Implementation of Endogenous and Exogenous Mesenchymal Progenitor Cells for Skeletal Tissue Regeneration and Repair
Abstract
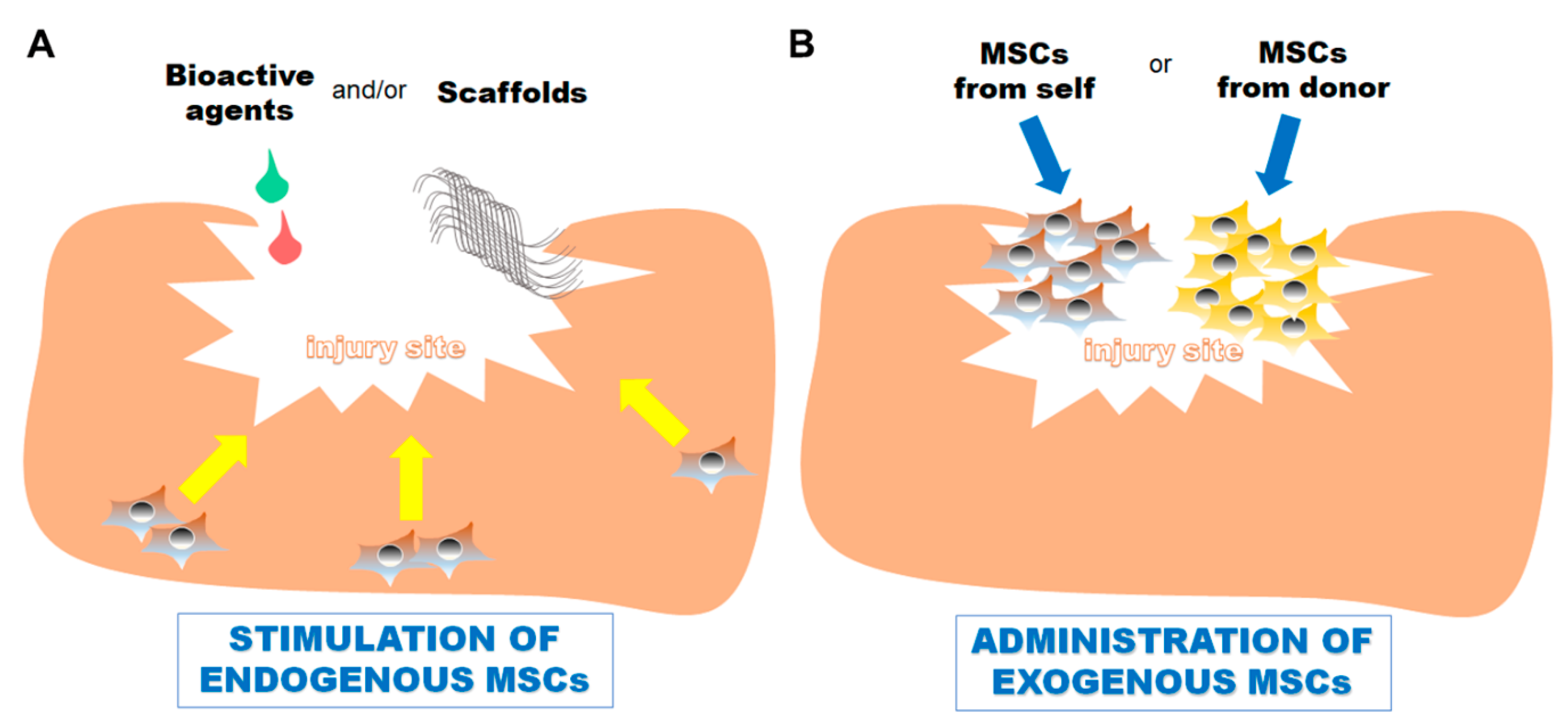
1. Introduction
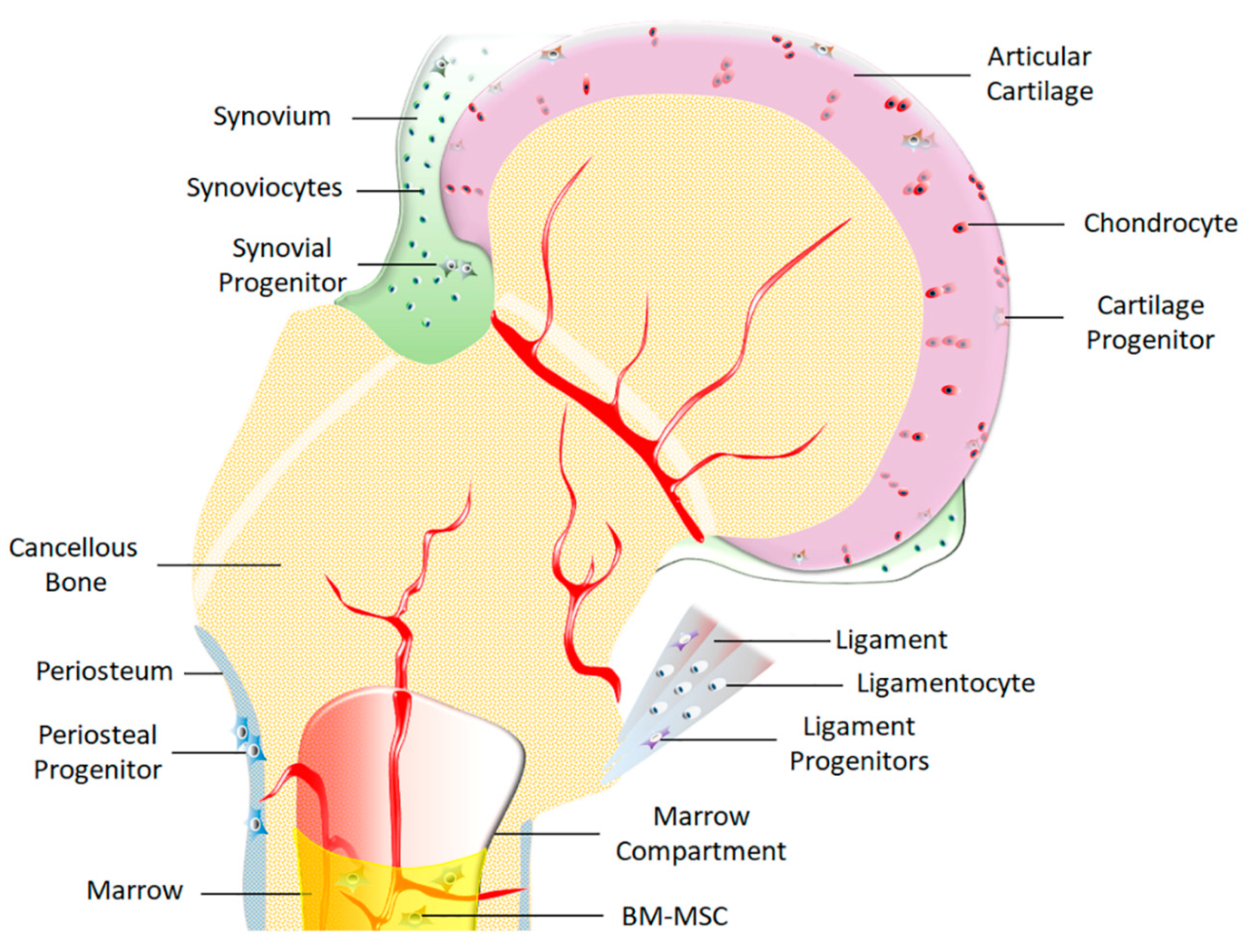
2. Common Progenitor/Stem Cells Utilized for Skeletal Tissue Repair
3. Stimulating Bone Repair
4. Stimulating Tendon and Ligament Repair
5. Stimulating Articular Cartilage Repair
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steele, R.; Collins, E.-M. Wnt signaling determines body axis polarity in regenerating Hydra tissue. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mohri, K.; Tanaka, R.; Nagano, S. Live cell imaging of cell movement and transdifferentiation during regeneration of an amputated multicellular body of the social amoeba Dictyostelium discoideum. Dev. Biol. 2020, 457, 140–149. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Galliot, B.; Crescenzi, M.; Jacinto, A.; Tajbakhsh, S. Trends in tissue repair and regeneration. Developmenmt 2017, 144, 357–364. [Google Scholar] [CrossRef]
- Stroncek, J.D.; Reichert, W.M. Overview of wound healing in different tissue types. In Indwelling Neural Implants: Strategies for Contending with the in Vivo Environment; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007; ISBN 9781420009309. [Google Scholar] [CrossRef]
- Tidball, J.G. Mechanisms of muscle injury, repair, and regeneration. Compr. Physiol. 2011, 1, 2029–2062. [Google Scholar] [CrossRef] [PubMed]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, V.; Tasso, R.; Cancedda, R.; Descalzi, F. Mesenchymal stem cell paracrine activity is modulated by platelet lysate: Induction of an inflammatory response and secretion of factors maintaining macrophages in a proinflammatory phenotype. Stem Cells Dev. 2014, 23, 1858–1869. [Google Scholar] [CrossRef]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.; Xiao, Y. Formation of Blood Clot on Biomaterial Implants Influences Bone Healing. Tissue Eng. Part B Rev. 2014, 20, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Hoogduijn, M.J.; Lombardo, E. Mesenchymal Stromal Cells Anno 2019: Dawn of the Therapeutic Era? Concise Review. Stem Cells Transl. Med. 2019, 8, 1126–1134. [Google Scholar] [CrossRef]
- Linero, I.; Chaparro, O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.G.; Wu, Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.H.; Laurencin, C.T. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, C.T.; Chen, Y.; Liu, W.; Chen, Q. The influence of tissue microenvironment on stem cell–based cartilage repair. Ann. N. Y. Acad. Sci. 2016, 1383, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Embree, M.C.; Chen, M.; Pylawka, S.; Kong, D.; Iwaoka, G.M.; Kalajzic, I.; Yao, H.; Shi, C.; Sun, D.; Sheu, T.J.; et al. Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nat. Commun. 2016, 7, 13073. [Google Scholar] [CrossRef]
- Ozeki, N.; Muneta, T.; Koga, H.; Nakagawa, Y.; Mizuno, M.; Tsuji, K.; Mabuchi, Y.; Akazawa, C.; Kobayashi, E.; Matsumoto, K.; et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthr. Cartil. 2016, 24, 1061–1070. [Google Scholar] [CrossRef]
- Meeson, R.; Sanghani-Keri, A.; Coathup, M.; Blunn, G. VEGF with AMD3100 endogenously mobilizes mesenchymal stem cells and improves fracture healing. J. Orthop. Res. 2019, 37, 1294–1302. [Google Scholar] [CrossRef]
- Devana, S.K.; Kelley, B.V.; McBride, O.J.; Kabir, N.; Jensen, A.R.; Park, S.J.; Eliasberg, C.D.; Dar, A.; Mosich, G.M.; Kowalski, T.J.; et al. Adipose-derived human perivascular stem cells may improve achilles tendon healing in rats. Clin. Orthop. Relat. Res. 2018, 476, 2091–2100. [Google Scholar] [CrossRef]
- Black, C.; Kanczler, J.M.; de Andrés, M.C.; White, L.J.; Savi, F.M.; Bas, O.; Saifzadeh, S.; Henkel, J.; Zannettino, A.; Gronthos, S.; et al. Characterisation and evaluation of the regenerative capacity of Stro-4+ enriched bone marrow mesenchymal stromal cells using bovine extracellular matrix hydrogel and a novel biocompatible melt electro-written medical-grade polycaprolactone scaffold. Biomaterials 2020, 247, 119998. [Google Scholar] [CrossRef]
- Loebel, C.; Burdick, J.A. Engineering Stem and Stromal Cell Therapies for Musculoskeletal Tissue Repair. Cell Stem Cell 2018, 22, 325–339. [Google Scholar] [CrossRef]
- De Bari, C.; Roelofs, A.J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharmacol. 2018, 40, 74–80. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, J.; Han, J.; Zhang, W.; Ma, J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am. J. Transl. Res. 2019, 11, 6275–6289. [Google Scholar] [PubMed]
- Scarfì, S. Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J. Stem Cells 2016, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Steinert, A.F.; Rackwitz, L.; Gilbert, F.; Nöth, U.; Tuan, R.S. Concise Review: The Clinical Application of Mesenchymal Stem Cells for Musculoskeletal Regeneration: Current Status and Perspectives. Stem Cells Transl. Med. 2012, 1, 237–247. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Murray, M.M.; Flutie, B.M.; Kalish, L.A.; Ecklund, K.; Fleming, B.C.; Proffen, B.L.; Micheli, L.J. The Bridge-Enhanced Anterior Cruciate Ligament Repair (BEAR) Procedure: An Early Feasibility Cohort Study. Orthop. J. Sport. Med. 2016, 4. [Google Scholar] [CrossRef]
- Murray, M.M.; Kalish, L.A.; Fleming, B.C.; Flutie, B.; Freiberger, C.; Henderson, R.N.; Perrone, G.S.; Thurber, L.G.; Proffen, B.L.; Ecklund, K.; et al. Bridge-Enhanced Anterior Cruciate Ligament Repair: Two-Year Results of a First-in-Human Study. Orthop. J. Sport. Med. 2019, 7. [Google Scholar] [CrossRef]
- Hurd, J.L.; Facile, T.R.; Weiss, J.; Hayes, M.; Hayes, M.; Furia, J.P.; Maffulli, N.; Winnier, G.E.; Alt, C.; Schmitz, C.; et al. Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: A prospective, randomized, controlled first-in-human pilot study. J. Orthop. Surg. Res. 2020, 15, 122–128. [Google Scholar] [CrossRef]
- Bruder, S.P.; Fink, D.J.; Caplan, A.I. Mesenchymal stem cells in bone development, bone repair, and skeletal regenaration therapy. J. Cell. Biochem. 1994, 56, 283–294. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef]
- Nöth, U.; Osyczka, A.M.; Tuli, R.; Hickok, N.J.; Danielson, K.G.; Tuan, R.S. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J. Orthop. Res. 2002, 20, 1060–1069. [Google Scholar] [CrossRef]
- Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Miner. Res. 2009, 24, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Owston, H.E.; Ganguly, P.; Tronci, G.; Russell, S.J.; Giannoudis, P.V.; Jones, E.A. Colony Formation, Migratory, and Differentiation Characteristics of Multipotential Stromal Cells (MSCs) From “Clinically Accessible” Human Periosteum Compared to Donor-Matched Bone Marrow MSCs. Stem Cells Int. 2019, 2019, 6074245. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Khan, I.M.; Richardson, K.; Nelson, L.; McCarthy, H.E.; Analbelsi, T.; Singhrao, S.K.; Dowthwaite, G.P.; Jones, R.E.; Baird, D.M.; et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS ONE 2010, 5, e13246. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.E.; Yasuhara, R.; Iwamoto, M.; Enomoto-Iwamoto, M. Resident mesenchymal progenitors of articular cartilage. Matrix Biol. 2014, 39, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, C.T.; Twomey-Kozak, J.; Newberry, J.; Desai, S.; Feltman, P.; Franco, J.R.; Li, N.; Terek, R.; Ehrlich, M.G.; Owens, B.D. Human Cartilage-Derived Progenitors Resist Terminal Differentiation and Require CXCR4 Activation to Successfully Bridge Meniscus Tissue Tears. Stem Cells 2019, 37, 102–114. [Google Scholar] [CrossRef]
- Ding, Z.; Huang, H. Mesenchymal stem cells in rabbit meniscus and bone marrow exhibit a similar feature but a heterogeneous multi-differentiation potential: Superiority of meniscus as a cell source for meniscus repair Evolutionary developmental biology and morphology. BMC Musculoskelet. Disord. 2015, 16, 65. [Google Scholar] [CrossRef]
- Liu, S.; Liang, H.; Lee, S.M.; Li, Z.; Zhang, J.; Fei, Q. Isolation and Identification of Stem Cells From Degenerated Human Intervertebral Discs and Their Migration Characteristics. Acta Biochim. Biophys. Sin. 2017, 49, 101–109. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Jo, C.H.; Lim, H.J.; Yoon, K.S. Characterization of Tendon-Specific Markers in Various Human Tissues, Tenocytes and Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2019, 16, 151–159. [Google Scholar] [CrossRef]
- Lee, K.J.; Clegg, P.D.; Comerford, E.J.; Canty-Laird, E.G. Ligament-Derived Stem Cells: Identification, Characterisation, and Therapeutic Application. Stem Cells Int. 2017, 2017, 1919845. [Google Scholar] [CrossRef]
