Mineralization of Biomaterials for Bone Tissue Engineering
Abstract
1. Introduction
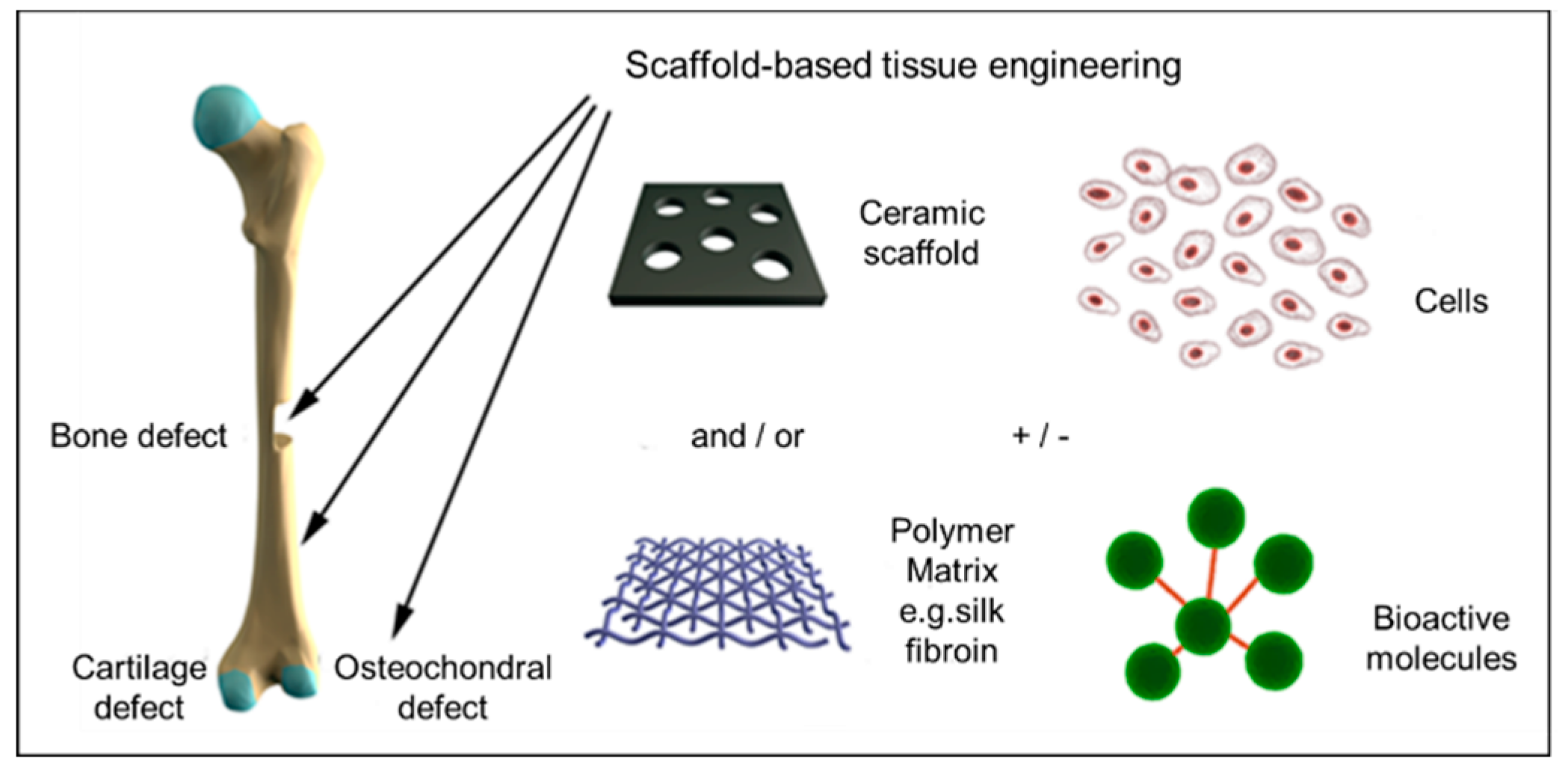
1.1. Engineering Bone Scaffolds
1.2. Conventional Scaffolds for Bone Tissue Engineering
1.3. Limitations of Currently Available Scaffolds
1.4. Mineralized Scaffolds
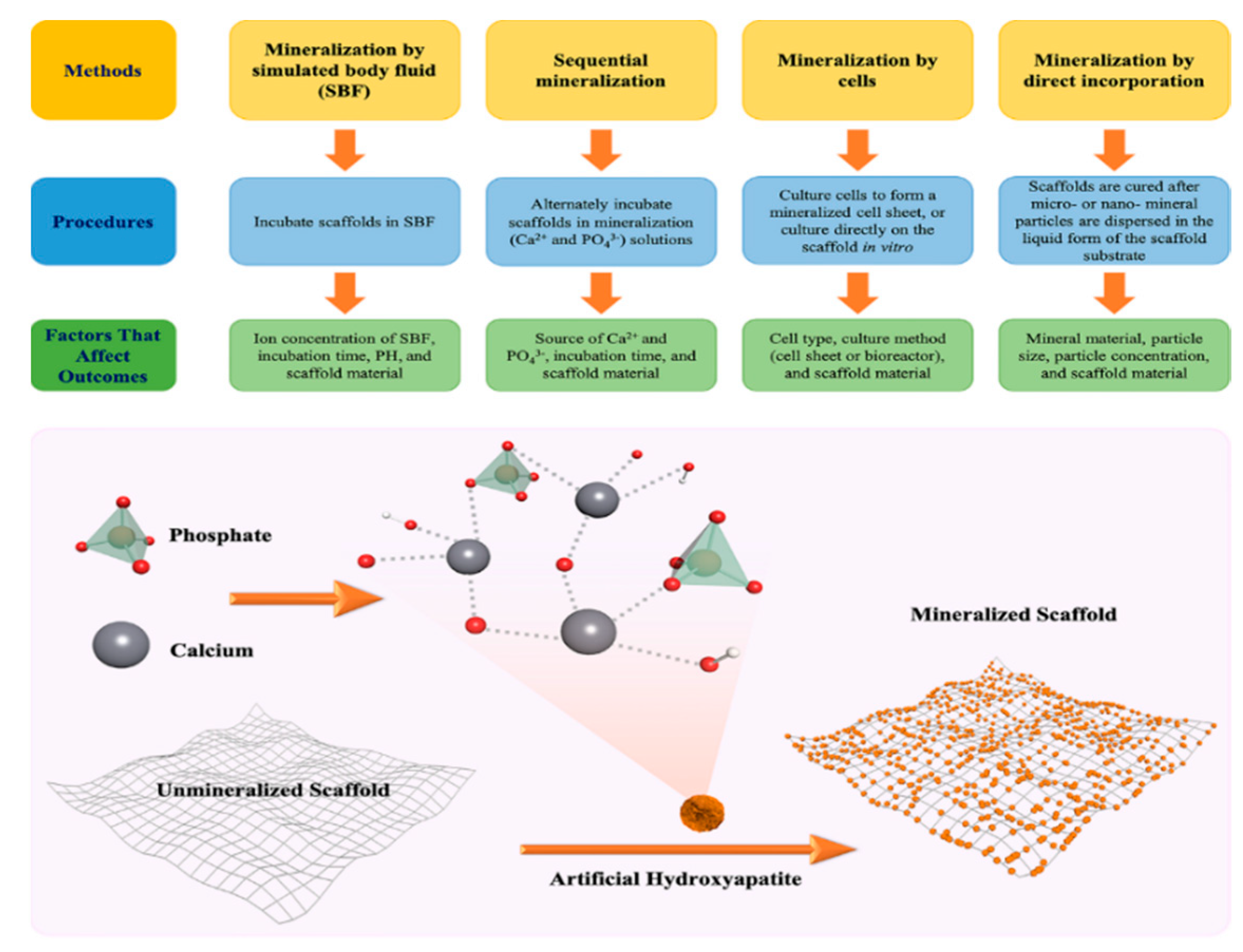
2. Fabrication of Mineralized Biomaterials
2.1. Mineralization by Simulated Body Fluid (SBF)
2.2. Sequential Mineralization via Deposition of Calcium (Ca2+) and Phosphate (PO43−)
2.3. Mineralization by Cells
2.4. Mineralization by Direct Incorporation of Minerals
3. Assessment of Mineral Deposition
3.1. Quantification of Mineral Deposition
3.2. Characterization of the Chemical Composition
3.3. Characterization of the Crystal Structure
3.4. Surface Characterization
3.5. High-Resolution 3D Imaging
3.6. Mechanical Testing
4. Applications of Mineralized Biomaterials in Bone Tissue Engineering
4.1. Mineralized Bone Scaffolds with Improved Osteoconductive and Osteoinductive Activity
4.2. Drug Delivery
5. Conclusions and Further Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef] [PubMed]
- Bolander, M.E. Regulation of Fracture Repair by Growth Factors. Exp. Biol. Med. 1992, 200, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium Enhances Osteogenic Differentiation of Mesenchymal Stem Cells and In Vivo Bone Formation by Activating Wnt/Catenin Signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.Y.; Nusse, R. The Wnt Signaling Pathway in Development and Disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A. The Cell and Molecular Biology of Fracture Healing. Clin. Orthop. Relat. Res. 1998, 355, S7–S21. [Google Scholar] [CrossRef]
- McKibbin, B. The biology of fracture healing in long bones. J. Bone Jt. Surgery. 1978, 60, 150–162. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef]
- Lo, S.-C.; Li, K.-C.; Chang, Y.; Hsu, M.-N.; Sung, L.-Y.; Vu, T.A.; Hu, Y.-C. Enhanced critical-size calvarial bone healing by ASCs engineered with Cre/loxP-based hybrid baculovirus. Biomaterials 2017, 124, 1–11. [Google Scholar] [CrossRef]
- Song, J.; Saiz, E.; Bertozzi, C.R. A New Approach to Mineralization of Biocompatible Hydrogel Scaffolds: An Efficient Process toward 3-Dimensional Bonelike Composites. J. Am. Chem. Soc. 2003, 125, 1236–1243. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Laromaine, A.; Hong, E.; Derda, R.; Whitesides, G.M. Biomineralization Guided by Paper Templates. Sci. Rep. 2016, 6, 27693. [Google Scholar] [CrossRef]
- Arora, A.; Katti, D.S. Understanding the influence of phosphorylation and polysialylation of gelatin on mineralization and osteogenic differentiation. Mater. Sci. Eng. C 2016, 65, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Suvarnapathaki, S.; Nguyen, M.A.; Wu, X.; Nukavarapu, S.P.; Camci-Unal, G. Synthesis and characterization of photocrosslinkable hydrogels from bovine skin gelatin. RSC Adv. 2019, 9, 13016–13025. [Google Scholar] [CrossRef]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef]
- Golebiowska, M.A.A.; Kim, H.S.; Camci-Unal, G.; Nukavarapu, S.P. Integration of Technologies for Bone Tissue Engineering. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Hancock, M.J.; Piraino, F.; Camci-Unal, G.; Rasponi, M.; Khademhosseini, A. Anisotropic material synthesis by capillary flow in a fluid stripe. Biomaterials 2011, 32, 6493–6504. [Google Scholar] [CrossRef] [PubMed]
- Suvarnapathaki, S.; Wu, X.; Lantigua, D.; Nguyen, M.A.; Camci-Unal, G. Breathing life into engineered tissues using oxygen-releasing biomaterials. NPG Asia Mater. 2019, 11, 1–18. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Cheng, M.-H.; Engel, H.; Kao, S.-W.; Larson, J.C.; Gupta, S.; Brey, E.M. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials 2011, 32, 6045–6051. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A.; Wong, W.J.; Teoh, S.-H. Surface modification of PCL-TCP scaffolds in rabbit calvaria defects: Evaluation of scaffold degradation profile, biomechanical properties and bone healing patterns. J. Biomed. Mater. Res. Part A 2010, 93, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Lim, J.W.; Naren, R.; Yun, H.-S.; Park, E.K. Effect of the biodegradation rate controlled by pore structures in magnesium phosphate ceramic scaffolds on bone tissue regeneration in vivo. Acta Biomater. 2016, 44, 155–167. [Google Scholar] [CrossRef]
