3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs
Abstract
1. Introduction
2. 3D Bioprinting Techniques
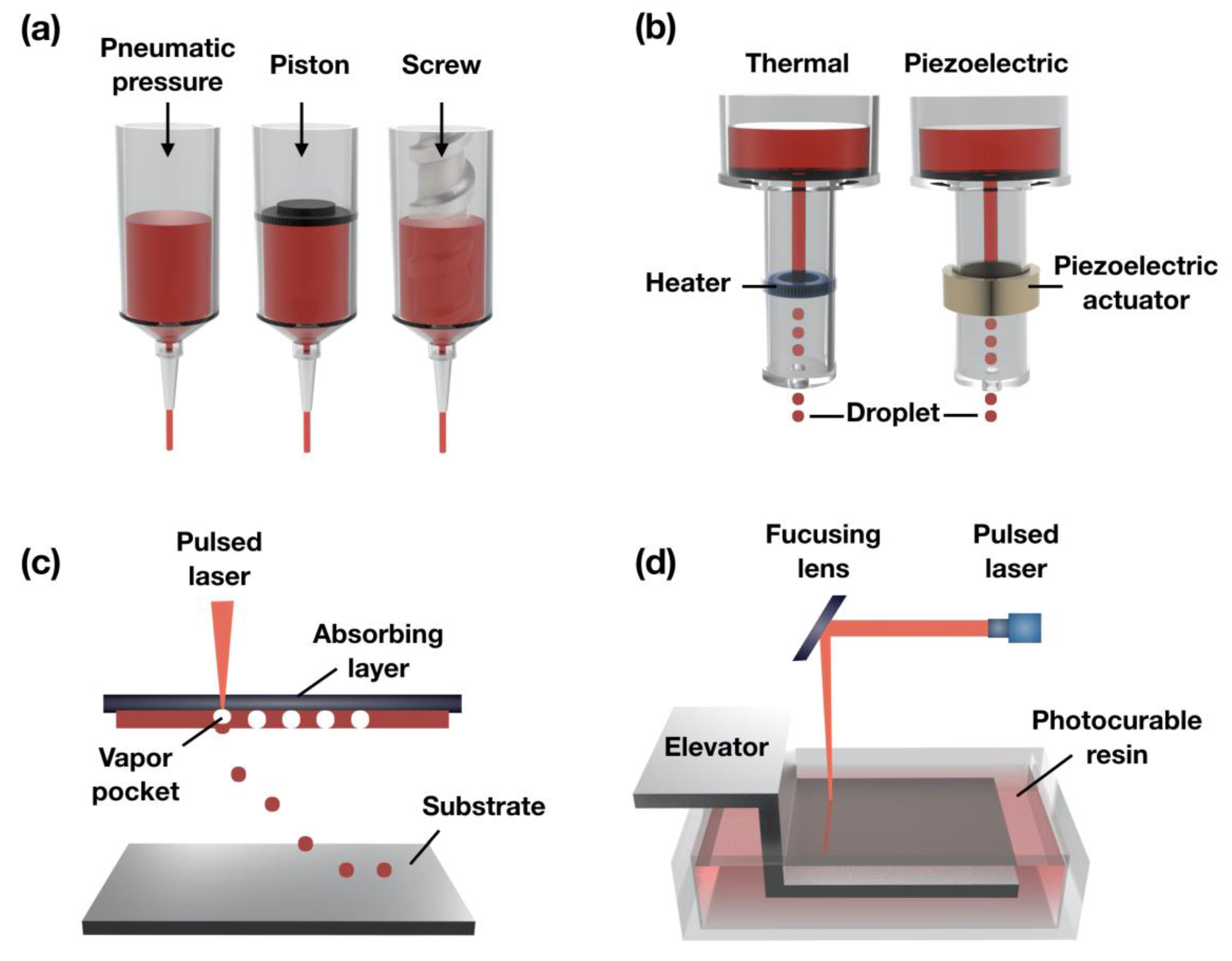
2.1. Extrusion-Based Bioprinting Systems
2.2. Ink-Jet Bioprinting Systems
2.3. Laser-Assisted Bioprinting
2.4. Stereolithography-Based Bioprinting
3. Printable Biocomposite Inks for Various 3D Bioprinting Techniques
3.1. Natural Polymers
3.1.1. Collagen
3.1.2. Gelatin
3.1.3. dECM Ink
3.1.4. Alginate
3.2. Synthetic Polymer
3.2.1. Polycaprolactone
3.2.2. Polylactic Acid
3.2.3. Polyglycolic Acid
3.3. Functional Polymer
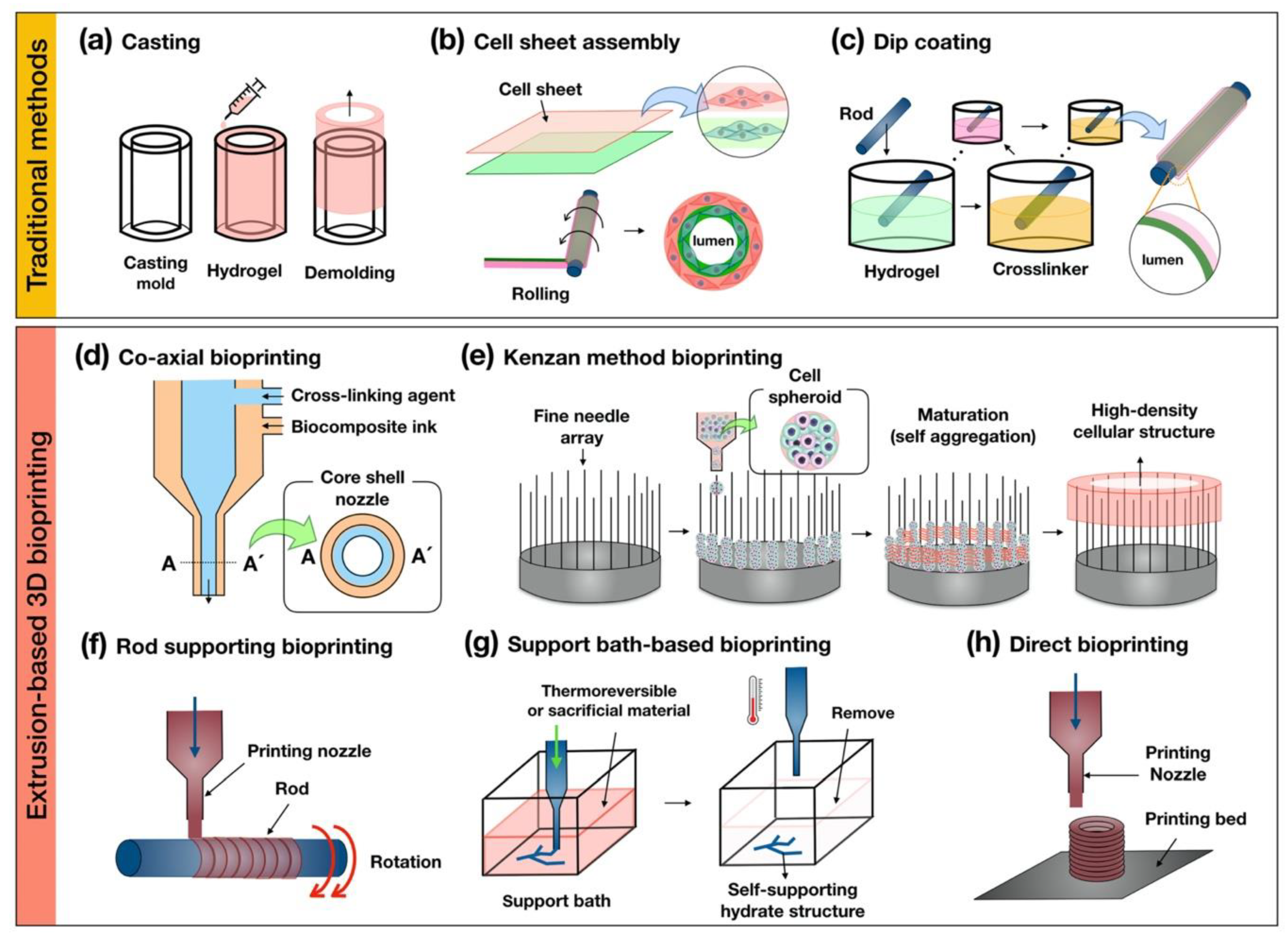
4. Recent Design Approaches for Engineering Tubular Structures
5. Application of the 3D Printed Tubular-Organs with Various Biocomposite Inks
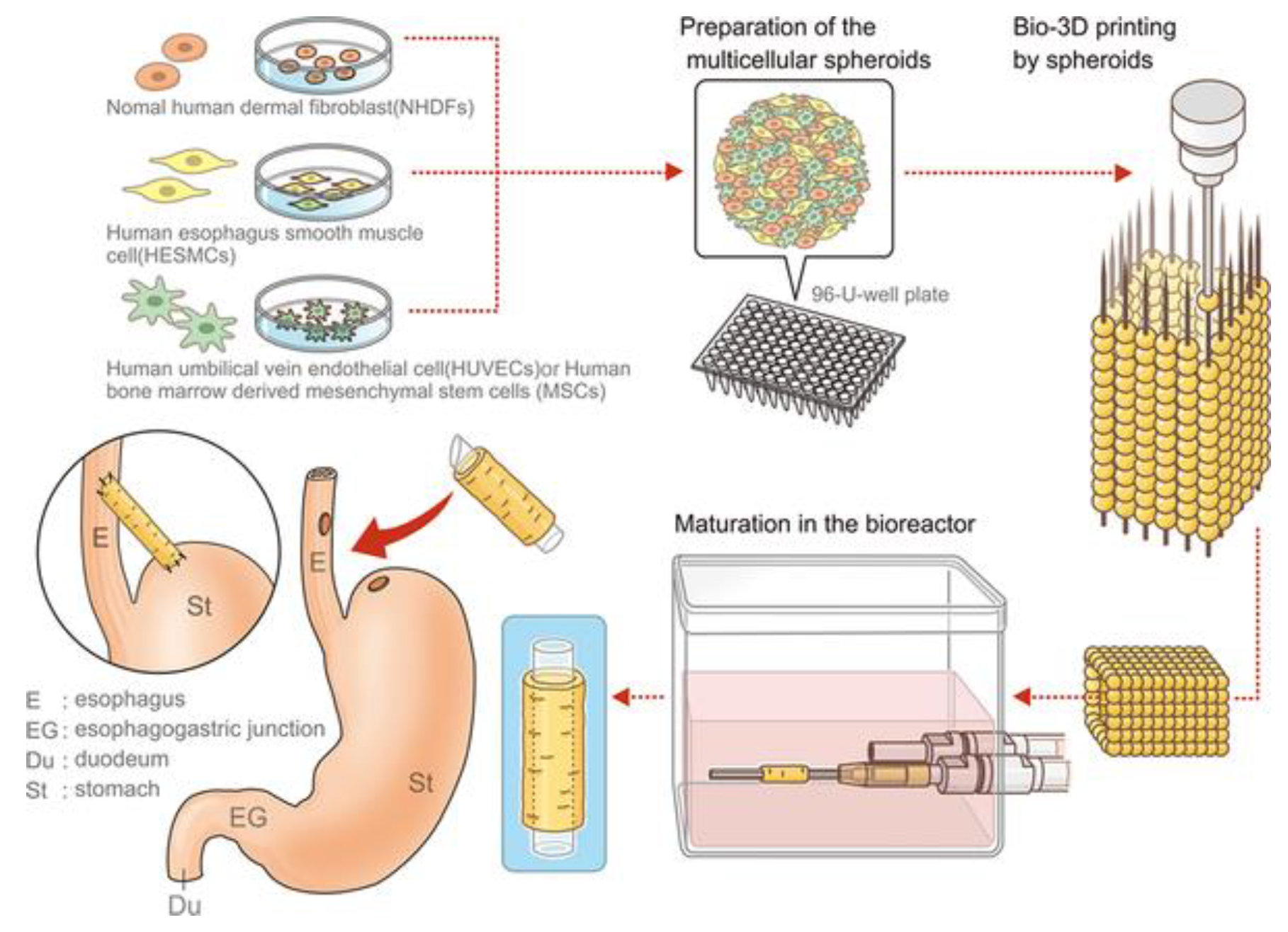
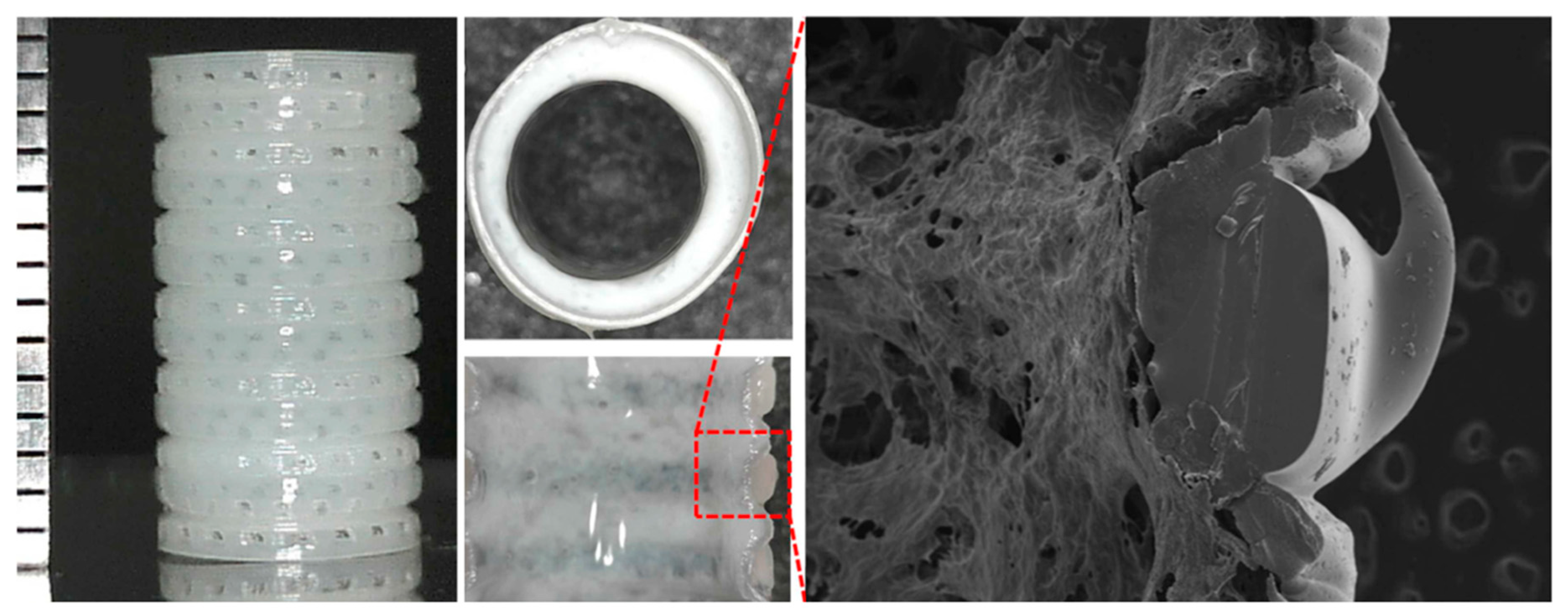
5.1. Esophagus
5.2. Blood Vessel
5.3. Trachea
6. Future Perspectives and Concluding Remark
Author Contributions
Funding
Conflicts of Interest
References
- Saksena, R.; Gao, C.Y.; Wicox, M.; de Mel, A. Tubular organ epithelialisation. J. Tissue Eng. 2016, 7, 2041731416683950. [Google Scholar] [CrossRef]
- Alcantara, P.S.; Spencer-Netto, F.A.; Silva-Junior, J.F.; Soares, L.A.; Pollara, W.M.; Bevilacqua, R.G. Gastro-esophageal isoperistaltic bypass in the palliation of irresectable thoracic esophageal cancer. Int. Surg. 1997, 82, 249–253. [Google Scholar]
- Badylak, S.F.; Vorp, D.A.; Spievack, A.R.; Simmons-Byrd, A.; Hanke, J.; Freytes, D.O.; Thapa, A.; Gilbert, T.W.; Nieponice, A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J. Surg. Res. 2005, 128, 87–97. [Google Scholar] [CrossRef]
- Gawad, K.A.; Hosch, S.B.; Bumann, D.; Lubeck, M.; Moneke, L.C.; Bloechle, C.; Knoefel, W.T.; Busch, C.; Kuchler, T.; Izbicki, J.R. How important is the route of reconstruction after esophagectomy: A prospective randomized study. Am. J. Gastroenterol. 1999, 94, 1490–1496. [Google Scholar] [CrossRef]
- Galliger, Z.; Vogt, C.D.; Panoskaltsis-Mortari, A. 3D bioprinting for lungs and hollow organs. Transl. Res. 2019, 211, 19–34. [Google Scholar] [CrossRef]
- Gora, A.; Pliszka, D.; Mukherjee, S.; Ramakrishna, S. Tubular Tissues and Organs of Human Body-Challenges in Regenerative Medicine. J. Nanosci. Nanotechnol. 2016, 16, 19–39. [Google Scholar] [CrossRef]
- Gangemi, A.; Tzvetanov, I.G.; Beatty, E.; Oberholzer, J.; Testa, G.; Sankary, H.N.; Kaplan, B.; Benedetti, E. Lessons Learned in Pediatric Small Bowel and Liver Transplantation From Living-Related Donors. Transplantation 2009, 87, 1027–1030. [Google Scholar] [CrossRef]
- Napier, K.J.; Scheerer, M.; Misra, S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J. Gastrointest. Oncol. 2014, 6, 112–120. [Google Scholar] [CrossRef]
- Atala, A. Tissue engineering of human bladder. Br. Med. Bull. 2011, 97, 81–104. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, S.H.; Jung, Y. Current status of three-dimensional printing inks for soft tissue regeneration. Tissue Eng. Regen. Med. 2016, 13, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, H.J.; Kim, S.W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Ju, H.W.; Yeon, Y.K.; Lee, J.S.; Lee, Y.J.; Seo, Y.B.; Hum, P.C. Development of an omentum-cultured oesophageal scaffold reinforced by a 3D-printed ring: Feasibility of an in vivo bioreactor. Artif. Cell Nanomed. Biotechnol. 2018, 46, S885–S895. [Google Scholar] [CrossRef]
- Kim, I.G.; Wu, Y.; Park, S.A.; Cho, H.; Choi, J.J.; Kwon, S.K.; Shin, J.W.; Chung, E.J. Tissue-Engineered Esophagus via Bioreactor Cultivation for Circumferential Esophageal Reconstruction. Tissue Eng. Part A 2019, 25, 1478–1492. [Google Scholar] [CrossRef]
- Wu, Y.; Kang, Y.G.; Kim, I.G.; Kim, J.E.; Lee, E.J.; Chung, E.J.; Shin, J.W. Mechanical stimuli enhance simultaneous differentiation into oesophageal cell lineages in a double-layered tubular scaffold. J. Tissue Eng. Regen. Med. 2019, 13, 1394–1405. [Google Scholar] [CrossRef]
- Zhu, Y.B.; Leong, M.F.; Ong, W.F.; Chan-Park, M.B.; Chian, K.S. Esophageal epithelium regeneration on fibronectin grafted poly(L-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials 2007, 28, 861–868. [Google Scholar] [CrossRef]