- Ogata, Y.; Mabuchi, Y.; Shinoda, K.; Horiike, Y.; Mizuno, M.; Otabe, K.; Suto, E.G.; Suzuki, N.; Sekiya, I.; Akazawa, C. Anterior cruciate ligament-derived mesenchymal stromal cells have a propensity to differentiate into the ligament lineage. Regen. Ther. 2018, 8, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Zhuge, Y.; Velazquez, O.C. Trafficking and differentiation of mesenchymal stem cells. J. Cell. Biochem. 2009, 106, 984–991. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Kachroo, U.; Vinod, E. Comparative analysis of gene expression between articular cartilage-derived cells to assess suitability of fibronectin adhesion assay to enrich chondroprogenitors. Knee 2020, 27, 755–759. [Google Scholar] [CrossRef]
- Grogan, S.P.; Duffy, S.F.; Pauli, C.; Lotz, M.K.; D’Lima, D.D. Gene expression profiles of the meniscus avascular phenotype: A guide for meniscus tissue engineering. J. Orthop. Res. 2018, 36, 1947–1958. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front. Cell Dev. Biol. 2019, 7. [Google Scholar] [CrossRef]
- Liang, Y.; Idrees, E.; Andrews, S.H.J.; Labib, K.; Szojka, A.; Kunze, M.; Burbank, A.D.; Mulet-Sierra, A.; Jomha, N.M.; Adesida, A.B. Plasticity of Human Meniscus Fibrochondrocytes: A Study on Effects of Mitotic Divisions and Oxygen Tension. Sci. Rep. 2017, 7, 12148. [Google Scholar] [CrossRef]
- Angelozzi, M.; Penolazzi, L.; Mazzitelli, S.; Lambertini, E.; Lolli, A.; Piva, R.; Nastruzzi, C. Dedifferentiated chondrocytes in composite microfibers as tool for cartilage repair. Front. Bioeng. Biotechnol. 2017, 5, 35. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, C.; Wei, X.; Hou, Y.; Zhao, L.; Fu, X.; Zhang, J.; Yu, C. Articular cartilage repair using dedifferentiated articular chondrocytes and bone morphogenetic protein 4 in a rabbit model of articular cartilage defects. Arthritis Rheum. 2008, 58, 1067–1075. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shen, S.; Fu, H.; Wang, Z.; Li, X.; Sui, X.; Yuan, M.; Liu, S.; Wang, G.; Guo, Q. Immunomodulatory functions of mesenchymal stem cells in tissue engineering. Stem Cells Int. 2019, 2019, 9671206. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56.e21. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhang, Y. Genetic and epigenetic variations in iPSCs: Potential causes and implications for application. Cell Stem Cell 2013, 13, 149–159. [Google Scholar] [CrossRef]
- Arvidson, K.; Abdallah, B.M.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef] [PubMed]
- Bussard, K.M.; Gay, C.V.; Mastro, A.M. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008, 27, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.M. Overview of the fracture healing cascade. Injury 2005, 36 (Suppl. 3), S5–S7. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, B.C.; Bahney, C.S. Origin of Reparative Stem Cells in Fracture Healing. Curr. Osteoporos. Rep. 2018, 16, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Majeska, R.J.; Rush, E.B.; Levine, P.M.; Horowitz, M.C. The expression of cytokine activity by fracture callus. J. Bone Miner. Res. 1995, 10, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Cho, T.J.; Kon, T.; Aizawa, T.; Tsay, A.; Fitch, J.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Impaired fracture healing in the absence of TNF-α signaling: The role of TNF-α in endochondral cartilage resorption. J. Bone Miner. Res. 2003, 18, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Tsiridis, E.; Upadhyay, N.; Giannoudis, P. Molecular aspects of fracture healing:Which are the important molecules? Injury 2007, 38 (Suppl. 1), S11–S25. [Google Scholar] [CrossRef]
- Deckers, M.M.L.; Van Bezooijen, R.L.; Van Geertje Horst, D.E.R.; Hoogendam, J.; Van Chris Bent, D.E.R.; Papapoulos, S.E.; Löwik, C.W.G.M. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology 2002, 143, 1545–1553. [Google Scholar] [CrossRef]
- Keating, J.F.; Simpson, A.H.; Robinson, C.M. The management of fractures with bone loss. J. Bone Jt. Surg. 2005, 87, 142–150. [Google Scholar] [CrossRef]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45, S3–S7. [Google Scholar] [CrossRef]
- Marsh, D. Concepts of fracture union, delayed union, and nonunion. Clin. Orthop. Relat. Res. 1998, 355, S22–S30. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics the bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Younger, E.M.; Chapman, M.W. Morbidity at bone graft donor sites. J. Orthop. Trauma 1989, 3, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Aro, H.T.; Aho, A.J. Clinical use of bone allografts. Ann. Med. 1993, 25, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.F.; Bucchi, C.; Navarro, P.; Beltrán, V.; Borie, E. Bone grafts utilized in dentistry: An analysis of patients’ preferences. BMC Med. Ethics 2015, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Offner, D.; de Grado, G.F.; Meisels, I.; Pijnenburg, L.; Fioretti, F.; Benkirane-Jessel, N.; Musset, A.-M. Bone Grafts, Bone Substitutes and Regenerative Medicine Acceptance for the Management of Bone Defects Among French Population: Issues about Ethics, Religion or Fear? Cell Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Shapiro, F.D. Expression of bone-specific genes by hypertrophic chondrocytes: Implications of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J. Cell. Biochem. 1996, 62. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Cruceta, J.; Shea, C.M.; Sampath, K.; Barnes, G.L.; Einhorn, T.A. Chondrocytes Provide Morphogenic Signals That Selectively Induce Osteogenic Differentiation of Mesenchymal Stem Cells. J. Bone Miner. Res. 2002, 17, 221–230. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Barnes, G.L.; Shea, C.M.; Einhorn, T.A. Osteogenic differentiation is selectively promoted by morphogenetic signals from chondrocytes and synergized by a nutrient rich growth environment. Connect. Tissue Res. 2003, 44, 85–91. [Google Scholar] [CrossRef]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef]
- Liu, D.; Qin, H.; Yang, J.; Yang, L.; He, S.; Chen, S.; Bao, Q.; Zhao, Y.; Zong, Z. Different effects of Wnt/β-catenin activation and PTH activation in adult and aged male mice metaphyseal fracture healing. BMC Musculoskelet. Disord. 2020, 21, 110–113. [Google Scholar] [CrossRef]
- Sandberg, O.; Bernhardsson, M.; Aspenberg, P. Earlier effect of alendronate in mouse metaphyseal versus diaphyseal bone healing. J. Orthop. Res. 2017, 35, 793–799. [Google Scholar] [CrossRef]
- Bernhardsson, M.; Aspenberg, P. Abaloparatide versus teriparatide: A head to head comparison of effects on fracture healing in mouse models. Acta Orthop. 2018, 89, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Bjørgul, K.; Reigstad, A. Atypical fracture of the ulna associated with alendronate use. Acta Orthop. 2011, 82, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Odvina, C.V.; Zerwekh, J.E.; Rao, D.S.; Maalouf, N.; Gottschalk, F.A.; Pak, C.Y.C. Severely suppressed bone turnover: A potential complication of alendronate therapy. J. Clin. Endocrinol. Metab. 2005, 90, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Ang, B.F.H.; Koh, J.S.B.; Ng, A.C.M.; Howe, T.S. Bilateral ulna fractures associated with bisphosphonate therapy. Osteoporos. Int. 2013, 24, 1523–1525. [Google Scholar] [CrossRef]
- Kumar, S.; Ponnazhagan, S. Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone 2012, 50, 1012–1018. [Google Scholar] [CrossRef]
- Mehrotra, M.; Williams, C.R.; Ogawa, M.; LaRue, A.C. Hematopoietic stem cells give rise to osteo-chondrogenic cells. Blood Cells Mol. Dis. 2013, 50, 41–49. [Google Scholar] [CrossRef]
- Murao, H.; Yamamoto, K.; Matsuda, S.; Akiyama, H. Periosteal cells are a major source of soft callus in bone fracture. J. Bone Miner. Metab. 2013, 31, 390–398. [Google Scholar] [CrossRef]
- Hadjiargyrou, M.; O’Keefe, R.J. The convergence of fracture repair and stem cells: Interplay of genes, aging, environmental factors and disease. J. Bone Miner. Res. 2014, 29, 2307–2322. [Google Scholar] [CrossRef]
- Palombella, S.; Lopa, S.; Gianola, S.; Zagra, L.; Moretti, M.; Lovati, A.B. Bone Marrow-Derived Cell Therapies to Heal Long-Bone Nonunions: A Systematic Review and Meta-Analysis-Which Is the Best Available Treatment? Stem Cells Int. 2019, 2019, 3715964. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar] [PubMed]
- Takagi, K.; Urist, M.R. The role of bone marrow in bone morphogenetic protein-induced repair of femoral massive diaphyseal defects. Clin. Orthop. Relat. Res. 1982, 171, 224–231. [Google Scholar] [CrossRef]
