Antioxidant and Antibacterial Peptides from Soybean Milk through Enzymatic- and Membrane-Based Technologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Membrane Bioreactor
2.3. Preparation of Soybean Milk
2.4. Enzymatic Hydrolysis of Soybean Milk Protein in Bioreactor
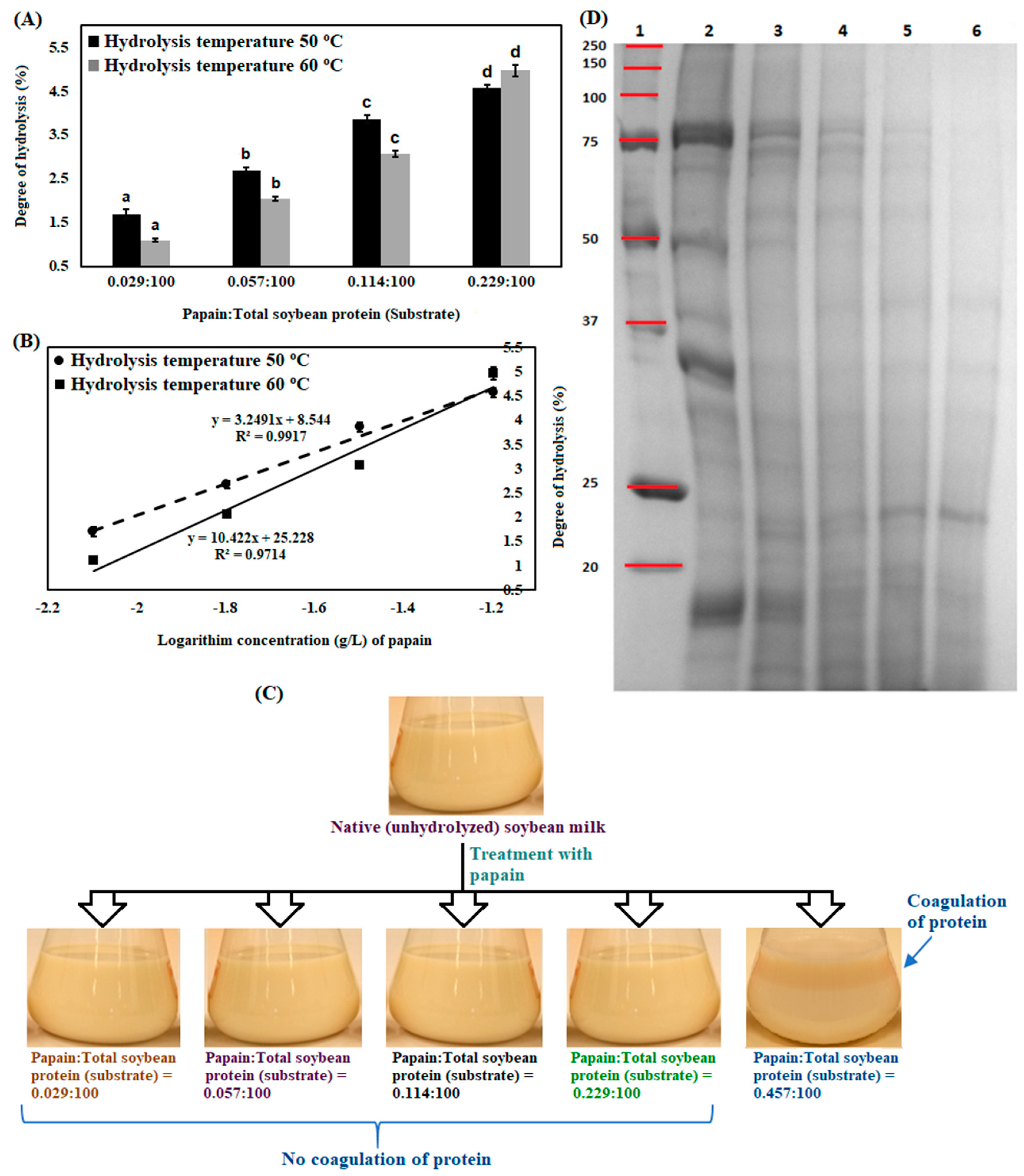
2.5. Estimation of Degree of Hydrolysis of Soybean Milk Protein
2.6. Production of Antioxidant and Antibacterial Peptides by Membrane Bioreactor
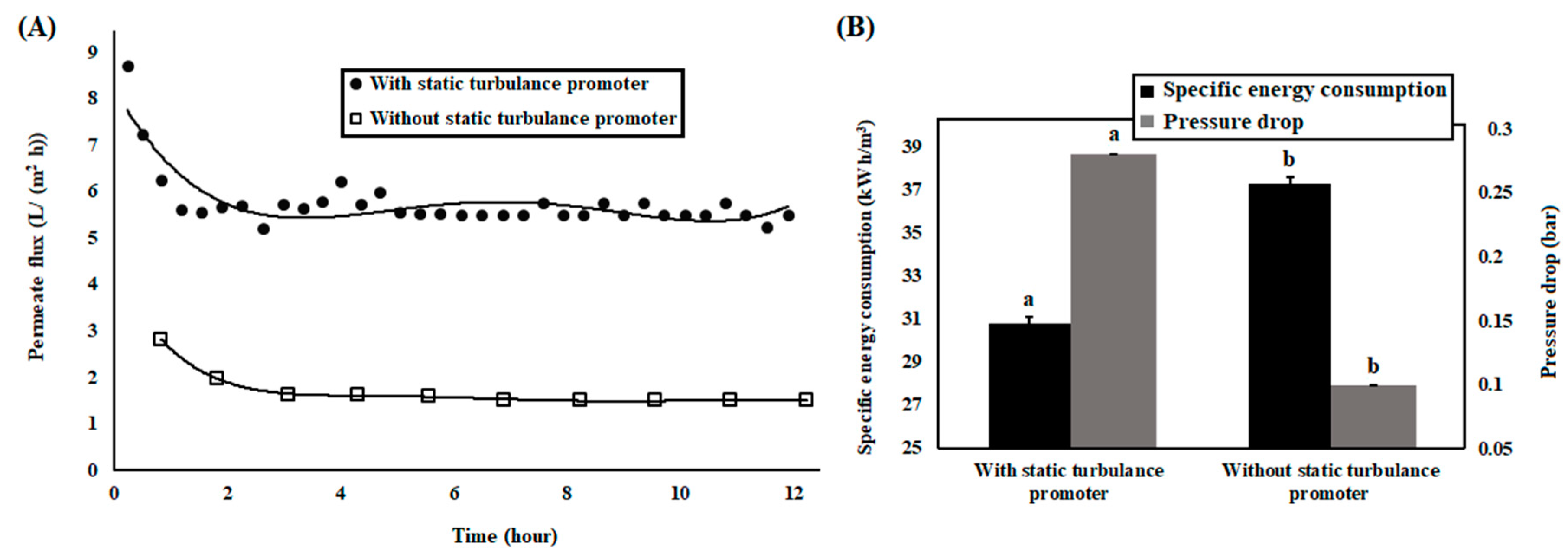
2.7. Specific Energy Consumption of Membrane Filtration
2.8. Membrane Cleaning
2.9. Determination of Protein Concentration
2.10. Determination of Total Carbohydrate
2.11. Determination of Total Fat
2.12. Determination of Total Antioxidant Capacity
2.13. Microbiological Assay
2.14. Determination of Molecular Weight of Soybean Milk Proteins and Soybean-Based Peptides by Gel Electrophoresis