- Kuppan, P.; Sethuraman, S.; Krishnan, U.M. PCL and PCL-Gelatin Nanofibers as Esophageal Tissue Scaffolds: Optimization, Characterization and Cell-Matrix Interactions. J. Biomed. Nanotechnol. 2013, 9, 1540–1555. [Google Scholar] [CrossRef]
- Soliman, S.; Laurent, J.; Kalenjian, L.; Burnette, K.; Hedberg, B.; La Francesca, S. A multilayer scaffold design with spatial arrangement of cells to modulate esophageal tissue growth. J. Biomed. Mater. Res. B 2019, 107, 324–331. [Google Scholar] [CrossRef]
- L’Heureux, N.; Paquet, S.; Labbe, R.; Germain, L.; Auger, F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998, 12, 47–56. [Google Scholar]
- Masuda, S.; Shimizu, T.; Yamato, M.; Okano, T. Cell sheet engineering for heart tissue repair. Adv. Drug Deliv. Rev. 2008, 60, 277–285. [Google Scholar] [CrossRef]
- Geng, W.X.; Ma, D.Y.; Yan, X.R.; Liu, L.Q.; Cui, J.H.; Xie, X.; Li, H.M.; Chen, F.L. Engineering tubular bone using mesenchymal stem cell sheets and coral particles. Biochem. Biophys. Res. Commun. 2013, 433, 595–601. [Google Scholar] [CrossRef]
- Weinberg, C.B.; Bell, E. A blood vessel model constructed from collagen and cultured vascular cells. Science 1986, 231, 397–400. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Costa, J.B.; da Silva Morais, A.; Lopez-Cebral, R.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M. Tunable Enzymatically Cross-Linked Silk Fibroin Tubular Conduits for Guided Tissue Regeneration. Adv. Healthc. Mater. 2018, 7, e1800186. [Google Scholar] [CrossRef]
- Schutte, S.C.; Chen, Z.; Brockbank, K.G.; Nerem, R.M. Cyclic strain improves strength and function of a collagen-based tissue-engineered vascular media. Tissue Eng. Part A 2010, 16, 3149–3157. [Google Scholar] [CrossRef]
- Boccafoschi, F.; Rajan, N.; Habermehl, J.; Mantovani, D. Preparation and characterization of a scaffold for vascular tissue engineering by direct-assembling of collagen and cells in a cylindrical geometry. Macromol. Biosci. 2007, 7, 719–726. [Google Scholar] [CrossRef]
- Park, J.H.; Jang, J.; Lee, J.S.; Cho, D.W. Current advances in three-dimensional tissue/organ printing. Tissue Eng. Regen. Med. 2016, 13, 612–621. [Google Scholar] [CrossRef]
- Jang, J.; Yi, H.G.; Cho, D.W. 3D Printed Tissue Models: Present and Future. ACS Biomater. Sci. Eng. 2016, 2, 1722–1731. [Google Scholar] [CrossRef]
- Park, J.Y.; Jang, J.; Kang, H.W. 3D Bioprinting and its application to organ-on-a-chip. Microelectron. Eng. 2018, 200, 1–11. [Google Scholar] [CrossRef]
- Gao, G.; Kim, B.S.; Jang, J.; Cho, D.W. Recent Strategies in Extrusion-Based Three-Dimensional Cell Printing toward Organ Biofabrication. ACS Biomater. Sci. Eng. 2019, 5, 1150–1169. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Z.J.; Lin, Z.W.; Qiu, J.J.; Liu, Y.; Liu, A.; Wang, Y.D.; Xiang, M.X.; Chen, B.; Fu, J.Z.; et al. 3D Bioprinting of Vessel-like Structures with Multilevel Fluidic Channels. ACS Biomater. Sci. Eng. 2017, 3, 399–408. [Google Scholar] [CrossRef]
- Jang, J. 3D Bioprinting and In Vitro Cardiovascular Tissue Modeling. Bioengineering 2017, 4, 71. [Google Scholar] [CrossRef]
- Shim, J.H.; Lee, J.S.; Kim, J.Y.; Cho, D.W. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J. Micromech. Microeng. 2012, 22, 08501410. [Google Scholar] [CrossRef]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. B 2011, 98, 160–170. [Google Scholar] [CrossRef]
- Shor, L.; Guceri, S.; Chang, R.; Gordon, J.; Kang, Q.; Hartsock, L.; An, Y.H.; Sun, W. Precision extruding deposition (PED) fabrication of polycaprolactone (PCL) scaffolds for bone tissue engineering. Biofabrication 2009, 1, 015003. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, X.H.; Wang, S.F.; Steffen, R.; Ackermann, M.; Haep, N.D.; Schroder, H.C.; Muller, W.E.G. 3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017, 64, 377–388. [Google Scholar] [CrossRef]
- Jeong, H.J.; Gwak, S.J.; Kang, N.U.; Hong, M.W.; Kim, Y.Y.; Cho, Y.S.; Lee, S.J. Bioreactor mimicking knee-joint movement for the regeneration of tissue-engineered cartilage. J. Mech. Sci. Technol. 2019, 33, 1841–1850. [Google Scholar] [CrossRef]