- Kadiyala, S. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997, 6, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chanda, D.; Ponnazhagan, S. Therapeutic potential of genetically modified mesenchymal stem cells. Gene Ther. 2008, 15, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Gaur, S.; Sharma, S. Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthop. 1993, 64, 671–672. [Google Scholar] [CrossRef]
- Sim, R.; Liang, T.S.; Tay, B.K. Autologous marrow injection in the treatment of delayed and non-union in long bones. Singapore Med. J. 1993, 34, 412–417. [Google Scholar]
- Kim, S.J.; Shin, Y.W.; Yang, K.H.; Kim, S.B.; Yoo, M.J.; Han, S.K.; Im, S.A.; Won, Y.D.; Sung, Y.B.; Jeon, T.S.; et al. A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast(OssronTM) injection to treat fractures. BMC Musculoskelet. Disord. 2009, 10, 20. [Google Scholar] [CrossRef]
- Mareschi, K.; Ferrero, I.; Rustichelli, D.; Aschero, S.; Gammaitoni, L.; Aglietta, M.; Madon, E.; Fagioli, F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J. Cell. Biochem. 2006, 97, 744–754. [Google Scholar] [CrossRef]
- Both, S.K.; Van Apeldoorn, A.A.; Jukes, J.M.; Englund, M.C.O.; Hyllner, J.; Van Blitterswijk, C.A.; De Boer, J. Differential bone-forming capacity of osteogenic cells from either embryonic stem cells or bone marrow-derived mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2011, 5, 180–190. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Mankani, M.H.; Gronthos, S.; Satomura, K.; Bianco, P.; Robey, P.G. Circulating skeletal stem cells. J. Cell Biol. 2001, 153, 1133–1140. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Mankani, M.H.; Leet, A.I.; Ziran, N.; Gronthos, S.; Robey, P.G. Circulating Connective Tissue Precursors: Extreme Rarity in Humans and Chondrogenic Potential in Guinea Pigs. Stem Cells 2007, 25, 1830–1839. [Google Scholar] [CrossRef]
- Levi, B.; James, A.W.; Nelson, E.R.; Vistnes, D.; Wu, B.; Lee, M.; Gupta, A.; Longaker, M.T. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS ONE 2010, 5, e11177. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.M.; Shi, Y.Y.; Aalami, O.O.; Chou, Y.F.; Mari, C.; Thomas, R.; Quarto, N.; Contag, C.H.; Wu, B.; Longaker, M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 2004, 22, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Kerkis, A.; Dozortsev, D.; Stukart-Parsons, G.C.; Gomes Massironi, S.M.; Pereira, L.V.; Caplan, A.I.; Cerruti, H.F. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 2007, 184, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, R.; Papaccio, G.; Laino, G.; Graziano, A. Dental pulp stem cells: A promising tool for bone regeneration. Stem Cell Rev. 2008, 4, 21–26. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chan, Y.H.; Hsieh, S.C.; Lew, W.Z.; Feng, S.W. Comparing the osteogenic potentials and bone regeneration capacities of bone marrow and dental pulp mesenchymal stem cells in a rabbit calvarial bone defect model. Int. J. Mol. Sci. 2019, 20, 5015. [Google Scholar] [CrossRef]
- Gandia, C.; Armiñan, A.; García-Verdugo, J.M.; Lledó, E.; Ruiz, A.; Miñana, M.D.; Sanchez-Torrijos, J.; Payá, R.; Mirabet, V.; Carbonell-Uberos, F.; et al. Human Dental Pulp Stem Cells Improve Left Ventricular Function, Induce Angiogenesis, and Reduce Infarct Size in Rats with Acute Myocardial Infarction. Stem Cells 2008, 26, 638–645. [Google Scholar] [CrossRef]
- Kerkis, I.; Ambrosio, C.E.; Kerkis, A.; Martins, D.S.; Zucconi, E.; Fonseca, S.A.S.; Cabral, R.M.; Maranduba, C.M.C.; Gaiad, T.P.; Morini, A.C.; et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: Local or systemic? J. Transl. Med. 2008, 6, 35. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura-Yamada, S.; Kusano, K.; Baba, S. Clinical potential and current progress of dental pulp stem cells for various systemic diseases in regenerative medicine: A concise review. Int. J. Mol. Sci. 2019, 20, 1132. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, L.; Zhang, Y.; Sun, Y.; Li, G. Systemic and local administration of allogeneic bone marrow-derived mesenchymal stem cells promotes fracture healing in rats. Cell Transplant. 2015, 24, 2643–2655. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Gou, W.; Xu, X.; Wang, Y.; Wang, A.; Xu, W.; Guo, Q.; Liu, S.; Lu, Q.; et al. The optimal time to inject bone mesenchymal stem cells for fracture healing in a murine model 11 Medical and Health Sciences 1103 Clinical Sciences. Stem Cell Res. Ther. 2018, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fang, T.; Qi, Y.; Yin, X.; Di, T.; Feng, G.; Lei, Z.; Zhang, Y.; Huang, Z. Combined use of mesenchymal stromal cell sheet transplantation and local injection of SDF-1 for bone repair in a rat nonunion model. Cell Transplant. 2016, 25, 1801–1817. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Yao, W.; Liu, R.; Lam, K.S.; Nolta, J.; Jia, J.; Panganiban, B.; Meng, L.; Zhou, P.; Shahnazari, M.; et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat. Med. 2012, 18, 456–462. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.H.C. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet. Disord. 2010, 11, 10. [Google Scholar] [CrossRef]
- Huang, T.F.; Yew, T.L.; Chiang, E.R.; Ma, H.L.; Hsu, C.Y.; Hsu, S.H.; Hsu, Y.T.; Hung, S.C. Mesenchymal stem cells from a hypoxic culture improve and engraft achilles tendon repair. Am. J. Sports Med. 2013, 41, 1117–1125. [Google Scholar] [CrossRef]
- Murray, M.M.; Spindler, K.P.; Devin, C.; Snyder, B.S.; Muller, J.; Takahashi, M.; Ballard, P.; Nanney, L.B.; Zurakowski, D. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J. Orthop. Res. 2006, 24, 820–830. [Google Scholar] [CrossRef]
- Murray, M.M.; Spindler, K.P.; Abreu, E.; Muller, J.A.; Nedder, A.; Kelly, M.; Frino, J.; Zurakowski, D.; Valenza, M.; Snyder, B.D.; et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J. Orthop. Res. 2007, 25, 81–91. [Google Scholar] [CrossRef]
- Tarafder, S.; Brito, J.A.; Minhas, S.; Effiong, L.; Thomopoulos, S.; Lee, C.H. In situ tissue engineering of the tendon-to-bone interface by endogenous stem/progenitor cells. Biofabrication 2019, 12, 015008. [Google Scholar] [CrossRef]
- Al-Ani, M.K.; Xu, K.; Sun, Y.; Pan, L.; Xu, Z.; Yang, L. Study of Bone Marrow Mesenchymal and Tendon-Derived Stem Cells Transplantation on the Regenerating Effect of Achilles Tendon Ruptures in Rats. Stem Cells Int. 2015, 2015, 984146. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.A.; Butler, D.L.; Boivin, G.P.; Smith, F.N.L.; Malaviya, P.; Huibregtse, B.; Caplan, A.I. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999, 5, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Liu, H.; Wong, E.J.W.; Toh, S.L.; Goh, J.C.H. In vivo study of anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold. Biomaterials 2008, 29, 3324–3337. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Fang, W.L.; Jin, B.; Xu, S.C.; Zheng, X.; Hu, Y.G. Enhancement of tendon-bone healing after rotator cuff injuries using combined therapy with mesenchymal stem cells and platelet rich plasma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9075–9084. [Google Scholar] [PubMed]
- Lee, S.Y.; Kwon, B.; Lee, K.; Son, Y.H.; Chung, S.G. Therapeutic Mechanisms of Human Adipose-Derived Mesenchymal Stem Cells in a Rat Tendon Injury Model. Am. J. Sports Med. 2017, 45, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Frauz, K.; Teodoro, L.F.; Carneiro, G.; da Veiga, F.C.; Ferrucci, D.L.; Bombeiro, A.L.; Simões, P.W.; Alvares, L.E.; de Oliveira, L.R.; Vicente, C.P.; et al. Transected Tendon Treated with a New Fibrin Sealant Alone or Associated with Adipose-Derived Stem Cells. Cells 2019, 8, 56. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, F.Y.; Tarafder, S.; Kao, K.; Jun, Y.; Yang, G.; Mao, J.J. Harnessing endogenous stem/progenitor cells for tendon regeneration. J. Clin. Investig. 2015, 125, 2690–2701. [Google Scholar] [CrossRef]
- Uggen, C.; Dines, J.; McGarry, M.; Grande, D.; Lee, T.; Limpisvasti, O. The effect of recombinant human platelet-derived growth factor BB-coated sutures on rotator cuff healing in a sheep model. J. Arthrosc. Relat. Surg. 2010, 26, 1456–1462. [Google Scholar] [CrossRef]
- Ko, J.Y.; Kim, K.I.; Park, S.; Im, G.I. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials 2014, 35, 3571–3581. [Google Scholar] [CrossRef]
