, 58, 331–336. [Google Scholar] [CrossRef]
- Uraoka, T.; Saito, Y.; Yamamoto, K.; Fujii, T. Submucosal injection solution for gastrointestinal tract endoscopic mucosal resection and endoscopic submucosal dissection. Drug Des. Dev. Ther. 2008, 2, 131. [Google Scholar] [CrossRef]
- Jin Hyun, J.; Rae Chun, H.; Jai Chun, H.; Tae Jeen, Y.; Won Baeck, C.; Kyun Yu, S.; Sik Kim, Y.; Sik Lee, H.; Ho Um, S.; Woo Lee, S.; et al. Comparison of the characteristics of submucosal injection solutions used in endoscopic mucosal resection. Scand. J. Gastroenterol. 2006, 41, 488–492. [Google Scholar] [CrossRef]




| 30 Degrees | 60 Degrees | |||||
|---|---|---|---|---|---|---|
| Eleview | Orise | Glycerol | Eleview | Orise | Glycerol | |
| 0.1 s | 8.1814 mm | 7.6182 mm | 7.2428 mm | 6.2480 mm | 6.3075 mm | 6.0108 mm |
| 0.15 s | 8.9531 mm | 8.6402 mm | 8.1605 mm | 7.2356 mm | 7.2502 mm | 6.9108 mm |
| 0.2 s | 9.5788 mm | 9.2659 mm | 9.2659 mm | 7.8217 mm | 7.8535 mm | 7.4495 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghaie Meybodi, M.; Saini, R.; Mehdizadeh, A.; Hejazi, R. Computational Fluid Dynamics (CFD)-Based Optimization of Injection Process during Endoscopic Mucosal Therapy. Bioengineering 2020, 7, 136. https://doi.org/10.3390/bioengineering7040136
Aghaie Meybodi M, Saini R, Mehdizadeh A, Hejazi R. Computational Fluid Dynamics (CFD)-Based Optimization of Injection Process during Endoscopic Mucosal Therapy. Bioengineering. 2020; 7(4):136. https://doi.org/10.3390/bioengineering7040136
Chicago/Turabian StyleAghaie Meybodi, Mohamad, Rohit Saini, Amirfarhang Mehdizadeh, and Reza Hejazi. 2020. "Computational Fluid Dynamics (CFD)-Based Optimization of Injection Process during Endoscopic Mucosal Therapy" Bioengineering 7, no. 4: 136. https://doi.org/10.3390/bioengineering7040136
APA StyleAghaie Meybodi, M., Saini, R., Mehdizadeh, A., & Hejazi, R. (2020). Computational Fluid Dynamics (CFD)-Based Optimization of Injection Process during Endoscopic Mucosal Therapy. Bioengineering, 7(4), 136. https://doi.org/10.3390/bioengineering7040136