- Mironov, A.V.; Grigoryev, A.M.; Krotova, L.I.; Skaletsky, N.N.; Popov, V.K.; Sevastianov, V.I. 3D printing of PLGA scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A 2017, 105, 104–109. [Google Scholar] [CrossRef]
- La, W.G.; Jang, J.; Kim, B.S.; Lee, M.S.; Cho, D.W.; Yang, H.S. Systemically replicated organic and inorganic bony microenvironment for new bone formation generated by a 3D printing technology. RSC Adv. 2016, 6, 11546–11553. [Google Scholar] [CrossRef]
- Serra, T.; Planell, J.A.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Song, W.; Yee, K.; Lee, K.Y.; Tagarielli, V.L. Measurements of the mechanical response of unidirectional 3D-printed PLA. Mater. Des. 2017, 123, 154–164. [Google Scholar] [CrossRef]
- Li, K.; Liu, J.K.; Chen, W.S.; Zhang, L. Controllable printing droplets on demand by piezoelectric inkjet: Applications and methods. Microsyst. Technol. 2018, 24, 879–889. [Google Scholar] [CrossRef]
- Derby, B. Bioprinting: Inkjet printing proteins and hybrid cell-containing materials and structures. J. Mater. Chem. 2008, 18, 5717–5721. [Google Scholar] [CrossRef]
- Odde, D.J.; Renn, M.J. Laser-guided direct writing for applications in biotechnology. Trends Biotechnol. 1999, 17, 385–389. [Google Scholar] [CrossRef]
- Michael, S.; Sorg, H.; Peck, C.T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef]
- Schiele, N.R.; Corr, D.T.; Huang, Y.; Raof, N.A.; Xie, Y.; Chrisey, D.B. Laser-based direct-write techniques for cell printing. Biofabrication 2010, 2, 032001. [Google Scholar] [CrossRef]
- Wust, S.; Muller, R.; Hofmann, S. Controlled Positioning of Cells in Biomaterials-Approaches Towards 3D Tissue Printing. J. Funct. Biomater. 2011, 2, 119–154. [Google Scholar] [CrossRef]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Wang, Z.J.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef]
- Heller, C.; Schwentenwein, M.; Russmueller, G.; Varga, F.; Stampfl, J.; Liska, R. Vinyl Esters: Low Cytotoxicity Monomers for the Fabrication of Biocompatible 3D Scaffolds by Lithography Based Additive Manufacturing. J. Polym. Sci. Polym. Chem. 2009, 47, 6941–6954. [Google Scholar] [CrossRef]
- Lee, K.W.; Wang, S.F.; Fox, B.C.; Ritman, E.L.; Yaszemski, M.J.; Lu, L.C. Poly(propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: Effects of resin formulations and laser parameters. Biomacromolecules 2007, 8, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.C.; Grover, L.M. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol. Lett. 2010, 32, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, M.; Negrini, N.C.; Scialla, S.; Marelli, B.; Fare, S.; Danti, S.; Buehler, M. Additive Manufacturing Approaches for Hydroxyapatite-Reinforced Composites. Adv. Funct. Mater. 2019, 29, 1903055. [Google Scholar] [CrossRef]
- Sabir, M.I.; Xu, X.X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Wu, W.; DeConinck, A.; Lewis, J.A. Omnidirectional Printing of 3D Microvascular Networks. Adv. Mater. 2011, 23, H178–H183. [Google Scholar] [CrossRef]
- Kim, B.S.; Jang, J.; Chae, S.; Gao, G.; Kong, J.S.; Ahn, M.; Cho, D.W. Three-dimensional bioprinting of cell-laden constructs with polycaprolactone protective layers for using various thermoplastic polymers. Biofabrication 2016, 8, 035013. [Google Scholar] [CrossRef]
- Gao, G.; Lee, J.H.; Jang, J.; Lee, D.H.; Kong, J.S.; Kim, B.S.; Choi, Y.J.; Jang, W.B.; Hong, Y.J.; Kwon, S.M.; et al. Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease. Adv. Funct. Mater. 2017, 27, 1700798. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.Y.; Mao, Z.W.; Zhou, J.; Shen, J.C.; Hu, X.Q.; Han, C.M. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds. Stem Cell Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, R. The human type I collagen mutation database. Nucleic Acids Res. 1997, 25, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.L.; Gawlitta, D.; Nerem, R.M.; Stegemann, J.P. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials 2004, 25, 3699–3706. [Google Scholar] [CrossRef]