- Xing, D.; Wu, J.; Wang, B.; Liu, W.; Liu, W.; Zhao, Y.; Wang, L.; Li, J.J.; Liu, A.; Zhou, Q.; et al. Intra-articular delivery of umbilical cord-derived mesenchymal stem cells temporarily retard the progression of osteoarthritis in a rat model. Int. J. Rheum. Dis. 2020, 23, 778–787. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Yin, Z.; Zhou, S.; Qiu, Y. SUMO-Modified bone marrow mesenchymal stem cells promoted the repair of articular cartilage in rats. Cell Biol. Int. 2020, 44, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Jin, C.; Li, X.; Li, J.; Du, X.; Yan, C.; Lu, S.; Wei, B.; Xu, Y.; Wang, L. Evaluation of an autologous bone mesenchymal stem cell-derived extracellular matrix scaffold in a rabbit and minipig model of cartilage repair. Med. Sci. Monit. 2019, 25, 7342–7350. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Newby, S.; Misk, N.; Donnell, R.; Dhar, M. Xenogenic implantation of equine synovial fluid-derived mesenchymal stem cells leads to articular cartilage regeneration. Stem Cells Int. 2018, 2018, 1073705. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Cook, J.L.; Mendelson, A.; Moioli, E.K.; Yao, H.; Mao, J.J. Regeneration of the articular surface of the rabbit synovial joint by cell homing: A proof of concept study. Lancet 2010, 376, 440–448. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, T.; Huang, S.; Suen, C.W.W.; Cheng, X.; Li, J.; Hou, H.; She, G.; Zhang, H.; Wang, H.; et al. Sustained Release SDF-1α/TGF-β1-Loaded Silk Fibroin-Porous Gelatin Scaffold Promotes Cartilage Repair. ACS Appl. Mater. Interfaces 2019, 11, 14608–14618. [Google Scholar] [CrossRef]
- Maehara, H.; Sotome, S.; Yoshii, T.; Torigoe, I.; Kawasaki, Y.; Sugata, Y.; Yuasa, M.; Hirano, M.; Mochizuki, N.; Kikuchi, M.; et al. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2). J. Orthop. Res. 2010, 28, 677–686. [Google Scholar] [CrossRef]
- Nourissat, G.; Berenbaum, F.; Duprez, D. Tendon injury: From biology to tendon repair. Nat. Rev. Rheumatol. 2015, 11, 223–233. [Google Scholar] [CrossRef]
- Fenwick, S.A.; Hazleman, B.L.; Riley, G.P. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002, 4, 252–260. [Google Scholar] [CrossRef]
- Bray, R.C.; Rangayyan, R.M.; Frank, C.B. Normal and healing ligament vascularity: A quantitative histological assessment in the adult rabbit medial collateral ligament. J. Anat. 1996, 188, 87. [Google Scholar]
- Tozer, S.; Duprez, D. Tendon and ligament: Development, repair and disease. Birth Defects Res. Part C Embryo Today Rev. 2005, 75, 226–236. [Google Scholar] [CrossRef]
- Kannus, P. Structure of the tendon connective tissue. J. Med. Sci. Sport. 2000, 10, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Kastelic, J.; Galeski, A.; Baer, E. The multicomposite structure of tendon. Connect. Tissue Res. 1978, 6, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Riley, G.P.; Harrall, H.; Constant, C.R.; Chard, M.D.; Cawston, T.E.; Hazleman, B.L. Tendon degeneration and chronic shoulder pain: Changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann. Rheum. Dis. 1994, 53, 359–366. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Birch, H.L.; Clegg, P.D.; Screen, H.R. The Role of the Non-Collagenous Matrix in Tendon Function. Int. J. Exp. Pathol. 2013, 94, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.R.; Evans, E.B.; Matuszewski, P.E.; Chen, Y.L.; Satchel, L.N.; Elliott, D.M.; Soslowsky, L.J.; Dodge, G.R. Distributions of types I, II and III collagen by region in the human supraspinatus tendon. Connect. Tissue Res. 2013, 54, 374–379. [Google Scholar] [CrossRef]
- J.H., Y.; J., H. Tendon proteoglycans: Biochemistry and function. J. Musculoskelet. Neuronal Interact. 2005, 5, 22–34. [Google Scholar]
- Leong, N.L.; Kator, J.L.; Clemens, T.L.; James, A.; Enamoto-Iwamoto, M.; Jiang, J. Tendon and Ligament Healing and Current Approaches to Tendon and Ligament Regeneration. J. Orthop. Res. 2020, 38, 7–12. [Google Scholar] [CrossRef]
- Benjamin, M.; Ralphs, J.R. The cell and developmental biology of tendons and ligaments. Int. Rev. Cytol. 2000, 196, 85–130. [Google Scholar]
- Hope, M.; Saxby, T.S. Tendon Healing. Foot Ankle Clin. 2007, 12, 553–567. [Google Scholar] [CrossRef]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon Healing: Repair and Regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef]
- Molloy, T.; Wang, Y.; Murrell, G.A. The roles of growth factors in tendon and ligament healing. Sport. Med. 2003, 33, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Massimino, M.L.; Rapizzi, E.; Cantini, M.; Libera, L.D.; Mazzoleni, F.; Arslan, P.; Carraro, U. ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochem. Biophys. Res. Commun. 1997, 235, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Leadbetter, W.B. Cell-matrix response in tendon injury. Clin. Sports Med. 1992, 11, 533–578. [Google Scholar] [PubMed]
- James, R.; Kesturu, G.; Balian, G.; Chhabra, A.B. Tendon: Biology, Biomechanics, Repair, Growth Factors, and Evolving Treatment Options. J. Hand Surg. Am. 2008, 33, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N.; Maffulli, N. Tendon Structure Biology of Tendon Injury: Healing, Modeling and Remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar]
- Abrahamsson, S.-O.; Lundborg, G.; Lohmander, L.S. Long-term explant culture of rabbit flexor tendon: Effects of recombinant human insulin-like growth factor-I and serum on matrix metabolism. J. Orthop. Res. 1991, 9, 503–515. [Google Scholar] [CrossRef]
- Miyashita, H.; Ochi, M.; Ikuta, Y. Histological and biomechanical observations of the rabbit patellar tendon after removal of its central one-third. Arch. Orthop. Trauma Surg. 1997, 116, 454–462. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Thomopoulos, S.; Flanagan, C.L.; DeBano, C.M.; Soslowsky, L.J. Rotator cuff defect healing: A biomechanical and histologic analysis in an animal model. J. Shoulder Elb. Surg. 1998, 7, 599–605. [Google Scholar] [CrossRef]
- Liu, C.F.; Aschbacher-Smith, L.; Barthelery, N.J.; Dyment, N.; Butler, D.; Wylie, C. What we should know before using tissue engineering techniques to repair injured tendons: A developmental biology perspective. Tissue Eng. Part B Rev. 2011, 17, 165–176. [Google Scholar] [CrossRef]
- Uchida, R.; Jacob, G.; Shimomura, K.; Horibe, S.; Nakamura, N. Biological Augmentation of ACL Repair and Reconstruction: Current Status and Future Perspective. Sports Med. Arthrosc. 2020, 28, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Juncosa, N.; Dressler, M.R. Functional Efficacy of Tendon Repair Processes. Annu. Rev. Biomed. Eng. 2004, 6, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Teunis, T.; Lubberts, B.; Reilly, B.T.; Ring, D. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J. Shoulder Elb. Surg. 2014, 23, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Oh, L.S.; Wolf, B.R.; Hall, M.P.; Levy, B.A.; Marx, R.G. Indications for rotator cuff repair: A systematic review. Clin. Orthop. Relat. Res. 2007, 455, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Evans, E.J.; Copp, L. The histology of tendon attachments to bone in man. J. Anat. 1986, 149, 89–100. [Google Scholar] [PubMed]
- Benjamin, M.; Toumi, H.; Ralphs, J.R.; Bydder, G.; Best, T.M.; Milz, S. Where tendons and ligaments meet bone: Attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J. Anat. 2006, 208, 471–490. [Google Scholar] [CrossRef]
- Gerber, C.; Schneeberger, A.G.; Perren, S.M.; Nyffeler, R.W. Experimental rotator cuff repair. A preliminary study. J. Bone Jt. Surg. Ser. A 1999, 81, 1281–1290. [Google Scholar] [CrossRef]
- Frank, C.B.; Jackson, D.W. Current Concepts Review—The Science of Reconstruction of the Anterior Cruciate Ligament. J. Bone Jt. Surg. 2005, 1556–1576. [Google Scholar] [CrossRef]
- Murray, M.M.; Martin, S.D.; Martin, T.L.; Spector, M. Histological changes in the human anterior cruciate ligament after rupture. J. Bone Jt. Surg. Ser. A 2000, 82, 1387–1397. [Google Scholar] [CrossRef]
- Gagliardi, A.G.; Carry, P.M.; Parikh, H.B.; Traver, J.L.; Howell, D.R.; Albright, J.C. ACL Repair With Suture Ligament Augmentation Is Associated With a High Failure Rate Among Adolescent Patients. Am. J. Sports Med. 2019, 47, 560–566. [Google Scholar] [CrossRef]
- Buss, D.D.; Warren, R.F.; Wickiewicz, T.L.; Galinat, B.J.; Panariello, R. Arthroscopically assisted reconstruction of the anterior cruciate ligament with use of autogenous patellar-ligament grafts. Results after twenty-four to forty-two months. J. Bone Jt. Surg. Ser. A 1993, 75, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Chiou, H.M.; Chang, M.C.; Lo, W.H. One-stage reconstruction of skin defect and patellar tendon rupture after total knee arthroplasty: A new technique. J. Arthroplast. 1997, 12, 575–579. [Google Scholar] [CrossRef]