- Taylor, B.; Indano, S.; Yankannah, Y.; Patel, P.; Perez, X.I.; Freeman, J.W. Decellularized Cortical Bone Scaffold Promotes Organized Neovascularization In Vivo. Tissue Eng. Part. A 2019, 25, 964–977. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Li, J.J.; Kaplan, D.L.; Zreiqat, H. Scaffold-based regeneration of skeletal tissues to meet clinical challenges. J. Mater. Chem. B 2014, 2, 7272–7306. [Google Scholar] [CrossRef]
- Hjortnaes, J.; Goettsch, C.; Hutcheson, J.D.; Camci-Unal, G.; Lax, L.; Scherer, K.; Body, S.; Schoen, F.J.; Kluin, J.; Khademhosseini, A.; et al. Simulation of early calcific aortic valve disease in a 3D platform: A role for myofibroblast differentiation. J. Mol. Cell. Cardiol. 2016, 94, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Aidun, A.; Zamanian, A.; Ghorbani, F. Novel bioactive porous starch-siloxane matrix for bone regeneration: Physicochemical, mechanical, and in vitro properties. Biotechnol. Appl. Biochem. 2018, 66, 43–52. [Google Scholar] [CrossRef]
- Madihally, S.V.; Matthew, H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Landis, W.J.; Silver, F.H.; Freeman, J.W. Collagen as a scaffold for biomimetic mineralization of vertebrate tissues. J. Mater. Chem. 2006, 16, 1495–1503. [Google Scholar] [CrossRef]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2015, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.E. Bone Regeneration within a Coralline Hydroxyapatite Implant. Plast. Reconstr. Surg. 1979, 63, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, C.A.; Bonassar, L.J.; Vacanti, M.P.; Shufflebarger, J. Replacement of an Avulsed Phalanx with Tissue-Engineered Bone. N. Engl. J. Med. 2001, 344, 1511–1514. [Google Scholar] [CrossRef]
- Yuan, H.; Kurashina, K.; De Bruijn, J.D.; Li, Y.; De Groot, K.; Zhang, X. A preliminary study on osteoinduction of two kinds of calcium phosphate ceramics. Biomaterials 1999, 20, 1799–1806. [Google Scholar] [CrossRef]
- Flatley, T.J.; Lynch, K.L.; Benson, M. Tissue Response to Implants of Calcium Phosphate Ceramic in the Rabbit Spine. Clin. Orthop. Relat. Res. 1983, 179, 246–252. [Google Scholar] [CrossRef]
- Graves, G.A.; Noyes, F.R.; Villanueva, A.R. The influence of compositional variations on bone ingrowth of implanted porous calcium aluminate ceramics. J. Biomed. Mater. Res. 1975, 9, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; El-Amin, S.F.; Ibim, S.E.; Willoughby, D.A.; Attawia, M.; Allcock, H.R.; Ambrosio, A.A. A highly porous 3-dimensional polyphosphazene polymer matrix for skeletal tissue regeneration. J. Biomed. Mater. Res. 1996, 30, 133–138. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone tissue engineering scaffolding: Computer-aided scaffolding techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef]
- Zhou, G.; Chang, W.; Yu, X. Nanofibrous Nerve Conduits with Pre-seeded Bone Marrow Stromal Cells and Cultured by Bioreactor for Enhancing Peripheral Nerve Regeneration. Regen. Eng. Transl. Med. 2018, 4, 154–161. [Google Scholar] [CrossRef]
- Black, J. Biological Performance of Materials; CRC Press: Cleveland, OH, USA, 2005. [Google Scholar] [CrossRef]
- Gkioni, K.; Leeuwenburgh, S.C.; Douglas, T.E.; Mikos, A.G.; Jansen, J.A. Mineralization of Hydrogels for Bone Regeneration. Tissue Eng. Part. B Rev. 2010, 16, 577–585. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Y.; Liu, X.; Chen, J.; Zhang, Q. Biomimetic mineralization on natural and synthetic polymers to prepare hybrid scaffolds for bone tissue engineering. Colloids Surf. B Biointerfaces 2019, 178, 222–229. [Google Scholar] [CrossRef]
- Yu, S.-H.; Cölfen, H. Bio-inspired crystal morphogenesis by hydrophilic polymers. J. Mater. Chem. 2004, 14, 2124–2147. [Google Scholar] [CrossRef]
- Yang, F.; Wolke, J.; Jansen, J. Biomimetic calcium phosphate coating on electrospun poly(ε-caprolactone) scaffolds for bone tissue engineering. Chem. Eng. J. 2008, 137, 154–161. [Google Scholar] [CrossRef]
- Kim, C.W.; Kim, S.E.; Kim, Y.W.; Lee, H.J.; Choi, H.W.; Chang, J.H.; Choi, J.; Kim, K.J.; Shim, K.B.; Jeong, Y.-K.; et al. Fabrication of hybrid composites based on biomineralization of phosphorylated poly(ethylene glycol) hydrogels. J. Mater. Res. 2009, 24, 50–57. [Google Scholar] [CrossRef]
- Racquel, Z. Calcium Phosphates in Oral Biology and Medicine. Monogr. Oral Sci. 1991, 15, 88–92. [Google Scholar]