- Han, C.M.; Zhang, L.P.; Sun, J.Z.; Shi, H.F.; Zhou, J.; Gao, C.Y. Application of collagen-chitosan/fibrin glue asymmetric scaffolds in skin tissue engineering. J. Zhejiang Univ.-Sci. B 2010, 11, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Ahn, S.; Kim, Y.; Cho, Y.; Chun, W. Coaxial structured collagen-alginate scaffolds: Fabrication, physical properties, and biomedical application for skin tissue regeneration. J. Mater. Chem. 2011, 21, 6165–6172. [Google Scholar] [CrossRef]
- Ulrich, T.A.; Jain, A.; Tanner, K.; MacKay, J.L.; Kumar, S. Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices. Biomaterials 2010, 31, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Jeong, S.J.; Lee, W.K. Fabrication of porous scaffold by ternary combination of chitosan, gelatin, and calcium phosphate for tissue engineering. J. Ind. Eng. Chem. 2019, 80, 862–869. [Google Scholar] [CrossRef]
- Wang, X.H.; Yan, Y.N.; Pan, Y.Q.; Xiong, Z.; Liu, H.X.; Cheng, B.; Liu, F.; Lin, F.; Wu, R.D.; Zhang, R.J.; et al. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006, 12, 83–90. [Google Scholar] [CrossRef]
- Dulnik, J.; Denis, P.; Sajkiewicz, P.; Kolbuk, D.; Choinska, E. Biodegradation of bicomponent PCL/gelatin and PCL/collagen nanofibers electrospun from alternative solvent system. Polym. Degrad. Stab. 2016, 130, 10–21. [Google Scholar] [CrossRef]
- Fu, W.; Liu, Z.L.; Feng, B.; Hu, R.J.; He, X.M.; Wang, H.; Yin, M.; Huang, H.M.; Zhang, H.B.; Wang, W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomed. 2014, 9, 2335–2344. [Google Scholar] [CrossRef]
- Roehm, K.D.; Madihally, S.V. Bioprinted chitosan-gelatin thermosensitive hydrogels using an inexpensive 3D printer. Biofabrication 2018, 10, 015002. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kang, H.W. The Development of Gelatin-Based Bio-Ink for Use in 3D Hybrid Bioprinting. Int. J. Precis. Eng. Manuf. 2018, 19, 767–771. [Google Scholar] [CrossRef]
- Sharma, R.; Smits, I.P.M.; De La Vega, L.; Lee, C.; Willerth, S.M. 3D Bioprinting Pluripotent Stem Cell Derived Neural Tissues Using a Novel Fibrin Bioink Containing Drug Releasing Microspheres. Front. Bioeng. Biotechnol. 2020, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yao, R.; Mao, S.; Chen, X.; Na, J.; Sun, W. Three-dimensional bioprinting of embryonic stem cells directs highly uniform embryoid body formation. Biofabrication 2015, 7, 044101. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Pati, F.; Choi, Y.J.; Rijal, G.; Shim, J.H.; Kim, S.W.; Ray, A.R.; Cho, D.W.; Ghosh, S. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015, 11, 233–246. [Google Scholar] [CrossRef]
- Xiong, S.; Zhang, X.; Lu, P.; Wu, Y.; Wang, Q.; Sun, H.; Heng, B.C.; Bunpetch, V.; Zhang, S.; Ouyang, H. A Gelatin-sulfonated Silk Composite Scaffold based on 3D Printing Technology Enhances Skin Regeneration by Stimulating Epidermal Growth and Dermal Neovascularization. Sci. Rep. 2017, 7, 4288. [Google Scholar] [CrossRef]
- Gauvin, R.; Chen, Y.C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.C.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, H.; Gao, G.; Jang, J.; Cho, D.W. Decellularized extracellular matrix: A step towards the next generation source for bioink manufacturing. Biofabrication 2017, 9, 034104. [Google Scholar] [CrossRef]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D.W. 3D Cell Printing of Perfusable Vascularized Human Skin Equivalent Composed of Epidermis, Dermis, and Hypodermis for Better Structural Recapitulation of Native Skin. Adv. Healthc. Mater. 2019, 8, 1801019. [Google Scholar] [CrossRef]
- Lee, H.; Han, W.; Kim, H.; Ha, D.H.; Jang, J.; Kim, B.S.; Cho, D.W. Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules 2017, 18, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kumar, P.R.A.; Yoo, J.J.; Zahran, F.; Atala, A.; Lee, S.J. A Photo-Crosslinkable Kidney ECM-Derived Bioink Accelerates Renal Tissue Formation. Adv. Healthc. Mater. 2019, 8, 1800992. [Google Scholar] [CrossRef]
- Jang, J.; Park, J.Y.; Gao, G.; Cho, D.W. Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics. Biomaterials 2018, 156, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Cunniffe, G.M.; Gonzalez-Fernandez, T.; Daly, A.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. Three-Dimensional Bioprinting of Polycaprolactone Reinforced Gene Activated Bioinks for Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 891–900. [Google Scholar] [CrossRef]