- Schweitzer, R.; Chyung, J.H.; Murtaugh, L.C.; Brent, A.E.; Rosen, V.; Olson, E.N.; Lassar, A.; Tabin, C.J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001, 128, 3855–3866. [Google Scholar] [PubMed]
- Murchison, N.D.; Price, B.A.; Conner, D.A.; Keene, D.R.; Olson, E.N.; Tabin, C.J.; Schweitzer, R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 2007, 134, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Shukunami, C.; Takimoto, A.; Nishizaki, Y.; Yoshimoto, Y.; Tanaka, S.; Miura, S.; Watanabe, H.; Sakuma, T.; Yamamoto, T.; Kondoh, G.; et al. Scleraxis is a transcriptional activator that regulates the expression of Tenomodulin, a marker of mature tenocytes and ligamentocytes. Sci. Rep. 2018, 8, 3155. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.-M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Rui, Y.-F.; Lui, P.P.Y.; Li, G.; Fu, S.C.; Lee, Y.W.; Chan, K.M. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng. Part A 2010, 16, 1549–1558. [Google Scholar] [CrossRef]
- Lovati, A.B.; Corradetti, B.; Lange Consiglio, A.; Recordati, C.; Bonacina, E.; Bizzaro, D.; Cremonesi, F. Characterization and differentiation of equine tendon-derived progenitor cells. J. Biol. Regul. Homeost. Agents 2020, 25 (Suppl. 2), S75–S84. [Google Scholar]
- Sutter, W.W. Autologous Cell-Based Therapy for Tendon and Ligament Injuries. Clin. Tech. Equine Pract. 2007, 6, 198–208. [Google Scholar] [CrossRef]
- Gaspar, D.; Spanoudes, K.; Holladay, C.; Pandit, A.; Zeugolis, D. Progress in cell-based therapies for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Hirzinger, C.; Tauber, M.; Korntner, S.; Quirchmayr, M.; Bauer, H.C.; Traweger, A.; Tempfer, H. ACL injuries and stem cell therapy. Arch. Orthop. Trauma Surg. 2014, 134, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H.; Huard, J. Gene therapy approaches to regenerating the musculoskeletal system. Nat. Rev. Rheumatol. 2015, 11, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, M.; Behera, P.; Patel, S.; Shetty, V. Orthobiologics and platelet rich plasma. Indian J. Orthop. 2014, 48, 1–9. [Google Scholar] [CrossRef]
- Bedi, A.; Maak, T.; Walsh, C.; Rodeo, S.A.; Grande, D.; Dines, D.M.; Dines, J.S. Cytokines in rotator cuff degeneration and repair. J. Shoulder Elb. Surg. 2012, 21, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Morita, W.; Dakin, S.G.; Snelling, S.J.B.; Carr, A.J. Cytokines in tendon disease: A systematic review. Bone Jt. Res. 2017, 6, 656–664. [Google Scholar] [CrossRef]
- Irie, K.; Uchiyama, E.; Iwaso, H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee 2003, 10, 93–96. [Google Scholar] [CrossRef]
- Kobayashi, D.; Kurosaka, M.; Yoshiya, S.; Mizuno, K. Effect of basic fibroblast growth factor on the healing of defects in the canine anterior cruciate ligament. Knee Surg. Sports Traumatol. Arthrosc. 1997, 5, 189–194. [Google Scholar] [CrossRef]
- Vermeulen, S.; Vasilevich, A.; Tsiapalis, D.; Roumans, N.; Vroemen, P.; Beijer, N.R.M.; Dede Eren, A.; Zeugolis, D.; de Boer, J. Identification of topographical architectures supporting the phenotype of rat tenocytes. Acta Biomater. 2019, 83, 277–290. [Google Scholar] [CrossRef]
- Lui, P.P.Y.; Wong, C.M. Biology of Tendon Stem Cells and Tendon in Aging. Front. Genet. 2020, 10, 1338. [Google Scholar] [CrossRef]
- Zhou, Z.; Akinbiyi, T.; Xu, L.; Ramcharan, M.; Leong, D.J.; Ros, S.J.; Colvin, A.C.; Schaffler, M.B.; Majeska, R.J.; Flatow, E.L.; et al. Tendon-derived stem/progenitor cell aging: Defective self-renewal and altered fate. Aging Cell 2010, 9, 911–915. [Google Scholar] [CrossRef]
- Jang, K.-M.; Lim, H.; Bae, J. Mesenchymal Stem Cells for Enhancing Biologic Healing after Anterior Cruciate Ligament Injuries. Curr. Stem Cell Res. Ther. 2015, 10, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, L.V.; Kovacevic, D.; Ehteshami, J.R.; Dagher, E.; Packer, J.D.; Rodeo, S.A. Application of bone marrow-derived mesenchymal stem cells in a Rotator cuff repair model. Am. J. Sports Med. 2009, 37, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, L.V.; Kovacevic, D.; Packer, J.D.; Deng, X.H.; Rodeo, S.A. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am. J. Sports Med. 2011, 39, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Haddad-Weber, M.; Prager, P.; Kunz, M.; Seefried, L.; Jakob, F.; Murray, M.M.; Evans, C.H.; Nöth, U.; Steinert, A.F. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy 2010, 12, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Ricci, G.; D’Andrea, F.; Nicoletti, G.F.; Ferraro, G.A. Human Adipose Stem Cells: From Bench to Bedside. Tissue Eng. Part B Rev. 2015, 21, 572–584. [Google Scholar] [CrossRef]
- Park, A.; Hogan, M.V.; Kesturu, G.S.; James, R.; Balian, G.; Chhabra, A.B. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Eng. Part A 2010, 16, 2941–2951. [Google Scholar] [CrossRef]
- Uysal, C.A.; Tobita, M.; Hyakusoku, H.; Mizuno, H. Adipose-derived stem cells enhance primary tendon repair: Biomechanical and immunohistochemical evaluation. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, 1712–1719. [Google Scholar] [CrossRef]
- Oshita, T.; Tobita, M.; Tajima, S.; Mizuno, H. Adipose-Derived Stem Cells Improve Collagenase-Induced Tendinopathy in a Rat Model. Am. J. Sports Med. 2016, 44, 1983–1989. [Google Scholar] [CrossRef]
- Kokubu, S.; Inaki, R.; Hoshi, K.; Hikita, A. Adipose-derived stem cells improve tendon repair and prevent ectopic ossification in tendinopathy by inhibiting inflammation and inducing neovascularization in the early stage of tendon healing. Regen. Ther. 2020, 14, 103–110. [Google Scholar] [CrossRef]
- Norelli, J.B.; Plaza, D.P.; Stal, D.N.; Varghese, A.M.; Liang, H.; Grande, D.A. Tenogenically differentiated adipose-derived stem cells are effective in Achilles tendon repair in vivo. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef]
- Würgler-Hauri, C.C.; Dourte, L.A.M.; Baradet, T.C.; Williams, G.R.; Soslowsky, L.J. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J. Shoulder Elb. Surg. 2007, 16, S198–S203. [Google Scholar] [CrossRef] [PubMed]
- Heisterbach, P.E.; Todorov, A.; Flückiger, R.; Evans, C.H.; Majewski, M. Effect of BMP-12, TGF-β1 and autologous conditioned serum on growth factor expression in Achilles tendon healing. Knee Surgery Sport. Traumatol. Arthrosc. 2012, 20, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Flouzat Lachaniette, C.H.; Delambre, J.; Zilber, S.; Duffiet, P.; Chevallier, N.; Rouard, H. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: A case-controlled study. Int. Orthop. 2014, 38, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sung, C.H.; Chung, S.H.; Kwak, S.J.; Koh, Y.G. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am. J. Sports Med. 2017, 45, 2010–2018. [Google Scholar] [CrossRef]
- Centeno, C.J.; Pitts, J.; Al-Sayegh, H.; Freeman, M.D. Anterior cruciate ligament tears treated with percutaneous injection of autologous bone marrow nucleated cells: A case series. J. Pain Res. 2015, 8, 437–447. [Google Scholar]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Malinin, T.; Ouellette, E.A. Articular cartilage nutrition is mediated by subchondral bone: A long-term autograft study in baboons. Osteoarthr. Cartil. 2000, 8, 483–491. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, L.; Zeng, L.; He, D.; Wei, X. Nutrition and degeneration of articular cartilage. Knee Surgery Sport. Traumatol. Arthrosc. 2013, 21, 1751–1762. [Google Scholar] [CrossRef]
- Akkiraju, H.; Nohe, A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef]
- Smith, B.; Sigal, I.R.; Grande, D.A. Immunology and cartilage regeneration. Immunol. Res. 2015, 63, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Shi, K.; Ding, Q.; Qu, Y.; Luo, F.; Qian, Z. Recent developments in scaffold-guided cartilage tissue regeneration. J. Biomed. Nanotechnol. 2014, 10, 3085–3104. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Zhang, L.; Li, K.; Fan, H.; Fan, Y.; Liang, J.; Zhang, X. Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.A.; Correia, C.; Oliveira, J.M.; Sousa, R.A.; Espregueira-Mendes, J.; Reis, R.L. Cartilage Repair Using Hydrogels: A Critical Review of in Vivo Experimental Designs. ACS Biomater. Sci. Eng. 2015, 1, 726–739. [Google Scholar] [CrossRef]
- Muchedzi, T.A.; Roberts, S.B. A systematic review of the effects of platelet rich plasma on outcomes for patients with knee osteoarthritis and following total knee arthroplasty. Surgeon 2018, 16, 250–258. [Google Scholar] [CrossRef]
- Cancedda, R.; Dozin, B.; Giannoni, P.; Quarto, R. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol. 2003, 22, 81–91. [Google Scholar] [CrossRef]