- Ruhé, P.; Boerman, O.; Russel, F.G.M.; Spauwen, P.; Mikos, A.; Jansen, J. Controlled release of rhBMP-2 loaded poly(dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J. Control. Release 2005, 106, 162–171. [Google Scholar] [CrossRef]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, D. Detection of vitronectin in mineralized bone matrix. J. Histochem. Cytochem. 1996, 44, 275–280. [Google Scholar] [CrossRef]
- Gough, J.E.; Notingher, I.; Hench, L.L. Osteoblast attachment and mineralized nodule formation on rough and smooth 45S5 bioactive glass monoliths. J. Biomed. Mater. Res. 2004, 68, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic Mineralization of Biomaterials Using Simulated Body Fluids for Bone Tissue Engineering and Regenerative Medicine. Tissue Eng. Part. A 2017, 23, 1169–1180. [Google Scholar] [CrossRef]
- Veis, A.; Dorvee, J.R. Biomineralization Mechanisms: A New Paradigm for Crystal Nucleation in Organic Matrices. Calcif. Tissue Int. 2012, 93, 307–315. [Google Scholar] [CrossRef]
- Rowlands, A.S.; George, P.A.; Cooper-White, J.J. Directing osteogenic and myogenic differentiation of MSCs: Interplay of stiffness and adhesive ligand presentation. Am. J. Physiol. Physiol. 2008, 295, C1037–C1044. [Google Scholar] [CrossRef]
- Wolff, J. The Law of Bone Remodelling; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar] [CrossRef]
- Limmahakhun, S.; Oloyede, A.; Sitthiseripratip, K.; Xiao, Y.; Yan, C. Stiffness and strength tailoring of cobalt chromium graded cellular structures for stress-shielding reduction. Mater. Des. 2017, 114, 633–641. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
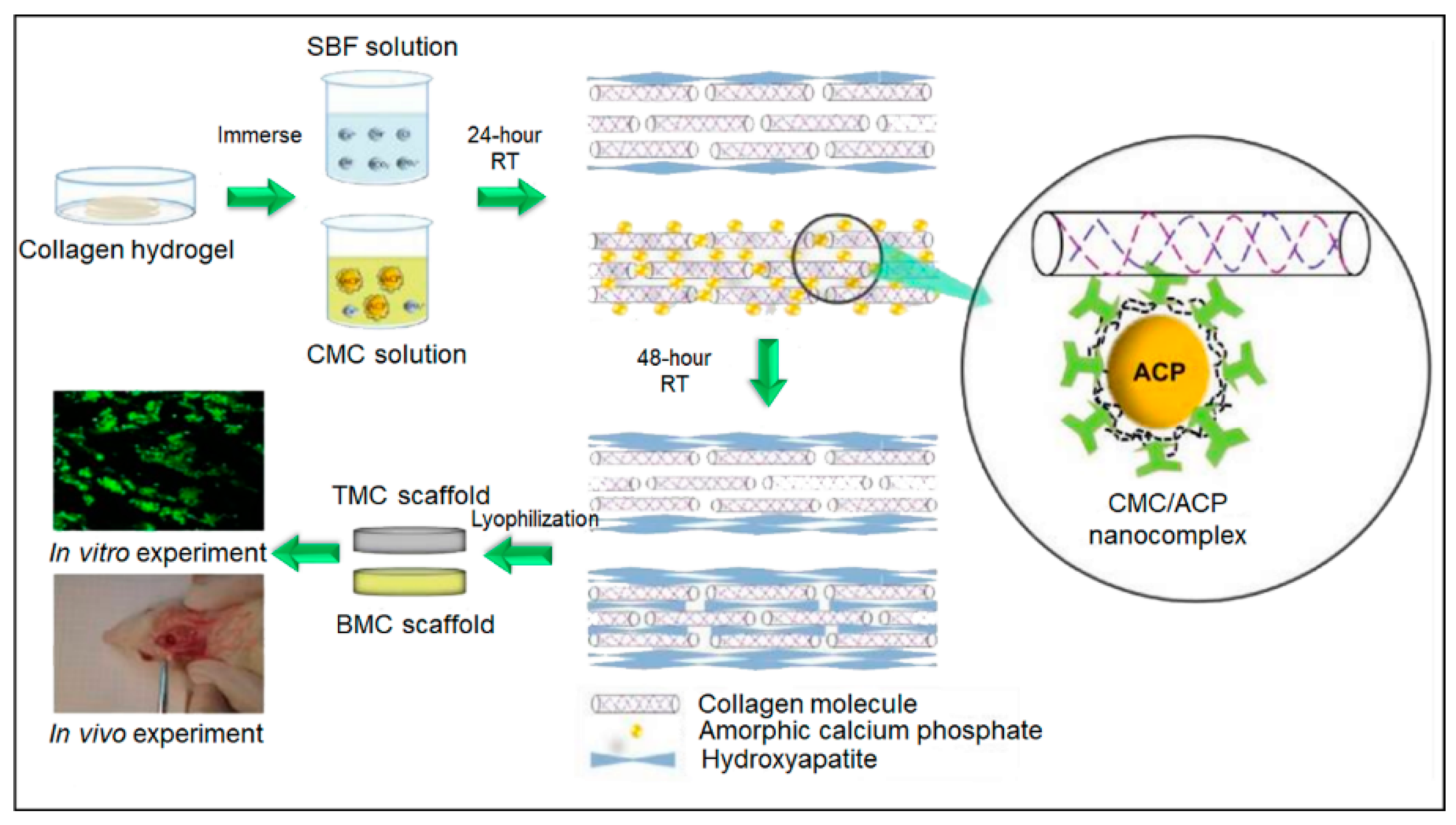
- Zhang, X.; Wang, Y.; Van Manh, N.; Wang, H.; Zhong, X.; Lia, C. Synergistic intrafibrillar/extrafibrillar mineralization of collagen scaffolds based on a biomimetic strategy to promote the regeneration of bone defects. Int. J. Nanomed. 2016, 11, 2053–2067. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Chu, C.C. Biomimetic mineralization of acid polysaccharide-based hydrogels: Towards porous 3-dimensional bone-like biocomposites. J. Mater. Chem. 2012, 22, 6080. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Joshi, M.K.; Tiwari, A.P.; Pant, H.R.; Shrestha, B.K.; Kim, H.J.; Park, C.H.; Kim, C.S. In Situ Generation of Cellulose Nanocrystals in Polycaprolactone Nanofibers: Effects on Crystallinity, Mechanical Strength, Biocompatibility, and Biomimetic Mineralization. ACS Appl. Mater. Interfaces 2015, 7, 19672–19683. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, Q.-W.; Sun, T.-W.; Chen, F.; Qi, C.; Wu, J.; Cai, Z.-Y.; Qian, Q.; Zhu, Y.-J. Amorphous calcium phosphate, hydroxyapatite and poly(d, l-lactic acid) composite nanofibers: Electrospinning preparation, mineralization and in vivo bone defect repair. Colloids Surf. B Biointerfaces 2015, 136, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-Inspired Polydopamine Coating as a Universal Route to Hydroxyapatite Crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Tanahashi, M.; Matsuda, T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J. Biomed. Mater. Res. 1997, 34, 305–315. [Google Scholar] [CrossRef]
- Yang, Q.; Song, F.; Zou, X.; Liao, L. Preparation and mineralization of a biocompatible double network hydrogel. J. Biomater. Sci. Polym. Ed. 2017, 28, 431–443. [Google Scholar] [CrossRef]
- Cowan, C.M.; Shi, Y.-Y.; O Aalami, O.; Chou, Y.-F.; Mari, C.; Thomas, R.; Quarto, N.; Contag, C.H.; Wu, B.; Longaker, M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 2004, 22, 560–567. [Google Scholar] [CrossRef]
- Kokubo, T. Apatite formation on surfaces of ceramics, metals and polymers in body environment. Acta Mater. 1998, 46, 2519–2527. [Google Scholar] [CrossRef]
- Rhee, S.-H.; Tanaka, J. Effect of citric acid on the nucleation of hydroxyapatite in a simulated body fluid. Biomaterials 1999, 20, 2155–2160. [Google Scholar] [CrossRef]
- Saiz, E.; Goldman, M.; Gomez-Vega, J.; Tomsia, A.; Marshall, G.; Marshall, S. In vitro behavior of silicate glass coatings on Ti6Al4V. Biomaterials 2002, 23, 3749–3756. [Google Scholar] [CrossRef]
- Du, C.; Cui, F.Z.; Zhang, W.; Feng, Q.L.; Zhu, X.D.; de Groot, K. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J. Biomed. Mater. Res. 2000, 50, 518–527. [Google Scholar] [CrossRef]