- Narayanan, L.K.; Huebner, P.; Fisher, M.B.; Spang, J.T.; Starly, B.; Shirwaiker, R.A. 3D-Bioprinting of Polylactic Acid (PLA) Nanofiber-Alginate Hydrogel Bioink Containing Human Adipose-Derived Stem Cells. ACS Biomater. Sci. Eng. 2016, 2, 1732–1742. [Google Scholar] [CrossRef]
- Gao, G.; Park, J.Y.; Kim, B.S.; Jang, J.; Cho, D.W. Coaxial Cell Printing of Freestanding, Perfusable, and Functional In Vitro Vascular Models for Recapitulation of Native Vascular Endothelium Pathophysiology. Adv. Healthc. Mater. 2018, 7, 1801102. [Google Scholar] [CrossRef]
- Wang, L.; Shelton, R.M.; Cooper, P.R.; Lawson, M.; Triffitt, J.T.; Barralet, J.E. Evaluation of sodium alginate for bone marrow cell tissue engineering. Biomaterials 2003, 24, 3475–3481. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H. Towards the development of polycaprolactone based amphiphilic block copolymers: Molecular design, self-assembly and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 620–634. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer-Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Guo, B.L.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Hutmacher, D.W.; Schantz, J.T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. Part A 2009, 90, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Savalani, M.M.; Teoh, S.H.; Hutmacher, D.W. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 034108. [Google Scholar] [CrossRef] [PubMed]
- Patricio, T.; Domingos, M.; Gloria, A.; D’Amora, U.; Coelho, J.F.; Bartolo, P.J. Fabrication and characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Rapid Prototyp. J. 2014, 20, 145–156. [Google Scholar] [CrossRef]
- Zhang, B.; Seong, B.; Nguyen, V.; Byun, D. 3D printing of high-resolution PLA-based structures by hybrid electrohydrodynamic and fused deposition modeling techniques. J. Micromech. Microeng. 2016, 26, 025015. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Andreopoulos, A.G.; Hatzi, E.C.; Doxastakis, M. Controlled release systems based on poly(lactic acid). An in vitro and in vivo study. J. Mater. Sci.-Mater. Med. 2000, 11, 393–397. [Google Scholar] [CrossRef]
- Stock, U.A.; Mayer, J.E., Jr. Tissue engineering of cardiac valves on the basis of PGA/PLA Co-polymers. J. Long Term Eff. Med. Implant. 2001, 11, 249–260. [Google Scholar] [CrossRef]
- Rouhollahi, F.; Hosseini, S.A.; Alihosseini, F.; Allafchian, A.; Haghighat, F. Investigation on the Biodegradability and Antibacterial Properties of Nanohybrid Suture Based on Silver Incorporated PGA-PLGA Nanofibers. Fiber Polym. 2018, 19, 2056–2065. [Google Scholar] [CrossRef]
- Park, J.Y.; Gao, G.; Jang, J.; Cho, D.W. 3D printed structures for delivery of biomolecules and cells: Tissue repair and regeneration. J. Mater. Chem. B 2016, 4, 7521–7539. [Google Scholar] [CrossRef]
- Angelopoulos, I.; Allenby, M.C.; Lim, M.; Zamorano, M. Engineering inkjet bioprinting processes toward translational therapies. Biotechnol. Bioeng. 2020, 117, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Wagberg, L.; Decher, G.; Norgren, M.; Lindstrom, T.; Ankerfors, M.; Axnas, K. The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 2008, 24, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Chinga-Carrasco, G.; Yu, Y.D.; Diserud, O. Quantitative Electron Microscopy of Cellulose Nanofibril Structures from Eucalyptus and Pinus radiata Kraft Pulp Fibers. Microsc. Microanal. 2011, 17, 563–571. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Avila, H.M.; Hagg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Eng. Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Ehman, N.V.; Pettersson, J.; Vallejos, M.E.; Brodin, M.W.; Felissia, F.E.; Hakansson, J.; Area, M.C. Pulping and Pretreatment Affect the Characteristics of Bagasse Inks for Three-dimensional Printing. ACS Sustain. Chem. Eng. 2018, 6, 4068–4075. [Google Scholar] [CrossRef]
- Basu, J.; Ludlow, J.W. Platform technologies for tubular organ regeneration. Trends Biotechnol. 2010, 28, 526–533. [Google Scholar] [CrossRef]