2.15. Statistical Analysis
3. Results and Discussion
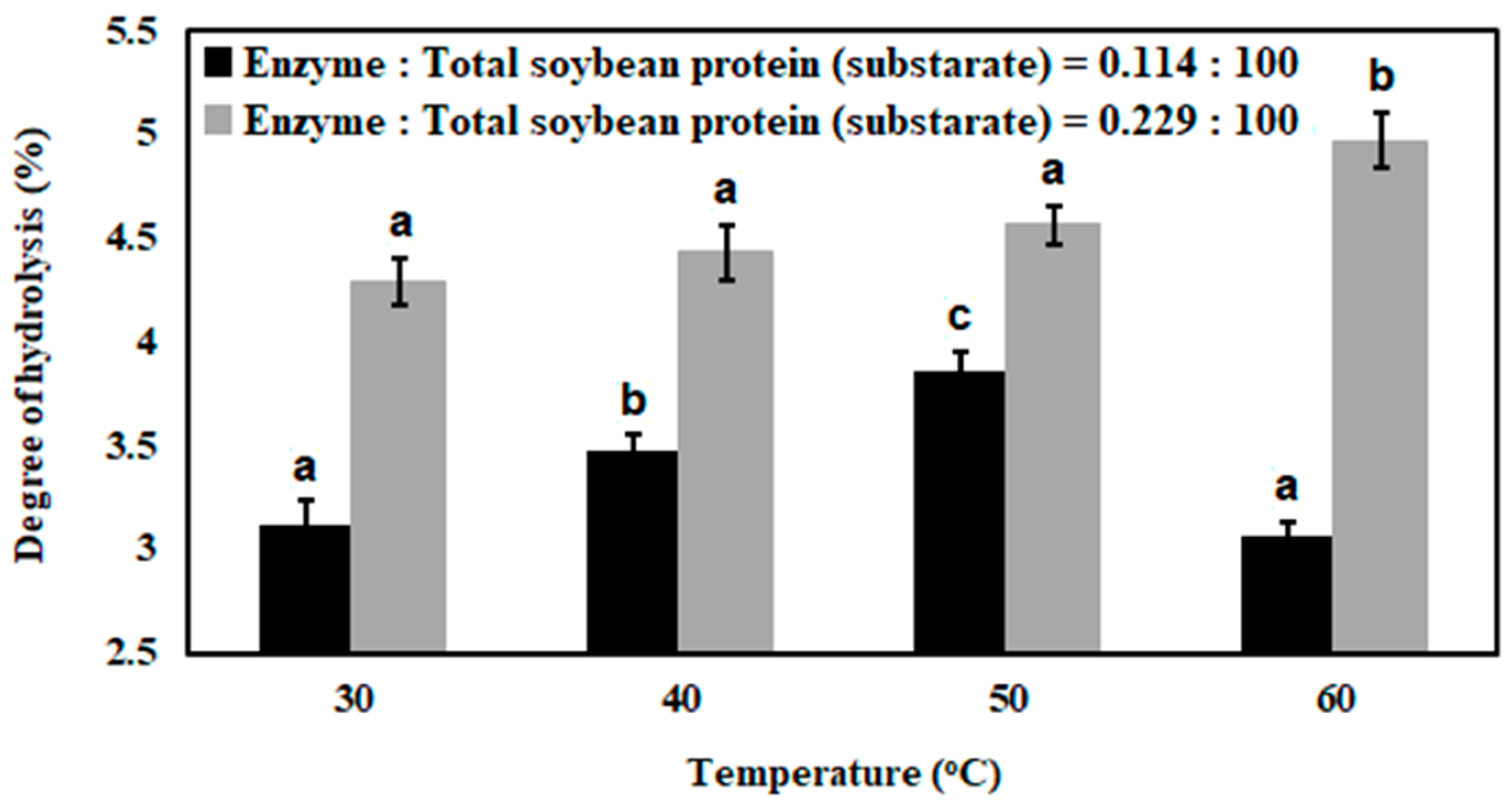
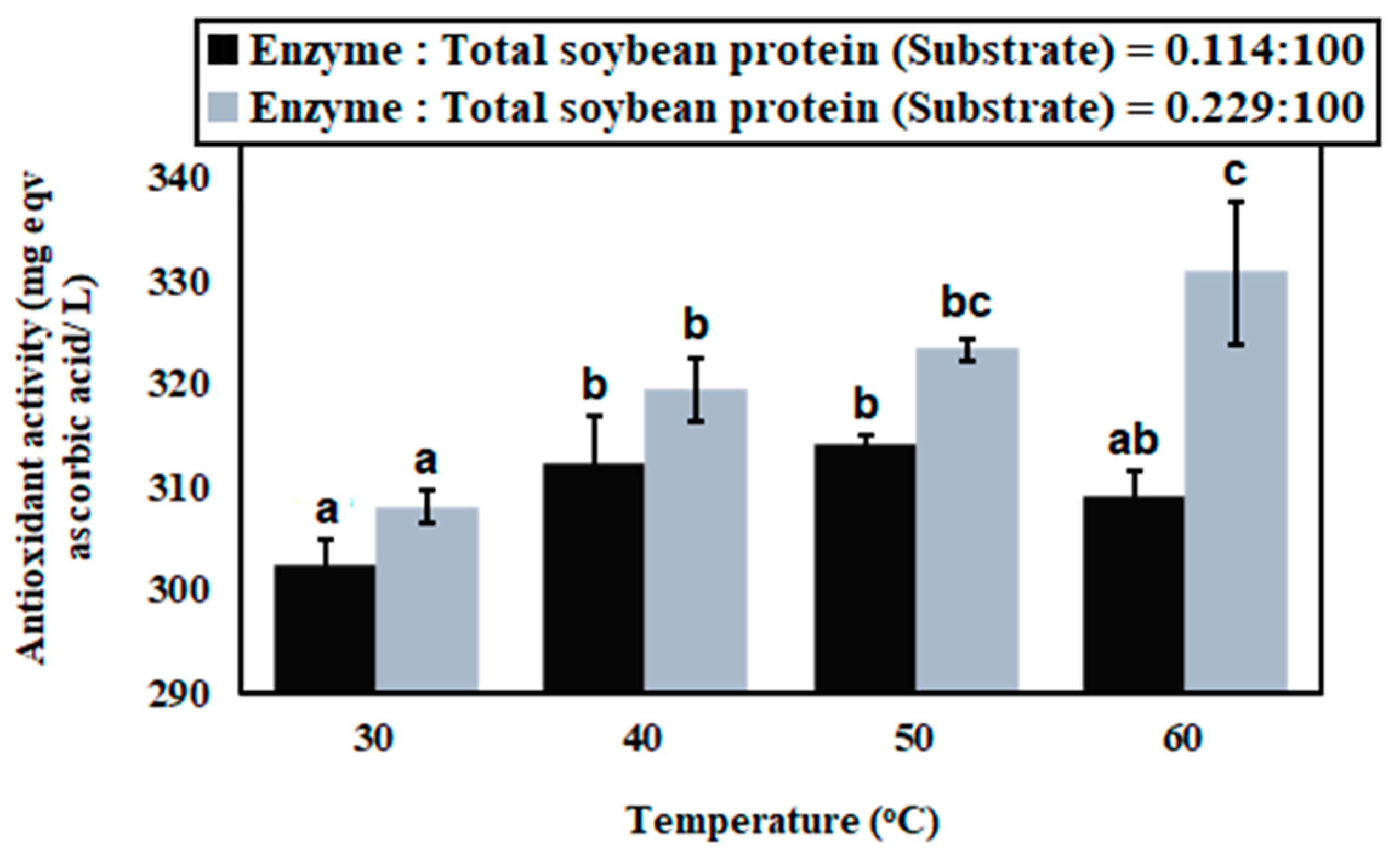
3.1. Enzymatic Hydrolysis of Soybean Milk Proteins with Batch Mode
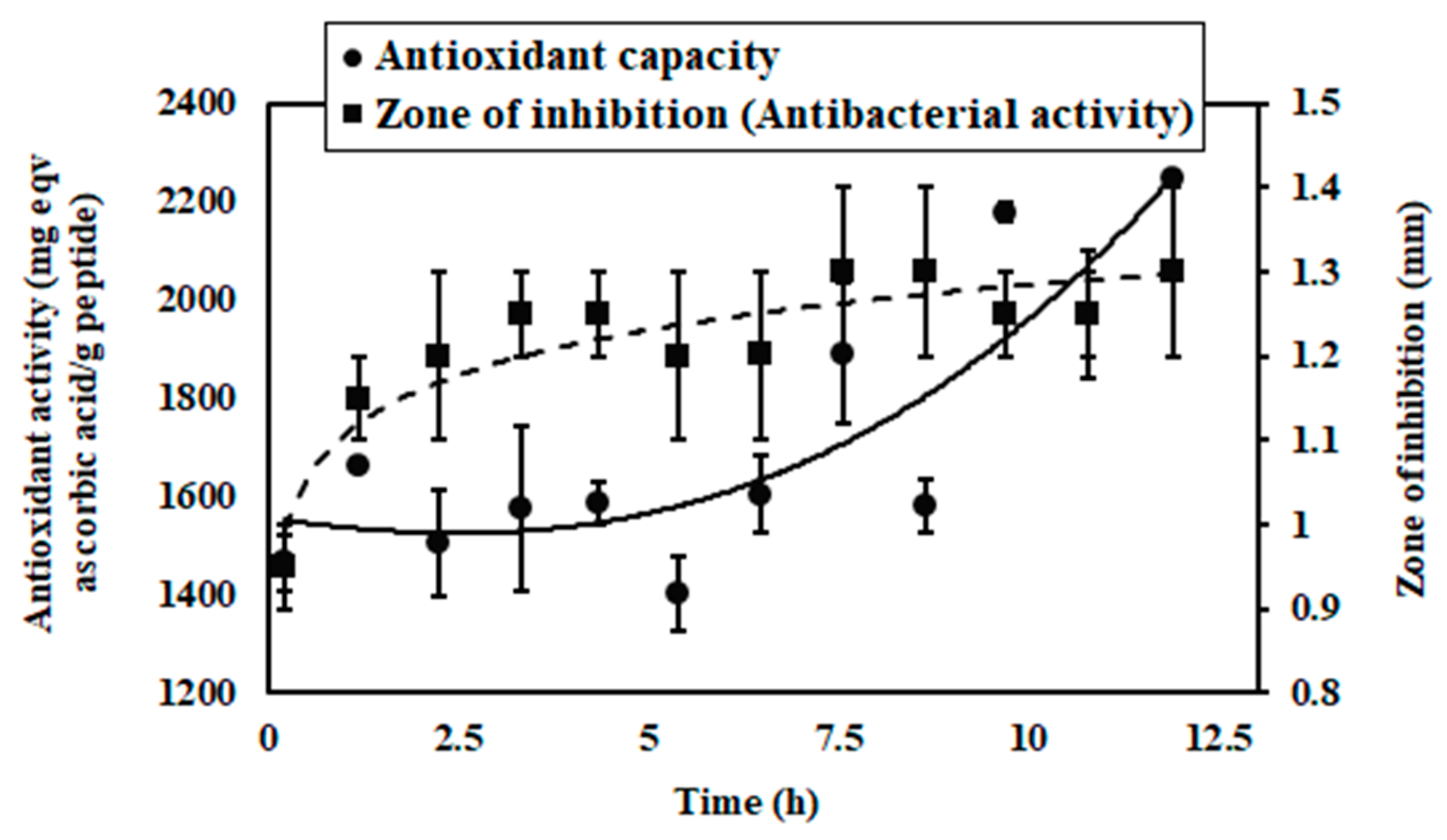
3.2. Membrane Bioreactor with Continuous Mode
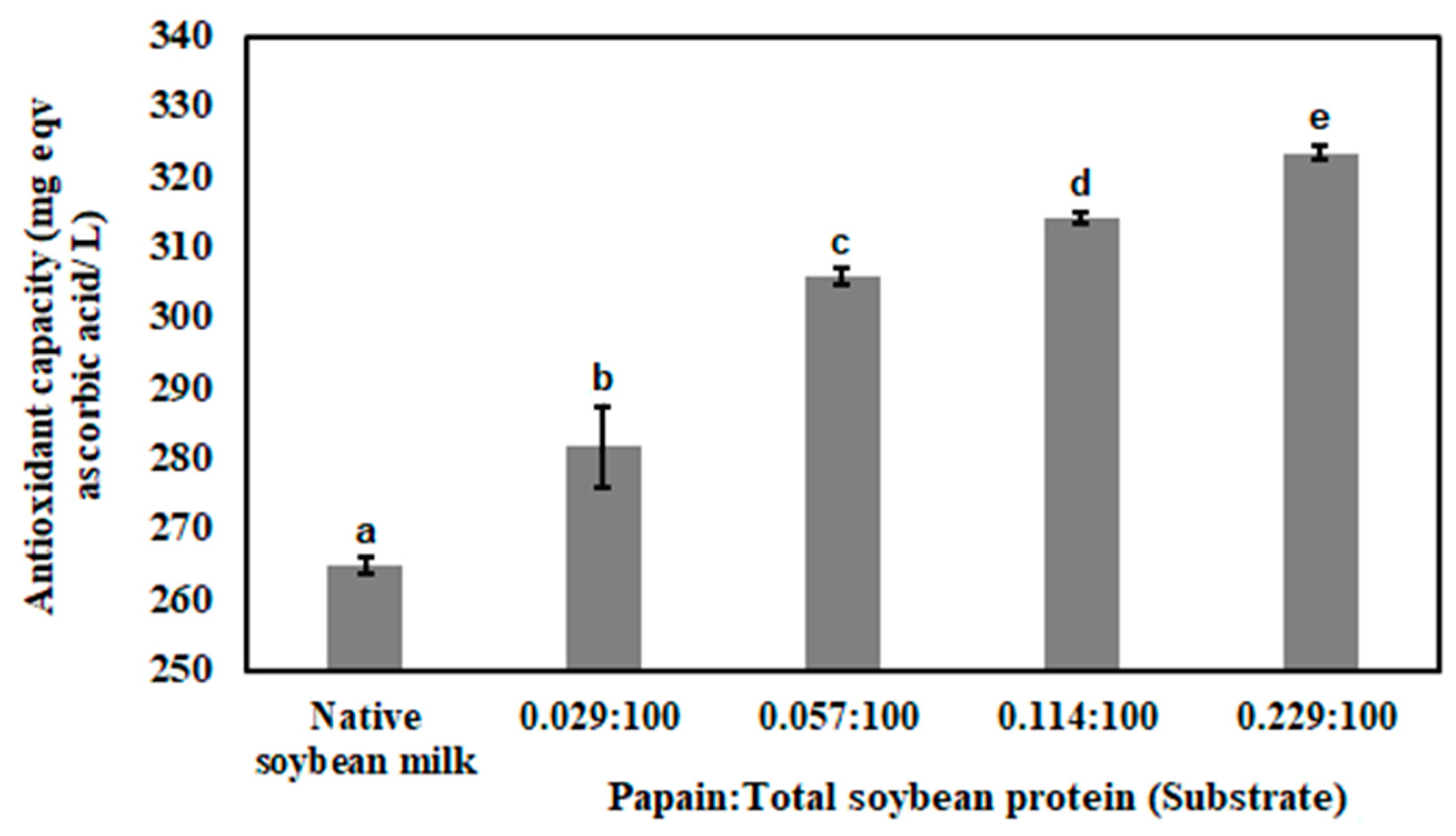
3.3. Antioxidant Capacity
3.4. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McKevith, B. Nutritional aspects of oilseeds. Nutr. Bull. 2005, 30, 13–26. [Google Scholar] [CrossRef]
- Jeong, H.; Park, S.; Pak, V.; Rhan, K.; Young, D. Fermented Soybean Products and Their Bioactive Compounds. In Soybean and Health; INTECH publication: Rijeka, Croatia, 2011. [Google Scholar]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean bioactive peptides