- Chen, Y.; Mak, A.F.T.; Wang, M.; Li, J. Composite coating of bonelike apatite particles and collagen fibers on poly L-lactic acid formed through an accelerated biomimetic coprecipitation process. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2006, 77, 315–322. [Google Scholar] [CrossRef]
- Hutchens, S.A.; Benson, R.S.; Evans, B.R.; Rawn, C.J.; O’Neill, H. A resorbable calcium-deficient hydroxyapatite hydrogel composite for osseous regeneration. Cellulose 2009, 16, 887–898. [Google Scholar] [CrossRef]
- Cao, Y.; Mei, M.L.; Li, Q.-L.; Chu, C.-H.; Chu, C.H. Agarose Hydrogel Biomimetic Mineralization Model for the Regeneration of Enamel Prismlike Tissue. ACS Appl. Mater. Interfaces 2013, 6, 410–420. [Google Scholar] [CrossRef]
- Furuichi, K.; Oaki, Y.; Ichimiya, H.; Komotori, J.; Imai, H. Preparation of hierarchically organized calcium phosphate–organic polymer composites by calcification of hydrogel. Sci. Technol. Adv. Mater. 2006, 7, 219–225. [Google Scholar] [CrossRef]
- Madhumathi, K.; Shalumon, K.; Rani, V.D.; Tamura, H.; Furuike, T.; Selvamurugan, N.; Nair, S.; Jayakumar, R. Wet chemical synthesis of chitosan hydrogel–hydroxyapatite composite membranes for tissue engineering applications. Int. J. Biol. Macromol. 2009, 45, 12–15. [Google Scholar] [CrossRef]
- Douglas, T.; Wlodarczyk, M.; Pamula, E.; A Declercq, H.; De Mulder, E.L.W.; Bucko, M.M.; Balcaen, L.; Vanhaecke, F.; Cornelissen, R.; Dubruel, P.; et al. Enzymatic mineralization of gellan gum hydrogel for bone tissue-engineering applications and its enhancement by polydopamine. J. Tissue Eng. Regen. Med. 2012, 8, 906–918. [Google Scholar] [CrossRef]
- Tomomatsu, O.; Tachibana, A.; Yamauchi, K.; Tanabe, T. A film of collagen/calcium phosphate composite prepared by enzymatic mineralization in an aqueous phase. J. Ceram. Soc. Jpn. 2008, 116, 10–13. [Google Scholar] [CrossRef]
- Flores, M.G.; Yashiro, R.; Washio, K.; Yamato, M.; Okano, T.; Ishikawa, I. Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J. Clin. Periodontol. 2008, 35, 1066–1072. [Google Scholar] [CrossRef]
- Lee, P.; Tran, K.; Zhou, G.; Bedi, A.; Shelke, N.B.; Yu, X.; Kumbar, S.G. Guided differentiation of bone marrow stromal cells on co-cultured cartilage and bone scaffolds. Soft Matter 2015, 11, 7648–7655. [Google Scholar] [CrossRef]
- Sikavitsas, V.I.; Bancroft, G.N.; Lemoine, J.J.; Liebschner, M.A.K.; Dauner, M.; Mikos, A.G. Flow Perfusion Enhances the Calcified Matrix Deposition of Marrow Stromal Cells in Biodegradable Nonwoven Fiber Mesh Scaffolds. Ann. Biomed. Eng. 2005, 33, 63–70. [Google Scholar] [CrossRef]
- Berner, A.; Henkel, J.; Woodruff, M.A.; Steck, R.; Nerlich, M.; Schuetz, M.A.; Hutmacher, D.W. Delayed minimally invasive injection of allogenic bone marrow stromal cell sheets regenerates large bone defects in an ovine preclinical animal model. Stem Cells Transl. Med. 2015, 4, 503–512. [Google Scholar] [CrossRef]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, N.; Wang, Z.; Wang, Y.; Liu, Y.; Ito, Y.; Zhang, P. Biodegradable Microcarriers of Poly(Lactide-co-Glycolide) and Nano-Hydroxyapatite Decorated with IGF-1 via Polydopamine Coating for Enhancing Cell Proliferation and Osteogenic Differentiation. Macromol. Biosci. 2015, 15, 1070–1080. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Y.; Cui, Y.; Jing, X.; Zhang, P.; Chen, X. The nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with l-lactic acid oligomer for bone repair. Acta Biomater. 2009, 5, 2680–2692. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Petite, H.; Viateau, V.; Bensaïd, W.; Meunier, A.; De Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef]
- Wu, X.; Stroll, S.I.; Lantigua, D.; Suvarnapathaki, S.; Camci-Unal, G. Eggshell particle-reinforced hydrogels for bone tissue engineering: An orthogonal approach. Biomater. Sci. 2019, 7, 2675–2685. [Google Scholar] [CrossRef]
- Menczel, J.D.; Prime, R.B. Thermal Analysis of Polymers; John Wiley Sons Inc.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Vyazovkin, S. Thermogravimetric Analysis. In Characterization of Materials; John Wiley Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Krawczyk, G.; Pamuła, E.; Declercq, H.A.; Schaubroeck, D.; Bucko, M.M.; Balcaen, L.; Van Der Voort, P.; Bliznuk, V.; Vreken, N.V.D.; et al. Generation of composites for bone tissue-engineering applications consisting of gellan gum hydrogels mineralized with calcium and magnesium phosphate phases by enzymatic means. J. Tissue Eng. Regen. Med. 2014, 10, 938–954. [Google Scholar] [CrossRef]