- Wilkens, C.A.; Rivet, C.J.; Akentjew, T.L.; Alverio, J.; Khoury, M.; Acevedo, J.P. Layer-by-layer approach for a uniformed fabrication of a cell patterned vessel-like construct. Biofabrication 2016, 9, 015001. [Google Scholar] [CrossRef]
- Harding, S.I.; Afoke, A.; Brown, R.A.; MacLeod, A.; Shamlou, P.A.; Dunnill, P. Engineering and cell attachment properties of human fibronectin-fibrinogen scaffolds for use in tissue engineered blood vessels. Bioprocess Biosyst. Eng. 2002, 25, 53–59. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, C.; Li, S.; Hu, Q. Composite vascular scaffold combining electrospun fibers and physically-crosslinked hydrogel with copper wire-induced grooves structure. J. Mech. Behav. Biomed. Mater. 2016, 61, 12–25. [Google Scholar] [CrossRef]
- Ouyang, L.; Burdick, J.A.; Sun, W. Facile Biofabrication of Heterogeneous Multilayer Tubular Hydrogels by Fast Diffusion-Induced Gelation. ACS Appl. Mater. Interfaces 2018, 10, 12424–12430. [Google Scholar] [CrossRef]
- Park, J.H.; Jang, J.; Lee, J.S.; Cho, D.W. Three-Dimensional Printing of Tissue/Organ Analogues Containing Living Cells. Ann. Biomed. Eng. 2017, 45, 180–194. [Google Scholar] [CrossRef]
- Sun, W.; Starly, B.; Daly, A.C.; Burdick, J.A.; Groll, J.; Skeldon, G.; Shu, W.; Sakai, Y.; Shinohara, M.; Nishikawa, M.; et al. The bioprinting roadmap. Biofabrication 2020, 12, 022002. [Google Scholar] [CrossRef]
- Luo, Y.; Lode, A.; Gelinsky, M. Direct plotting of three-dimensional hollow fiber scaffolds based on concentrated alginate pastes for tissue engineering. Adv. Healthc. Mater. 2013, 2, 777–783. [Google Scholar] [CrossRef]
- Takeoka, Y.; Matsumoto, K.; Taniguchi, D.; Tsuchiya, T.; Machino, R.; Moriyama, M.; Oyama, S.; Tetsuo, T.; Taura, Y.; Takagi, K.; et al. Regeneration of esophagus using a scaffold-free biomimetic structure created with bio-three-dimensional printing. PLoS ONE 2019, 14, e0211339. [Google Scholar] [CrossRef]
- Moldovan, N.I.; Hibino, N.; Nakayama, K. Principles of the Kenzan Method for Robotic Cell Spheroid-Based Three-Dimensional Bioprinting. Tissue Eng. Part B-Rev. 2017, 23, 237–244. [Google Scholar] [CrossRef]
- Moldovan, L.; Barnard, A.; Gil, C.H.; Lin, Y.; Grant, M.B.; Yoder, M.C.; Prasain, N.; Moldovan, N.I. iPSC-Derived Vascular Cell Spheroids as Building Blocks for Scaffold-Free Biofabrication. Biotechnol. J. 2017, 12, 1700444. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yanagi, Y.; Sheng, Z.J.; Nagata, K.; Nakayama, K.; Taguchi, T. Regeneration of diaphragm with bio-3D cellular patch. Biomaterials 2018, 167, 1–14. [Google Scholar] [CrossRef]
- Bae, S.W.; Lee, K.W.; Park, J.H.; Lee, J.; Jung, C.R.; Yu, J.; Kim, H.Y.; Kim, D.H. 3D Bioprinted Artificial Trachea with Epithelial Cells and Chondrogenic-Differentiated Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 1624. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef]
- Jin, Y.F.; Liu, C.C.; Chai, W.X.; Compaan, A.; Huang, Y. Self-Supporting Nanoclay as Internal Scaffold Material for Direct Printing of Soft Hydrogel Composite Structures in Air. ACS Appl. Mater. Interfaces 2017, 9, 17457–17466. [Google Scholar] [CrossRef]
- Talley, N.J. Introducing Expert Review of Gastroenterology and Hepatology. Expert Rev. Gastroenterol. Hepatol. 2007, 1, 1–2. [Google Scholar] [CrossRef]
- Yoshida, N.; Watanabe, M.; Baba, Y.; Iwagami, S.; Ishimoto, T.; Iwatsuki, M.; Sakamoto, Y.; Miyamoto, Y.; Ozaki, N.; Baba, H. Risk factors for pulmonary complications after esophagectomy for esophageal cancer. Surg. Today 2014, 44, 526–532. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Chauhan, V.; Sharma, V.; Singh, H. Gastric Conduit Perforation: A Late Fatal Complication after Esophagectomy. Cureus 2019, 11, e4987. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kofler, K.; Ainodhofer, H.; Hollwarth, M.E. Esophagus tissue engineering: Hybrid approach with esophageal epithelium and unidirectional smooth muscle tissue component generation in vitro. J. Gastrointest. Surg. 2009, 13, 1037–1043. [Google Scholar] [CrossRef]
- Saxena, A.K.; Ainoedhofer, H.; Hollwarth, M.E. Esophagus tissue engineering: In vitro generation of esophageal epithelial cell sheets and viability on scaffold. J. Pediatr. Surg. 2009, 44, 896–901. [Google Scholar] [CrossRef]