- Hjelle, K.; Solheim, E.; Strand, T.; Muri, R.; Brittberg, M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 2002, 18, 730–734. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Walker, K.J.; Madihally, S.V. Anisotropic temperature sensitive chitosan-based injectable hydrogels mimicking cartilage matrix. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1149–1160. [Google Scholar] [CrossRef]
- Chahla, J.; Cinque, M.E.; Godin, J.A.; Sanchez, G.; Lebus, G.F.; Whalen, J.M.; Price, M.D.; Kennedy, N.I.; Moatshe, G.; LaPrade, R.F.; et al. Meniscectomy and Resultant Articular Cartilage Lesions of the Knee Among Prospective National Football League Players: An Imaging and Performance Analysis. Am. J. Sports Med. 2018, 46, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Dorotka, R.; Windberger, U.; Macfelda, K.; Bindreiter, U.; Toma, C.; Nehrer, S. Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials 2005, 26, 3617–3629. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, K.; Mcadams, T.; Williams, R.J.; Kreuz, P.C.; Mandelbaum, B.R. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: An evidence-based systematic analysis. Am. J. Sports Med. 2009, 37, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Benazzo, F.; Cadossi, M.; Cavani, F.; Fini, M.; Giavaresi, G.; Setti, S.; Cadossi, R.; Giardino, R. Cartilage repair with osteochondral autografts in sheep: Effect of biophysical stimulation with pulsed electromagnetic fields. J. Orthop. Res. 2008, 26, 631–642. [Google Scholar] [CrossRef]
- McCarty, E.C.; Fader, R.R.; Mitchell, J.J.; Glenn, R.E.; Potter, H.G.; Spindler, K.P. Fresh osteochondral allograft versus autograft: Twelve-month results in isolated canine knee defects. Am. J. Sports Med. 2016, 44, 2354–2365. [Google Scholar] [CrossRef]
- DiBartola, A.C.; Wright, B.M.; Magnussen, R.A.; Flanigan, D.C. Clinical Outcomes After Autologous Chondrocyte Implantation in Adolescents’ Knees: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 1905–1916. [Google Scholar] [CrossRef]
- Niemeyer, P.; Pestka, J.M.; Kreuz, P.C.; Erggelet, C.; Schmal, H.; Suedkamp, N.P.; Steinwachs, M. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am. J. Sports Med. 2008, 36, 2091–2099. [Google Scholar] [CrossRef]
- Selmi, T.A.S.; Verdonk, P.; Chambat, P.; Dubrana, F.; Potel, J.F.; Barnouin, L.; Neyret, P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: Outcome at two years. J. Bone Jt. Surg. 2008, 90, 597–604. [Google Scholar] [CrossRef]
- Harris, J.D.; Siston, R.A.; Pan, X.; Flanigan, D.C. Autologous chondrocyte implantation: A systematic review. J. Bone Jt. Surg. Ser. 2010, 92, 2220–2233. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.; Vasiliadis, H.S.; Brittberg, M.; Lindahl, A. Autologous chondrocyte implantation: A long-term follow-up. Am. J. Sports Med. 2010, 38, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, M.; Villa, V.; Magnussen, R.A.; Neyret, P. MACI—A new era? Sport. Med. Arthrosc. Rehabil. Ther. Technol. 2011, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Devitt, B.M.; Bell, S.W.; Webster, K.E.; Feller, J.A.; Whitehead, T.S. Surgical treatments of cartilage defects of the knee: Systematic review of randomised controlled trials. Knee 2017, 24, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.; Shi, Y.; Liu, W.; Jia, Y.; Kong, L.; Zhang, T.; Han, C.; Ren, Y. Is implantation of autologous chondrocytes superior to microfracture for articular-cartilage defects of the knee? A systematic review of 5-year follow-up data. Int. J. Surg. 2019, 68, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Park, C.H. Microfracture for cartilage repair in the knee: Current concepts and limitations of systematic reviews. Ann. Transl. Med. 2019, 7, S108. [Google Scholar] [CrossRef] [PubMed]
- Aae, T.F.; Randsborg, P.H.; Lurås, H.; Årøen, A.; Lian, Ø.B. Microfracture is more cost-effective than autologous chondrocyte implantation: A review of level 1 and level 2 studies with 5 year follow-up. Knee Surgery Sport. Traumatol. Arthrosc. 2018, 26, 1044–1052. [Google Scholar] [CrossRef]
- Sherman, S.L.; Thyssen, E.; Nuelle, C.W. Osteochondral Autologous Transplantation. Clin. Sports Med. 2017, 36, 489–500. [Google Scholar] [CrossRef]
- Batty, L.; Dance, S.; Bajaj, S.; Cole, B.J. Autologous chondrocyte implantation: An overview of technique and outcomes. ANZ J. Surg. 2011, 81, 18–25. [Google Scholar] [CrossRef]
- Kizaki, K.; El-Khechen, H.A.; Yamashita, F.; Duong, A.; Simunovic, N.; Musahl, V.; Ayeni, O.R. Arthroscopic versus Open Osteochondral Autograft Transplantation (Mosaicplasty) for Cartilage Damage of the Knee: A Systematic Review. J. Knee Surg. 2019, 9, 2019. [Google Scholar] [CrossRef]
- Bao, W.; Li, M.; Yang, Y.; Wan, Y.; Wang, X.; Bi, N.; Li, C. Advancements and Frontiers in the High Performance of Natural Hydrogels for Cartilage Tissue Engineering. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Schnabel, M.; Marlovits, S.; Eckhoff, G.; Fichtel, I.; Gotzen, L.; Vécsei, V.; Schlegel, J. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthr. Cartil. 2002, 10, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Menage, J.; Sandell, L.J.; Evans, E.H.; Richardson, J.B. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee 2009, 16, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Breit, S.; Parsch, D.; Benz, K.; Steck, E.; Hauner, H.; Weber, R.M.; Ewerbeck, V.; Richter, W. Cartilage-like gene expression in differentiated human stem cell spheroids: A comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003, 48, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Fuggle, N.R.; Cooper, C.; Oreffo, R.O.C.; Price, A.J.; Kaux, J.F.; Maheu, E.; Cutolo, M.; Honvo, G.; Conaghan, P.G.; Berenbaum, F.; et al. Alternative and complementary therapies in osteoarthritis and cartilage repair. Aging Clin. Exp. Res. 2020, 32, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Ohba, S.; Hojo, H.; Chung, U. Il Bioactive factors for tissue regeneration: State of the art. Muscles Ligaments Tendons J. 2012, 2, 193–203. [Google Scholar] [PubMed]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef]
- Censi, R.; Dubbini, A.; Matricardi, P. Bioactive Hydrogel Scaffolds—Advances in Cartilage Regeneration Through Controlled Drug Delivery. Curr. Pharm. Des. 2015, 21, 1545–1555. [Google Scholar] [CrossRef]
- Dowthwaite, G.P.; Bishop, J.C.; Redman, S.N.; Khan, I.M.; Rooney, P.; Evans, D.J.R.; Haughton, L.; Bayram, Z.; Boyer, S.; Thomson, B.; et al. The surface of articular cartilage contains a progenitor cell populations. J. Cell Sci. 2004, 117, 889–897. [Google Scholar] [CrossRef]
- Yang, S.S.; Jin, L.H.; Park, S.H.; Kim, M.S.; Kim, Y.J.; Choi, B.H.; Lee, C.T.; Park, S.R.; Min, B.H. Extracellular matrix (ECM) multilayer membrane as a sustained releasing growth factor delivery system for rhTGF-β3 in articular cartilage repair. PLoS ONE 2016, 11, e0156292. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Kim, Y.H.; Jung, Y.; Kim, S.H.; Park, J.C.; Yoon, D.S.; Kim, S.H.; Lee, J.W. In Situ Recruitment of Human Bone Marrow-Derived Mesenchymal Stem Cells Using Chemokines for Articular Cartilage Regeneration. Cell Transplant. 2015, 24, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Brouillette, M.J.; Seol, D.; Zheng, H.; Buckwalter, J.A.; Martin, J.A. Use of recombinant human stromal cell-derived factor 1α-loaded fibrin/hyaluronic acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis Rheumatol. 2015, 67, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Boushell, M.K.; Mosher, C.Z.; Suri, G.K.; Doty, S.B.; Strauss, E.J.; Hunziker, E.B.; Lu, H.H. Polymeric mesh and insulin-like growth factor 1 delivery enhance cell homing and graft−cartilage integration. Ann. N. Y. Acad. Sci. 2019, 1442, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Guilak, F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy 2003, 5, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Wickham, M.Q.; Erickson, G.R.; Gimble, J.M.; Vail, T.P.; Guilak, F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin. Orthop. Relat. Res. 2003, 412, 196–212. [Google Scholar] [CrossRef]
- Estes, B.T.; Diekman, B.O.; Gimble, J.M.; Guilak, F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat. Protoc. 2010, 5, 1294–1311. [Google Scholar] [CrossRef]
- Huang, J.I.; Zuk, P.A.; Jones, N.F.; Zhu, M.; Lorenz, H.P.; Hedrick, M.H.; Benhaim, P. Chondrogenic potential of multipotential cells from human adipose tissue. Plast. Reconstr. Surg. 2004, 113, 585–594. [Google Scholar] [CrossRef]