- Salama, A.; Shukry, N.; El-Gendy, A.; El-Sakhawy, M. Bioactive cellulose grafted soy protein isolate towards biomimetic calcium phosphate mineralization. Ind. Crop. Prod. 2017, 95, 170–174. [Google Scholar] [CrossRef]
- Zhou, B.; He, M.; Wang, P.; Fu, H.; Yu, Y.; Wang, Q.; Fan, X. Synthesis of silk fibroin-g-PAA composite using H2O2-HRP and characterization of the in situ biomimetic mineralization behavior. Mater. Sci. Eng. C 2017, 81, 291–302. [Google Scholar] [CrossRef]
- Si, J.; Cui, Z.; Wang, Q.; Liu, Q.C.; Liu, C. Biomimetic composite scaffolds based on mineralization of hydroxyapatite on electrospun poly(ε-caprolactone)/nanocellulose fibers. Carbohydr. Polym. 2016, 143, 270–278. [Google Scholar] [CrossRef]
- Lee, S.-D.; Hsiue, G.-H.; Chang, P.C.-T.; Kao, C.-Y. Plasma-induced grafted polymerization of acrylic acid and subsequent grafting of collagen onto polymer film as biomaterials. Biomaterials 1996, 17, 1599–1608. [Google Scholar] [CrossRef]
- Chittur, K.K. FTIR/ATR for protein adsorption to biomaterial surfaces. Biomaterials 1998, 19, 357–369. [Google Scholar] [CrossRef]
- Vo, T.N.; Tatara, A.M.; Santoro, M.; Beucken, J.J.J.P.V.D.; Leeuwenburgh, S.; Jansen, J.A.; Mikos, A.G. Acellular mineral deposition within injectable, dual-gelling hydrogels for bone tissue engineering. J. Biomed. Mater. Res. Part A 2016, 105, 110–117. [Google Scholar] [CrossRef]
- Bosch-Reig, F.; Gimeno-Adelantado, J.V.; Bosch-Mossi, F.; Domenech, A. Quantification of minerals from ATR-FTIR spectra with spectral interferences using the MRC method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 181, 7–12. [Google Scholar] [CrossRef]
- Shindo, D.; Oikawa, T. Energy Dispersive X-ray Spectroscopy. In Analytical Electron Microscopy for Materials Science; Springer: Tokyo, Japan, 2002; pp. 81–102. [Google Scholar] [CrossRef]
- Liao, S.; Murugan, R.; Chan, C.K.; Ramakrishna, S. Processing nanoengineered scaffolds through electrospinning and mineralization suitable for biomimetic bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2008, 1, 252–260. [Google Scholar] [CrossRef]
- Li, B.; Kan, L.; Zhang, X.; Li, J.; Li, R.; Gui, Q.; Qiu, D.; He, F.; Ma, N.; Wang, Y.; et al. Biomimetic Bone-like Hydroxyapatite by Mineralization on Supramolecular Porous Fiber Networks. Langmuir 2017, 33, 8493–8502. [Google Scholar] [CrossRef]
- Stevie, F.A.; Vartuli, C.B.; Giannuzzi, L.A.; Shofner, T.L.; Brown, S.R.; Rossie, B.; Hillion, F.; Mills, R.H.; Antonell, M.; Irwin, R.B.; et al. Application of focused ion beam lift-out specimen preparation to TEM, SEM, STEM, AES and SIMS analysis. Surf. Interface Anal. 2001, 31, 345–351. [Google Scholar] [CrossRef]
- Cui, Y.; Chang, X.; Zhu, X.; Luo, H.; Hu, Z.; Zou, X.; He, Q. Chemically modified silica gel with p-dimethylaminobenzaldehyde for selective solid-phase extraction and preconcentration of Cr(III), Cu(II), Ni(II), Pb(II) and Zn(II) by ICP-OES. Microchem. J. 2007, 87, 20–26. [Google Scholar] [CrossRef]
- Douglas, T.; Pilarz, M.; Lopez-Heredia, M.; Brackman, G.; Schaubroeck, D.; Balcaen, L.; Bliznuk, V.; Dubruel, P.; Knabe-Ducheyne, C.; Vanhaecke, F.; et al. Composites of gellan gum hydrogel enzymatically mineralized with calcium-zinc phosphate for bone regeneration with antibacterial activity. J. Tissue Eng. Regen. Med. 2015, 11, 1610–1618. [Google Scholar] [CrossRef]
- Cassetta, A. X-Ray Diffraction (XRD). In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–3. [Google Scholar] [CrossRef]
- Chen, Q.; Wong, C.; Lu, W.W.; Cheung, K.M.; Leong, J.; Luk, K. Strengthening mechanisms of bone bonding to crystalline hydroxyapatite in vivo. Biomaterials 2004, 25, 4243–4254. [Google Scholar] [CrossRef]
- Chun, S.S.; Jeong, J.H.; Kim, K.H.; Kim, S.Y. Biodegradation Study of Amorphous and Crystalline Calcium Metaphosphate in the SBF and Tris-Buffer Solution. Key Eng. Mater. 2000, 192, 131–134. [Google Scholar] [CrossRef]
- Chauhan, A. Powder XRD Technique and its Applications in Science and Technology. J. Anal. Bioanal. Tech. 2014, 5. [Google Scholar] [CrossRef]
- Nijsure, M.P.; Pastakia, M.; Spano, J.; Fenn, M.B.; Kishore, V. Bioglass incorporation improves mechanical properties and enhances cell-mediated mineralization on electrochemically aligned collagen threads. J. Biomed. Mater. Res. Part A 2017, 105, 2429–2440. [Google Scholar] [CrossRef]