- Gong, C.; Hou, L.; Zhu, Y.; Lv, J.; Liu, Y.; Luo, L. In vitro constitution of esophageal muscle tissue with endocyclic and exolongitudinal patterns. ACS Appl. Mater. Interfaces 2013, 5, 6549–6555. [Google Scholar] [CrossRef]
- Lin, M.H.; Firoozi, N.; Tsai, C.T.; Wallace, M.B.; Kang, Y.Q. 3D-printed flexible polymer stents for potential applications in inoperable esophageal malignancies. Acta Biomater. 2019, 83, 119–129. [Google Scholar] [CrossRef]
- Dimitrievska, S.; Niklason, L.E. Historical Perspective and Future Direction of Blood Vessel Developments. CSH Perspect. Med. 2018, 8, a025742. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, X.H.; Tolba, E.; Dorweiler, B.; Schroder, H.C.; Link, T.; Diehl-Seifert, B.; Muller, W.E.G. Modular Small Diameter Vascular Grafts with Bioactive Functionalities. PLoS ONE 2015, 10, e0133632. [Google Scholar] [CrossRef]
- Wenger, R.; Giraud, M.N. 3D Printing Applied to Tissue Engineered Vascular Grafts. Appl. Sci. 2018, 8, 2631. [Google Scholar] [CrossRef]
- Carrabba, M.; Madeddu, P. Current Strategies for the Manufacture of Small Size Tissue Eng. Vascular Grafts. Front. Bioeng. Biotechnol. 2018, 6, 41. [Google Scholar] [CrossRef]
- Freeman, S.; Ramos, R.; Chando, P.A.; Zhou, L.X.; Reeser, K.; Jin, S.; Soman, P.; Ye, K.M. A bioink blend for rotary 3D bioprinting tissue engineered small-diameter vascular constructs. Acta Biomater. 2019, 95, 152–164. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, M.E.; Nah, H.; Seok, J.M.; Jeong, M.H.; Park, K.; Kwon, I.K.; Lee, J.S.; Park, S.A. Vascular endothelial growth factor immobilized on mussel-inspired three-dimensional bilayered scaffold for artificial vascular graft application: In vitro and in vivo evaluations. J. Colloid Interface Sci. 2019, 537, 333–344. [Google Scholar] [CrossRef]
- Savoji, H.; Davenport Huyer, L.; Mohammadi, M.H.; Lai, B.F.L.; Rafatian, N.; Bannerman, D.; Shoaib, M.; Bobicki, E.R.; Ramachandran, A.; Radisic, M. 3D Printing of Vascular Tubes Using Bioelastomer Prepolymers byFreeform Reversible Embedding. ACS Biomater. Sci. Eng. 2020, 6, 1333–1343. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef]
- Rock, J.R.; Randell, S.H.; Hogan, B.L.M. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Dis. Model. Mech. 2010, 3, 545–556. [Google Scholar] [CrossRef]
- Nam, H.; Choi, Y.; Jang, J. Vascularized Lower Respiratory-Physiology-On-A-Chip. Appl. Sci. 2020, 10, 900. [Google Scholar] [CrossRef]
- Foltinová, J.; Schrott-Fischer, A.; Zilínek, V.; Foltin, V.; Freysinger, W. Is the Trachea a Marker of the Type of Environmental Pollution? Larygoscope 2009, 112, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, M.F.; Kerns, D.B.; Bruch, D.; Reinitz, E.R.; Schwartz, R.A. Does the end-to-end venous anastomosis offer a functional advantage over the end-to-side venous anastomosis in high-output arteriovenous grafts? J. Vasc. Surg. 1990, 12, 676–688, discussion 688–690. [Google Scholar] [CrossRef] [PubMed]
- Kaihara, S.; Kim, S.; Benvenuto, M.; Kim, B.S.; Mooney, D.J.; Tanaka, K.; Vacanti, J.P. End-to-End Anastomosis between Tissue-Engineered Intestine and Native Small Bowel. Tissue Eng. Part A 2007, 5, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Ten Hallers, E.J.O.; Rakhorst, G.; Marres, H.A.M.; Jansen, J.A.; van Kooten, T.G.; Schutte, H.K.; van Loon, J.P.; van der Houwen, E.B.; Verkerke, G.J. Animal models for tracheal research. Biomaterials 2004, 25, 1533–1543. [Google Scholar] [CrossRef]
- Kaye, R.; Goldstein, T.; Zeltsman, D.; Grande, D.A.; Smith, L.P. Three dimensional printing: A review on the utility within medicine and otolaryngology. Int. J. Pediatr. Otorhinolaryngol. 2016, 89, 145–148. [Google Scholar] [CrossRef]
- Zhong, N.P.; Zhao, X. 3D printing for clinical application in otorhinolaryngology. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 4079–4089. [Google Scholar] [CrossRef]
- Taniguchi, D.; Matsumoto, K.; Tsuchiya, T.; Machino, R.; Takeoka, Y.; Elgalad, A.; Gunge, K.; Takagi, K.; Taura, Y.; Hatachi, G.; et al. Scaffold-free trachea regeneration by tissue engineering with bio-3D printing. Interact. Cardiovasc. Thorac. 2018, 26, 745–752. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, J.H.; Ahn, M.J.; Kim, S.W.; Cho, D.W. Long-term study on off-the-shelf tracheal graft: A conceptual approach for urgent implantation. Mater. Des. 2020, 185, 108218. [Google Scholar] [CrossRef]