| Tissue | Approach | Animal Model | Injury Model | Experimental Treatment | Outcomes/Results | References |
|---|---|---|---|---|---|---|
| Bone | Endogenous | Mice | Long Segmental Defect | Growth factor + AMD3100 treatment | 2 weeks: IGF1 showed increased proliferation and migration of isolated MSC as well as augmented bone growth. | Kumar et al., [86] 2012 |
| Bone | Exogenous | Nude mice | Calvarial defect (4 mm) | Undifferentiated Human ASCs + PLGA scaffold + (rh) BMP2 | 8 weeks: Gross analysis, microCT, and histological examination showed complete healing and trabecular bone formation in the hASCs treated group compared to the scaffold only group and empty defects. | Levi et al., [103] 2010 |
| Bone | Exogenous | Rats | Femoral fracture | BMSCs + skin fibroblasts | 5 weeks: Callus size and mechanical properties were significantly higher in the MSC injected group compared to fibroblast and the PBS control. Quantitative analysis showed GFP-positive MSCs were present in callus in MSC group at 5 weeks after fracture. | Huang et al., [113] 2015 |
| Bone | Exogenous | Mice | Femur fracture | Mouse BMSCs/RFP | 42 days: BMSCs injected on day 7 post-fracture accelerated fracture healing with improved callus and bone quality. | Wang et al., [114] 2018 |
| Bone | Exogenous | Rat | Bone nonunion | Primary MSCs sheet + SDF1 injection | 4 and 8 weeks: At 4 weeks, new formed bone tissue united the distal and proximal sites in the MSC sheet/SDF group compared to 5 other groups. At 8 weeks, the MSC sheet/SDF group showed complete bridging of the fracture site, forming hard bony union. | Chen et al., [115] 2016 |
| Bone | Exogenous + Endogenous | Mice | Osteogenesis impairment | Peptidomimetic ligand (LLP2A) + Alendronate (LLP2A-Ale) injection | 3 and 12 weeks: At 3 weeks, the hMSC cells injected intravenously in the xenotransplantation model were observed at the bone surface in the LLP2A-Ale group. At 12 weeks, the LLP2A-Ale group could augment bone formation in mice. | Guan et al., [116] 2012 |
| Tendon | Exogenous | Rats | Multi-differentiation potential | TSCs + Matrigel (gel-cells) | 8 weeks: Transplantation of TSCs subcutaneously resulted in the formation of tendon, cartilage and bone-like tissues. | Zhang et al., [117] 2010 |
| Tendon | Exogenous | Rat | Achilles Tendon | MSCs cultured in hypoxic and normoxic condition | 2 and 4 weeks: Tendon rupture site and biomechanical properties were superior in hypoxic MSC group compared to the normoxic and control. | Huang et al., [118] 2013 |
| Ligament | Endogenous; Growth factors | Canine | ACL defect | Collagen-Platelet Rich Plasma (PRP) Scaffold | 3 and 6 weeks: The percent filling defect was significantly higher in the treated group at both 3 and 6 weeks compared to the untreated defects. Mechanically, the ACL treated group had 40% increase in strength at 6 weeks, compared to untreated defects. | Murray et al., [119] 2006 |
| Ligament | Endogenous; Growth factors | Porcine | ACL defect | Suture + Collagen-Platelet Rich Plasma (PRP) hydrogel | 4 weeks: At 4 weeks, the collagen-PRP hydrogel group stimulated healing and improved biomechanical properties after suture repair, compared to suture repair alone. However, both groups remained significantly inferior to the intact ligament group. | Murray et al., [120] 2007 |
| Tendon | Endogenous; Growth factors | Rat | Rotator Cuff | 3D printed scaffold + Growth factors (CTGF, CTGF + TGF-b + BMP2) | 1 and 4 weeks GF embedded (+GF) scaffolds promoted recruitment of endogenous tendon progenitor cells and healed tendon-to-bone via formation of cartilaginous interface compared to –GF scaffold. | Solaiman et al., [121] 2019 |
| Tendon | Exogenous | Rat | Achilles Tendon | TDSCs and BMMSCs | 1, 2 and 4 weeks: TDSCs showed higher regenerative potential with high mechanical strength, better appearance density and well-organized longitudinal fibrous structure and BMSCs also showed positive results. | Al-Ani MK et al., [122] 2015 |
| Tendon | Exogenous | Rabbit | Patellar Tendon defect | BMSCs + Type I bovine collagen gel | 4 weeks: Mechanically and histologically, the MSC + gel group showed significantly greater material and structural properties compared to the collagen gel alone control. However, treatment group improvements were not impressive compared to the normal healthy tendon. | Awad et al., [123] 1999 |
| Ligament | Exogenous | Rabbit | ACL Reconstruction | BMSCs + Silk scaffold | 8, 16 and 24 weeks: The MSC/Scaffold group showed abundant ligament ECM (Col I was more prominent compared to Col III and Tenascin-C), compared to the scaffold alone control. The tensile strength was comparable to the mechanical properties of daily activities. | Fan et al., [124] 2008 |
| Tendon | Exogenous | Rat | Rotator Cuff injury | BMSCs + PRP | 4 and 8 weeks: Gene and protein detection at 4 weeks, showed that combined therapy enhanced the expression of growth factors and genes related to tendon repair (Col I, Tenomodulin, Scx). At 8 weeks, mechanical testing demonstrated that combined therapy was most efficient to promote tissue regeneration, compared to single therapy control (PRP alone and MSC alone). | Han et al., [125] 2019 |
| Tendon | Exogenous | Rat | Tendon injury | hASC + fibrin glue | 4 weeks: Treatment group of hASCs demonstrated enhanced tendon healing biomechanically, compared to the fibrin alone and sham group. Cells were showed to survive for 4 weeks, in vivo and secreted human-specific Col I and Tenascin-C. | Lee et al., [126] 2017 |
| Tendon | Exogenous | Rat | Partial Transection of Achilles Tendon | ASCs + Fibrin Sealant (FS) from serpent venom | 21 days: In vivo analysis at day 14 revealed higher quantification of the transplanted fluorescent ASCs in the tendon treated with ASCs + FS compared to ASC alone. The ASCs group up-regulated Tenomodulin expression compared to normal (without transection), transection alone and the FS group. TIMP-2 and Scx expression compared to N group. FS group demonstrated great organization of collagen fibers followed by ASCs + FS and ASCs alone in comparison to N | Frauz et al., [127] 2019 |
| Tendon | Endogenous; Growth factor | Rat | Patellar Tendon | TSCs (CD146+) + Fibrin glue + CTGF | 1, 2 and 4 weeks: CTFG treated CD146+ cells led to tendon regeneration with dense collagen fibers, compared to the untreated CD146+. By week 4, the CTGF group generated tendon with dense collagen fibers compared to the fibrin alone group and tensile property on the level of native tendon compared to CD146- and untreated CD146+. | Lee et al., [128] 2015 |
| Tendon | Endogenous; Growth factor | Sheep | Rotator Cuff injury | rhPDGF-BB coated sutures | 6 weeks: rhPDGF-BB coated sutures enhanced histologic scores of sheep rotator injury and enhanced tendon healing. However, load to failure was equivalent to standard suture repair. | Uggen et al., [129] 2010 |
| Cartilage | Exogenous | Rat | Osteo-chondral Defect | hiPSCs pellet or hiPSCs + alginate hydrogel | 12 weeks: Defects treated with chondro-induced hiPSCs implantation had smooth, firm tissue with good restoration of articular surface compared to control or alginate alone. However, histological appearance showed reduced amount of proteoglycan compared to the normal cartilage. | Ko et al., [130] 2014 |
| Cartilage | Exogenous | Rat | Osteoarthritis | Human umbilical MSCs + Hyaluronic acid (HA) | 6 and 12 weeks: Macroscopic observation of the femur surface at 6 weeks, showed signs of OA progression with cartilage surface roughness and osteophyte formation compared to preserved cartilage in MSC + HA group; at 12 weeks, joint surface showed OA progression in all 3 groups. Histologically at 6 weeks, the MSC + HA group showed abundant proteoglycan and reduced cartilage loss, whereas at 12 weeks, Saf-O staining was significantly reduced compared to 6 weeks Hence, single injection of hUC-MSCs had temporary effects to decelerate OA progression. | Xing et al., [131] 2020 |
| Cartilage | Exogenous | Rat | Full thickness cartilage defect (2mm) | BMSCs + SUMO1/SUMO2,3/SUMO1,2,3 | 4 weeks: BMSCs overexpressing SUMO1 differentiated into articular cartilage with hard surface; BMSCs overexpressing SUMO1,2 reduced inflammation and improved damaged cartilage microenvironment; BMSCs overexpressing SUMO1,2,3 showed better survival, less inflammatory response, and improved tissue repair. | Liu et al., [132] 2020 |