- Nanotechnology Research Methods for Foods and Bioproducts. Nanotechnol. Res. Methods Foods Bioprod. 2012. [CrossRef]
- Burghardt, R.; Droleskey, R. Transmission Electron Microscopy. Curr. Protoc. Microbiol. 2006, 3. [Google Scholar] [CrossRef]
- Inkson, B. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for materials characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Elsevier: Amsterdam, The Netherlands, 2016; pp. 17–43. [Google Scholar] [CrossRef]
- Merino, S.; Novillo, C.; De Diego, G.; Conde, J.J.; Folgado, M.A.; Ferreira-Aparicio, P.; Chaparro, A.M. Comparing Different Cross-Section Cutting Methods for SEM Analysis of Membrane-Electrodes Assemblies. ECS Trans. 2019, 92, 87–94. [Google Scholar] [CrossRef]
- Ritman, E.L. Micro-Computed Tomography—Current Status and Developments. Annu. Rev. Biomed. Eng. 2004, 6, 185–208. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Yeo, A.; Wong, W.J.; Khoo, H.H.; Teoh, S.H. Surface modification of PCL-TCP scaffolds improve interfacial mechanical interlock and enhance early bone formation: An in vitro and in vivo characterization. J. Biomed. Mater. Res. Part A 2010, 92, 311–321. [Google Scholar] [CrossRef]
- Lin, A.S.P.; Barrows, T.H.; Cartmell, S.H.; Guldberg, R.E. Microarchitectural and mechanical characterization of oriented porous polymer scaffolds. Biomaterials 2003, 24, 481–489. [Google Scholar] [CrossRef]
- Sijbers, J.; Postnov, A. Reduction of ring artefacts in high resolution micro-CT reconstructions. Phys. Med. Biol. 2004, 49, N247–N253. [Google Scholar] [CrossRef]
- Batiste, D.L.; Kirkley, A.; Laverty, S.; Thain, L.M.; Spouge, A.R.; Gati, J.S.; Foster, P.J.; Holdsworth, D.W. High-resolution MRI and micro-CT in an ex vivo rabbit anterior cruciate ligament transection model of osteoarthritis. Osteoarthr. Cartil. 2004, 12, 614–626. [Google Scholar] [CrossRef]
- Mehboob, H.; Chang, S.-H. Effect of structural stiffness of composite bone plate–scaffold assembly on tibial fracture with large fracture gap. Compos. Struct. 2015, 124, 327–336. [Google Scholar] [CrossRef]
- Moreo, P.; Garcia-Aznar, J.M.; Doblaré, M. Modeling mechanosensing and its effect on the migration and proliferation of adherent cells. Acta Biomater. 2008, 4, 613–621. [Google Scholar] [CrossRef]
- Menard, K.P.; Menard, N. Dynamic Mechanical Analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–25. [Google Scholar] [CrossRef]
- Wang, J.C. Young’s modulus of porous materials. J. Mater. Sci. 1984, 19, 801–808. [Google Scholar] [CrossRef]
- Ratassepp, M.; Rao, J.; Fan, Z. Quantitative imaging of Young’s modulus in plates using guided wave tomography. NDT E Int. 2018, 94, 22–30. [Google Scholar] [CrossRef]
- Chartoff, R.P.; Dillman, S.H. Dynamic Mechanical Analysis (DMA). In Thermal Analysis of Polymers; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; pp. 387–495. [Google Scholar] [CrossRef]
- Storage, T.M.; Brockman, R.A.; Tienda, K.M. Analysis of Data Reduction Strategy Used in TA Instruments Q800 DMA Test System. 2013. Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/a591605.pdf (accessed on 1 July 2013).
- Menard, K. Dynamic Mechanical Analysis; CRC Press: Cleveland, OH, USA, 1999. [Google Scholar] [CrossRef]
- Wadud, S.E.B. Dynamic Mechanical Analysis and Its Advantages for Deflection Temperature under Load Measurements. TA Instrum. 2016. Available online: http://www.tainstruments.com/pdf/literature/TA307.pdf. (accessed on 19 October 2020).
- Rauner, N.; Meuris, M.; Zoric, M.; Tiller, J.C. Enzymatic mineralization generates ultrastiff and tough hydrogels with tunable mechanics. Nat. Cell Biol. 2017, 543, 407–410. [Google Scholar] [CrossRef]
- Rath, B.; Nam, J.; Knobloch, T.J.; Lannutti, J.J.; Agarwal, S. Compressive forces induce osteogenic gene expression in calvarial osteoblasts. J. Biomech. 2008, 41, 1095–1103. [Google Scholar] [CrossRef]
- Sanzherrera, J.; García-Aznar, J.M.; Doblare, M. On scaffold designing for bone regeneration: A computational multiscale approach. Acta Biomater. 2009, 5, 219–229. [Google Scholar] [CrossRef]
- Pobloth, A.; Checa, S.; Razi, H.; Petersen, A.; Weaver, J.C.; Schmidt-Bleek, K.; Windolf, M.; Tatai, A.Á.; Roth, C.P.; Schaser, K.-D.; et al. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med. 2018, 10, eaam8828. [Google Scholar] [CrossRef]