- Gao, M.C.; Zhang, H.Y.; Dong, W.; Bai, J.; Gao, B.T.; Xia, D.K.; Feng, B.; Chen, M.L.; He, X.M.; Yin, M.; et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair. Sci. Rep.-UK 2017, 7, 5246. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Liao, C.Y.; Dai, N.T.; Tseng, C.S.; Yen, B.L.J.; Hsu, S.H. 3D printing of tubular scaffolds with elasticity and complex structure from multiple waterborne polyurethanes for tracheal tissue engineering. Appl. Mater. Today 2018, 12, 330–341. [Google Scholar] [CrossRef]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Moons, L.; Collen, D. Mouse models of angiogenesis, arterial stenosis, atherosclerosis and hemostasis. Cardiovasc. Res. 1998, 39, 8–33. [Google Scholar] [CrossRef]
- Rumbaut, R.E.; Slaff, D.W.; Burns, A.R. Microvascular thrombosis models in venules and arterioles in vivo. Microcirculation 2005, 12, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Wengerter, B.C.; Emre, G.; Park, J.Y.; Geibel, J. Three-dimensional Printing in the Intestine. Clin. Gastroenterol. Hepatol. 2016, 14, 1081–1085. [Google Scholar] [CrossRef]

| Extrusion-Based 3D Bioprinting | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Co-axial bioprinting |
|
| [59,88,114] |
| Kenzan method bioprinting |
|
| [115,116,117,118] |
| Rod supporting bioprinting |
|
| [31,119] |
| Support bath-based bioprinting |
|
| [120,121] |
| Direct bioprinting |
|
| [122,123] |
| 3D Bioprinting Technique | Biocomposite Ink | Reference |
|---|---|---|
| Kenzan method bioprinting | Cell spheroids with human dermal fibroblasts, human esophageal smooth muscle cells, human bone marrow-derived mesenchymal stem cells, human umbilical vein endothelial cells | [115] |
| Rod supporting bioprinting and electrospinning | Polyurethane (PU), polycaprolactone (PCL) | [15] |
| Rod supporting bioprinting and electrospinning | Polycaprolactone (PCL) | [14] |
| Direct bioprinting | Thermal polyurethane (TPU), polylactic acid (PLA) | [130] |
| 3D Bioprinting Technique | Biocomposite Ink | Reference |
|---|---|---|
| Co-axial bioprinting | Vascular-tissue-derived decellularized extracellular matrix (VdECM) with alginate | [59] |
| Rod supporting bioprinting | Fibrinogen and gelatin | [135] |
| Rod supporting bioprinting and electrospinning | Polycaprolactone (PCL) | [136] |
| Co-axial bioprinting and rod supporting bioprinting | Alginate | [31] |
| Support bath-based bioprinting | Alginate and gelatin slurry support bath | [120] |
| Co-axial printing and support bath-based bioprinting | Photocrosslinkable bioelastomer prepolymers ink (dimethyl itaconate. 1,8-ictanediol and triethyl citrate) and carbomer gel bath | [137] |
| Direct bioprinting | Pluronic 127 and gelatin methacrylate (GelMA) | [138] |
| 3D Bioprinting Technique | Biocomposite Ink | Reference |
|---|---|---|
| Kenzan method bioprinting | Cell spheroids with chondrocytes, endothelial cells, and mesenchymal stem cells | [147] |
| Direct bioprinting and rod-supporting bioprinting | Polycaprolactone (PCL), silicone | [148] |
| Direct bioprinting | Polyurethane (PU) | [149] |
| Direct bioprinting | Polycaprolactone (PCL) | [150] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.-J.; Nam, H.; Jang, J.; Lee, S.-J. 3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs. Bioengineering 2020, 7, 32. https://doi.org/10.3390/bioengineering7020032
Jeong H-J, Nam H, Jang J, Lee S-J. 3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs. Bioengineering. 2020; 7(2):32. https://doi.org/10.3390/bioengineering7020032
Chicago/Turabian StyleJeong, Hun-Jin, Hyoryung Nam, Jinah Jang, and Seung-Jae Lee. 2020. "3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs" Bioengineering 7, no. 2: 32. https://doi.org/10.3390/bioengineering7020032
APA StyleJeong, H.-J., Nam, H., Jang, J., & Lee, S.-J. (2020). 3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs. Bioengineering, 7(2), 32. https://doi.org/10.3390/bioengineering7020032