| Cartilage | Exogenous | Rabbit and Minipigs | Osteo-chondral defect | ECM group: autologous MSC-derived ECM scaffold; BMS group: Bone marrow stimulation | Rabbits: 6hrs, 3 and 7 days: Macroscopic appearance of exudate healing wounds showed less fibrosis and histology showed evenly distributed chondrocyte in the EMS group compared to the BMS. The CFU-F assay showed increased number of bone MSCs in the ECM group. Minipigs: 6 months: Macroscopic and MRI finding improved in the ECM compared to BMS group. Repaired tissue in ECM had similar histological characteristic to normal hyaline cartilage. | Tang et al., [133] 2019 |
| Cartilage | Exogenous | Rat | Full thickness cartilage defect (2 mm) | Equine BMSCs and Synovial Fluid-Derived MSC (SFMSCs) + agarose gel | 1 and 12 weeks: At 1 week, the knee joint showed the presence of MSCs at the injured site.Macroscopic and histological analysis demonstrated better healing of cartilage in MSC treated knees at 12 weeks, compared to the control. SFMSC treated showed significantly higher Col II, suggesting presence of hyaline cartilage at the defect site. | Zayed et al., [134] 2018 |
| Cartilage | Endogenous; Growth factors | Rabbit | Humeral Head incision | TGF-β3 adsorbed or TGF-β3-free + collagen hydrogel | 4 months: The TGF-β3 treated group had significantly greater matrix and articular cartilage thickness compared to the TGF-β3-free group, showing that the articular cartilage of the synovial joint was regenerated by homing endogenous cells. The TGF-β3 treated group also had consistent distribution of Col II and Aggrecan. | Lee et al., [135] 2010 |
| Cartilage | Endogenous; Growth factors | Rats | Osteo-chondral Defect (1.6mm) | Silk fibroin scaffold + SDF-1α + TGF-β1 | 12 weeks: Scaffold treated with + SDF-1α and TGF-β1 (GSTS) had the most significant cartilage regeneration compared to 4 other control groups. The GSTS group also produced more type II collagen compared to other groups, which generated fibrocartilage. | Chen et al., [136] 2019 |
| Cartilage | Endogenous; Growth factors | Rabbit | Osteo-chondral Defect (5 mm) | Hydroxyapatite collagen (Hap/Col) scaffold + FGF-2 with 10 and 100 µg/mL concentration collagen (HAp/Col) scaffold | 3,6, 12 and 24 weeks: Abundant bone formation observed in the Hap/Col group compared to the defect group. The FGF10 group demonstrated abundant bone regeneration as well as satisfactory cartilage regeneration with a hyaline-like appearance. | Maehara et al., [137] 2010 |
| Condition | NCT Identifier | Title | Status | Intervention |
|---|---|---|---|---|
| Non Union Fracture | NCT03325504 | A Comparative Study of 2 Doses of BM Autologous H-MSC + Biomaterial vs Iliac Crest AutoGraft for Bone Healing in Non-Union | Recruiting | Biological: Cultured Mesenchymal Stem Cells Procedure: Autologous iliac crest graft |
| Osteochondral Fracture of Talus | NCT03905824 | The Effectiveness of Adding Allogenic Stem Cells After Traditional Treatment of Osteochondral Lesions of the Talus | Recruiting | Biological: Allogenic stromal mesenchymal cells derived from the umbilical cord Procedure: Debridement and microfracture |
| Full Thickness Rotator Cuff Tear | NCT02484950 | Mesenchymal Stem Cell Augmentation in Patients Undergoing Arthroscopic Rotator Cuff Repair | Recruiting | Biological: Mesenchymal stem cell augmentation in rotator cuff repair Procedure: Standard arthroscopic rotator cuff repair |
| Rotator Cuff Tear Rotator Cuff Tendinitis | NCT03752827 | Autologous Adult Adipose-Derived Regenerative Cell Injection into Chronic Partial-Thickness Rotator Cuff Tears | Recruiting | Device: Adipose Derived Regenerative Cells Drug: Corticosteroid |
| Rotator Cuff Tear | NCT03688308 | Bone Marrow Derived Stem Cells for the Treatment of Rotator Cuff Tears | Recruiting | Procedure: Arthroscopic rotator cuff repair with bone marrow aspirate concentrate |
| Rotator Cuff Tear | NCT03551509 | LifeNet: Extracellular Matrix Graft in Rotator Cuff Repair | Recruiting | Biological: ArthroFLEX ECM scaffold graft Procedure: Control Biological: Crossover |
| Rotator Cuff Tears | NCT04325789 | Rotator Cuff Healing Using a Nanofiber Scaffold in Patients Greater Than 55 Years | Recruiting | Device: Rotium nanofiber graft |
| ACL—Anterior Cruciate Ligament Rupture | NCT03294720 | BioACL Reconstruction with Amnion Collagen Matrix Wrap and Stem Cells Case Series | Active, not recruiting | Procedure: Bio-ACL Device: amnion wrap and BMAC |
| ACL—Anterior Cruciate Ligament Rupture | NCT03294759 | Bio ACL Reconstruction Amnion Collagen Matrix Wrap and Stem Cells | Active, not recruiting | Other: Bio ACL Other: Control |
| Anterior Cruciate Ligament Tear | NCT02664545 | Bridge-Enhanced ACL Repair vs. ACL Reconstruction | Active, not recruiting | Device: BEAR Scaffold Procedure: Tendon Graft |
| Defect of Articular Cartilage Cartilage Injury Osteoarthritis, Knee | NCT02696876 | Synovium Brushing to Augmented Microfracture for Improved Cartilage Repair | Recruiting | Device: Arthroscopic synovial brushing Procedure: Microfracture |
| Degenerative Lesion of Articular Cartilage of Knee | NCT02090140 | Microfracture Versus Adipose Derived Stem Cells for the Treatment of Articular Cartilage Defects | Recruiting | Procedure: ADSC Application Procedure: Microfracture |
| Osteoarthritis, Knee | NCT04205656 | Prospective Evaluation of PRP and BMC Treatment to Accelerate Healing After ACL Reconstruction | Recruiting | Biological: Leukocyte-Poor Platelet Rich Plasma (LP-PRP) Biological: Bone Marrow Concentrate (BMC) Other: Control group (Placebo) |
| Osteoarthritis, Knee | NCT02805855 | Autologous Culture Expanded Mesenchymal Stromal Cells for Knee Osteoarthritis | Recruiting | Drug: Autologous Adipose-Derived Mesenchymal Stromal Cells |
| Knee Osteoarthritis | NCT03014401 | The Effect of Adipose-Derived Stem Cells for Knee Osteoarthritis | Recruiting | Procedure: Arthroscopic debridement with stem cell transplantation Procedure: Arthroscopic debridement only |
| Osteoarthritis, Knee Knee Pain | NCT03467919 | The Effect of Micro Fragmented Adipose Tissue (MFAT) on Knee Osteoarthritis | Recruiting | Procedure: Micro Fragmented Adipose Tissue Procedure: Corticosteroid injection |
| Post-Traumatic Osteoarthritis of Knee | NCT04222140 | Early Regenerative Intervention for Post-Traumatic Osteoarthritis | Not yet recruiting | Combination Product: ERIPTO Protocol Biological: BMAC Only |
| Knee Osteoarthritis | NCT04043819 | Evaluation of Safety and Exploratory Efficacy of an Autologous Adipose-derived Cell Therapy Product for Treatment of Single Knee Osteoarthritis | Active, not recruiting | Drug: PSC-01 |
| Musculoskeletal Pain Knee Osteoarthritis Cartilage Injury Cartilage Degeneration | NCT03477942 | Impact of Mesenchymal Stem Cells in Knee Osteoarthritis | Recruiting | Biological: Autologous Mesenchymal Stem Cells |
| Articular Cartilage Disorder of Knee Articular Cartilage; Degeneration | NCT03101163 | Efficacy and Safety Study of Intra-Articular Injections of Autologous Peripheral Blood Stem Cells Following Subchondral Drilling Surgery for the Treatment of Articular Cartilage Injury in the Knee | Recruiting | Biological: Autologous peripheral blood stem cells and hyaluronic acid Other: Hyaluronic acid |
| Osteoarthritis, Hip | NCT03608579 | Autologous Culture Expanded Adipose Derived MSCs for Treatment of Painful Hip OA | Recruiting | Drug: Autologous Adipose Derived Mesenchymal Stromal Cells |
| Osteoarthritis | NCT03818737 | Multicenter Trial of Stem Cell Therapy for Osteoarthritis (MILES) | Recruiting | Biological: Autologous Bone Marrow Concentrate (BMAC) Biological: Adipose-derived Stromal Vascular Fraction (SVF) Biological: Umbilical Cord Tissue Drug: Depomedrol and Normal saline (Corticosteroid injection) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, S.; Jayasuriya, C.T. Implementation of Endogenous and Exogenous Mesenchymal Progenitor Cells for Skeletal Tissue Regeneration and Repair. Bioengineering 2020, 7, 86. https://doi.org/10.3390/bioengineering7030086
Desai S, Jayasuriya CT. Implementation of Endogenous and Exogenous Mesenchymal Progenitor Cells for Skeletal Tissue Regeneration and Repair. Bioengineering. 2020; 7(3):86. https://doi.org/10.3390/bioengineering7030086
Chicago/Turabian StyleDesai, Salomi, and Chathuraka T. Jayasuriya. 2020. "Implementation of Endogenous and Exogenous Mesenchymal Progenitor Cells for Skeletal Tissue Regeneration and Repair" Bioengineering 7, no. 3: 86. https://doi.org/10.3390/bioengineering7030086
APA StyleDesai, S., & Jayasuriya, C. T. (2020). Implementation of Endogenous and Exogenous Mesenchymal Progenitor Cells for Skeletal Tissue Regeneration and Repair. Bioengineering, 7(3), 86. https://doi.org/10.3390/bioengineering7030086