- Mata, A.; Geng, Y.; Henrikson, K.J.; Aparicio, C.; Stock, S.R.; Satcher, R.L.; Stupp, S.I. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials 2010, 31, 6004–6012. [Google Scholar] [CrossRef]
- Nayef, L.; Mekhail, M.; Benameur, L.; Rendon, J.S.; Hamdy, R.; Tabrizian, M. A combinatorial approach towards achieving an injectable, self-contained, phosphate-releasing scaffold for promoting biomineralization in critical size bone defects. Acta Biomater. 2016, 29, 389–397. [Google Scholar] [CrossRef]
- Oyane, A.; Yokoyama, Y.; Uchida, M.; Ito, A. The formation of an antibacterial agent–apatite composite coating on a polymer surface using a metastable calcium phosphate solution. Biomaterials 2006, 27, 3295–3303. [Google Scholar] [CrossRef]
- Matsubayashi, M.; Terukina, T.; Hattori, Y.; Otsuka, M. Preparation of Calcium Phosphate Coated Simvastatin-Loaded PLGA Microspheres Dispersed Alginate Hydrogel Beads as a Controlled Drug Delivery Carrier. Key Eng. Mater. 2018, 782, 201–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Calcium phosphate/chitosan composite scaffolds for controlled in vitro antibiotic drug release. J. Biomed. Mater. Res. 2002, 62, 378–386. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Sreeja, S.; Muraleedharan, C.; Varma, P.H.; Sailaja, G. Surface-transformed osteoinductive polyethylene terephthalate scaffold as a dual system for bone tissue regeneration with localized antibiotic delivery. Mater. Sci. Eng. C 2020, 109, 110491. [Google Scholar] [CrossRef]
- Boda, S.K.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J. Mineralized nanofiber segments coupled with calcium-binding BMP-2 peptides for alveolar bone regeneration. Acta Biomater. 2019, 85, 282–293. [Google Scholar] [CrossRef]
- Gibon, E.; Lu, L.; Goodman, S.B. Aging, inflammation, stem cells, and bone healing. Stem Cell Res. Ther. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Singh, A.T.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-Based Sensors: Emerging Themes and Applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef]
- Lantigua, D.; Ni Kelly, Y.; Unal, B.; Camci-Unal, G. Engineered Paper-Based Cell Culture Platforms. Adv. Heal. Mater. 2017, 6, 1700619. [Google Scholar] [CrossRef]
- Wu, X.; Suvarnapathaki, S.; Walsh, K.; Camci-Unal, G. Paper as a scaffold for cell cultures: Teaching an old material new tricks. MRS Commun. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Newsome, D.; Eustace, B.K.; Whitesides, G.M. Fibroblasts Enhance Migration of Human Lung Cancer Cells in a Paper-Based Coculture System. Adv. Heal. Mater. 2015, 5, 641–647. [Google Scholar] [CrossRef]
- Yokoyama, A.; Gelinsky, M.; Kawasaki, T.; Kohgo, T.; König, U.; Pompe, W.; Watari, F. Biomimetic porous scaffolds with high elasticity made from mineralized collagen—An animal study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75, 464–472. [Google Scholar] [CrossRef]
| Scaffold Substrates | Mineralization Method | Main Experiments | Results | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
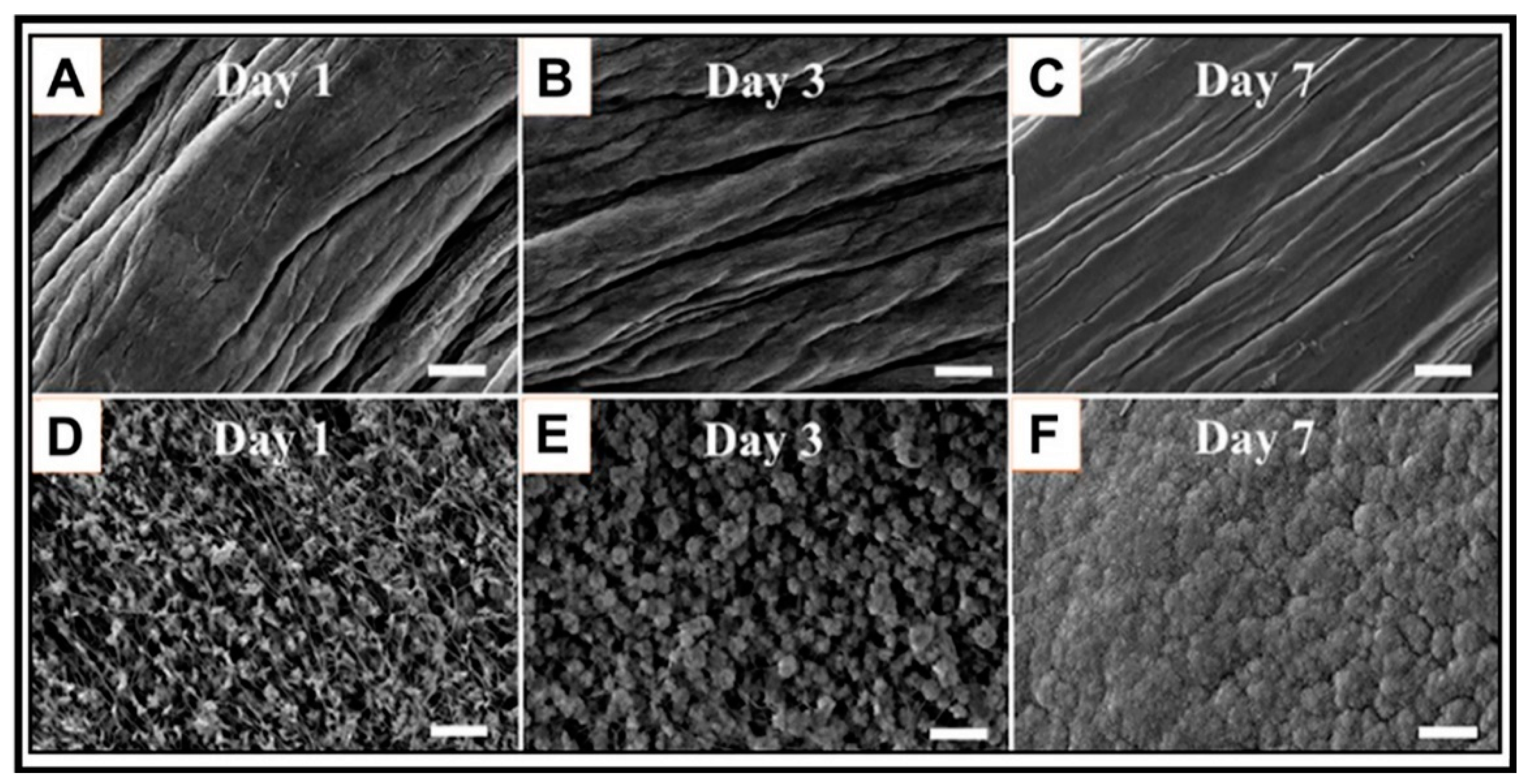
| Type I collagen fiber | Sequential mineralization by calcium and phosphate solutions " | Calculation of steric energies and elastic energy storage | The elastic modulus was improved, and more elastic energy was stored for the mineralized fiber | The method can be applied for various proteins to fabricate true biomimetic material | The mechanical properties are low for these fibers | Landis et al., 2006 [26] |
| Bacterial cellulose hydrogel | Sequential mineralization by calcium and phosphate solutions | Mechanical testing, XRD, FTIR, and SEM | Calcium-deficient hydroxyapatite was formed | The mineral structure similar to natural bone apatite | The tensile strength was decreased for the mineralized sample and the compressive strength was not tested | Hutchens et al., 2009 [69] |
| Ti, stainless steel, Si, gold, glass, poly(styrene)poly(methyl methacrylate), polydimethylsiloxane. Si3N4, filter paper, nylon membrane filter, and polytetrafluoroethylene | Polydopamine was coated on the surfaces of the substrates. The coated ones were then incubated in SBF | SEM, TEM, XRD, high-resolution dispersive Raman microscope and pre-osteoblast culture | Natural inorganic crystalline hydroxyapatite was formed on the surfaces of all substrates | This method enables the hydroxyapatite formation on various synthetic biomaterials with favorable cell viability | For some materials, this method is not the most effective way | Ryu et al., 2010 [60] |
| Peptide amphiphile (PA) fiber | Combining PA solution with CaCl2 solution | Regeneration of a critical-size femoral defect in a rat model | The phosphorylated serine residues promoted mineralization and therefore enhanced bone regeneration | The scaffold can regenerate bone tissue in a defect that will not heal on its own | The mechanical properties are low for these fibers | Mata et al., 2010 [129] |
| Chitosan/PEGDA hydrogel | Incubation in SBF | SEM, TEM, XRD, FTIR and TGA | Crystalline hydroxyapatite was form on the porous 3D hydrogel network | The bulk mineralization is achieved for the biomimetic scaffold | The in vitro or in vivo study should be performed for the osteoinductivity and osteoconductivity for the scaffold | Zhong and Chu, 2012 [56] |
| PCL/cellulose acetate nanofiber | Incubation in SBF | Cell viability assay and SEM | The mineralization improved mechanical properties and ensured the excellent cellular compatibility | Osteoblasts showed good attachment on the nanofibers and normal spreading morphology | The mature osteoblasts were directly used in this study. The osteoinductivity remains unknown for this scaffold | Joshi et al., 2015 [58] |
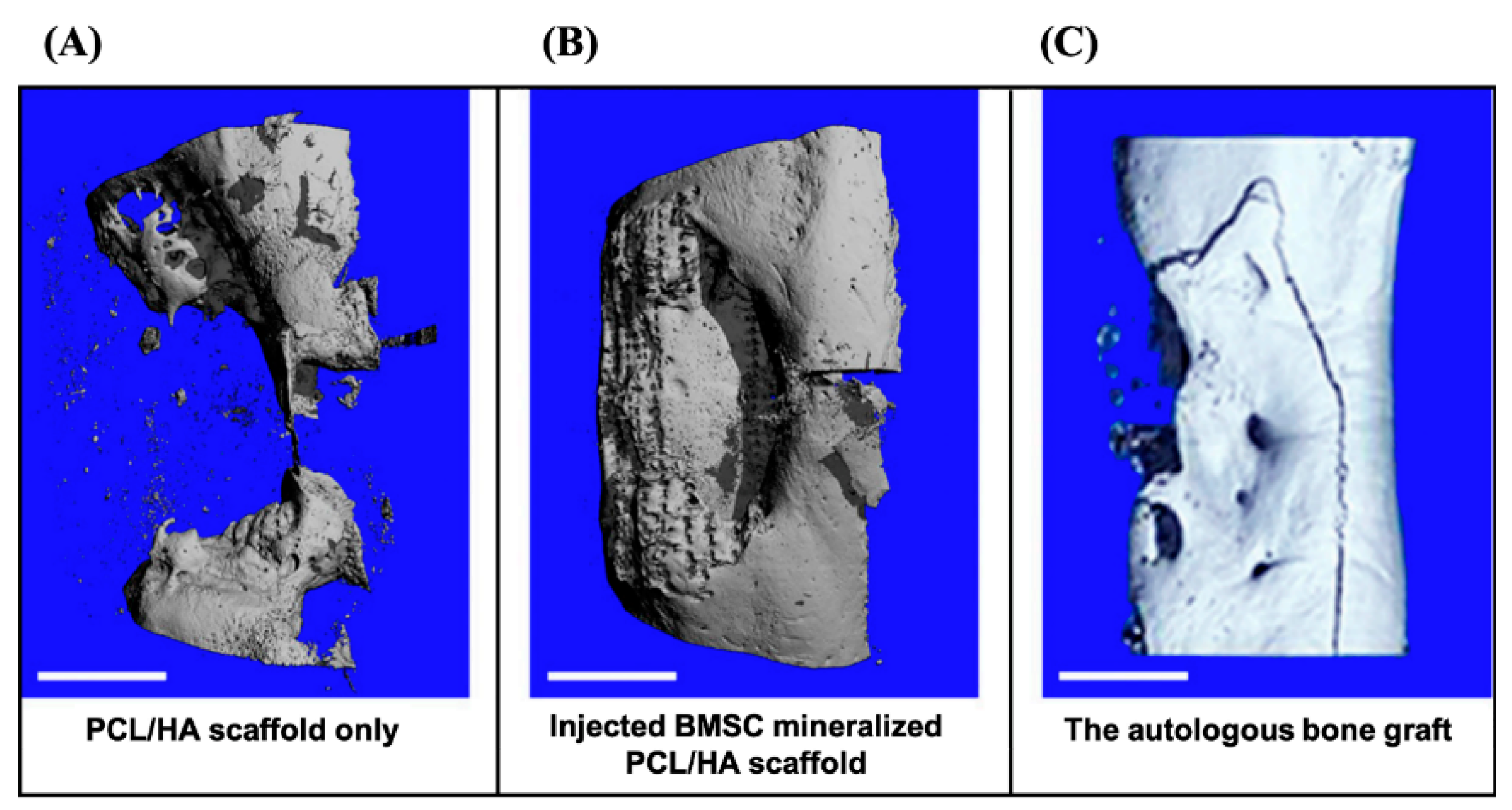
| PCL/HA coated with calcium phosphate | BMSCs from sheep were cultured and to engineer cell sheet. After the scaffold was implanted in the defect, the BMSCs were the injected to the defect | Regeneration of a critical-size tibia defect in a sheep model | Large amount of regenerated bone tissue and complete bridging of the defect for the BMSC group | This method not only provided the mineralization for scaffolds, but also delivered primary cells | A secondary surgery is needed. Though the cell sheet was injected, the animals were under anesthesia | Berner et al., 2015 [78] |
| Cellulose grafted soy protein isolate | Incubation in SBF | FTIR, TEM, XRD, TGA, and cytotoxicity tests | Rod-like nanocrystal hydroxyapatite was formed on the scaffold with good biocompatibility | The soy protein isolates successfully modified the cellulose and induced the mineralization | The mechanical testing and bioactivity of the scaffold remain unknown | Salama et al., 2017 [88] |
| Collagen, chitosan, poly(lactic acid), and poly(lactic-coglycolic acid) | Calcium and phosphate solutions were added to the polymer solution before crosslinking | XRD, SEM, TGA, mechanical testing and biocompatibility testing | The micro-sized porous structure with nanohydroxyapatite crystals were created for the scaffolds with good biocompatibility | The micro-nano structure is beneficial for combining bone morphogenetic protein which will enhance the bone regeneration | The degradation rat of different materials, as long as the formed nHA, may have unexpected impact on bone regeneration | Zou et al., 2019 [40] |
| Gelatin based hydrogel | Micro-sized eggshells were added to the polymer solution before crosslinking | SEM, mechanical testing, and real-time polymerase chain reaction for osteogenic differentiation makers | The eggshell micro-particles were well embedded in the hydrogel network and induced the osteogenic differentiation of pre-osteoblast | The eggshells improve the mechanical properties of the weak hydrogel substrate and provide natural minerals as a common kitchen waste | The high cell activity induced by the eggshells may result in a faster biodegradation rate of the hydrogel substrate | Wu et al., 2019 [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Walsh, K.; Hoff, B.L.; Camci-Unal, G. Mineralization of Biomaterials for Bone Tissue Engineering. Bioengineering 2020, 7, 132. https://doi.org/10.3390/bioengineering7040132
Wu X, Walsh K, Hoff BL, Camci-Unal G. Mineralization of Biomaterials for Bone Tissue Engineering. Bioengineering. 2020; 7(4):132. https://doi.org/10.3390/bioengineering7040132
Chicago/Turabian StyleWu, Xinchen, Kierra Walsh, Brianna L. Hoff, and Gulden Camci-Unal. 2020. "Mineralization of Biomaterials for Bone Tissue Engineering" Bioengineering 7, no. 4: 132. https://doi.org/10.3390/bioengineering7040132
APA StyleWu, X., Walsh, K., Hoff, B. L., & Camci-Unal, G. (2020). Mineralization of Biomaterials for Bone Tissue Engineering. Bioengineering, 7(4), 132. https://doi.org/10.3390/bioengineering7040132