and their functional properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D. Bioactive Proteins and Peptides from Soybeans. Recent Pat. Food Nutr. Agric. 2015, 7, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Collier, P.D.; Cromie, D.D.O.; Davies, A.P. Mechanism of formation of chloropropanols present in protein hydrolysates. J. Am. Oil Chem. Soc. 1991, 68, 785–790. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Chen, F.; Liu, K.; Zhu, T. Milk processing as a tool to reduce cow’s milk allergenicity: A mini-review. Dairy Sci. Technol. 2013, 93, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liang, X.; Wei, M.; Zhao, W.; He, B.; Lu, Q.; Huo, Q.; Ma, C. Optimization of glutamine peptide production from soybean meal and analysis of molecular weight distribution of hydrolysates. Int. J. Mol. Sci. 2012, 13, 7483–7495. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Szécsi, G.; Csehi, B.; Mednyánszky, Z.; Kiskó, G.; Bányai, É.; Dernovics, M.; Koris, A. Production of hypoallergenic antibacterial peptides from defatted soybean meal in membrane bioreactor: A bioprocess engineering study with comprehensive product characterization. Food Technol. Biotechnol. 2017, 55, 308–324. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Q.; Qu, Y.; Ye, F. Antioxidant activity and anti-exercise-fatigue effect of highly denatured soybean meal hydrolysate prepared using neutrase. J. Food Sci. Technol. 2015, 52, 1982–1992. [Google Scholar] [CrossRef]
- Barac, M.B.; Jovanovic, S.T.; Stanojevic, S.P.; Pesic, M.B. Effect of limited hydrolysis on traditional soy protein concentrate. Sensors 2006, 6, 1087–1101. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, Y. Limited hydrolysis of soybean protein concentrate and isolate with two proteases and the impact on emulsifying activity index of hydrolysates. Afr. J. Biotechnol. 2009, 8, 3314–3319. [Google Scholar]
- Cai, M.Y.; Gu, R.Z.; Li, C.Y.; Ma, Y.; Dong, Z.; Liu, W.Y.; Jin, Z.T.; Lu, J.; Yi, W.X. Pilot-scale production of soybean oligopeptides and antioxidant and antihypertensive effects in vitro and in vivo. J. Food Sci. Technol. 2014, 51, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.F.; Corrêa, A.P.F.; Coletto, D.; Daroit, D.J.; Cladera-Olivera, F.; Brandelli, A. Soy protein hydrolysis with microbial protease to improve antioxidant and functional properties. J. Food Sci. Technol. 2015, 52, 2668–2678. [Google Scholar] [CrossRef] [PubMed]
- Beermann, C.; Euler, M.; Herzberg, J.; Stahl, B. Anti-oxidative capacity of enzymatically released peptides from soybean protein isolate. Eur. Food Res. Technol. 2009, 229, 637–644. [Google Scholar] [CrossRef]
- Tsou, M.-J.; Lin, S.-B.; Chao, C.-H.; Chiang, W.-D. Enhancing the lipolysis-stimulating activity of soy protein using limited hydrolysis with Flavourzyme and ultrafiltration. Food Chem. 2012, 134, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Roblet, C.; Amiot, J.; Lavigne, C.; Marette, A.; Lessard, M.; Jean, J.; Ramassamy, C.; Moresoli, C.; Bazinet, L. Screening of in vitro bioactivities of a soy protein hydrolysate separated by hollow fiber and spiral-wound ultrafiltration membranes. Food Res. Int. 2012, 46, 237–249. [Google Scholar] [CrossRef]
- Hrčková, M.; Rusňáková, M.; Zemanovič, J. Enzymatic Hydrolysis of Defatted Soy Flour by Three Different Proteases and their Effect on the Functional Properties of Resulting Protein Hydrolysates. Czech J. Food Sci. 2002, 20, 7–14. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Reitmeier, C.; Murphy, P.A.; Johnson, L.A. Enzymatic hydrolysis of extruded-expelled soy flour and resulting functional properties. J. Am. Oil Chem. Soc. 2006, 83, 731–737. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Amorim, M.M.; Voss, G.B.; Nerli, B.B.; Picó, G.A.; Pintado, M.E. Bioactive properties of peptides obtained from Argentinian defatted soy flour protein by Corolase PP hydrolysis. Food Chem. 2016, 198, 36–44. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Zougman, A.; Masse, R.; Mulligan, C. Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food. Food Res. Int. 2004, 37, 123–131. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, J.S.; Baek, H.H.; Lee, H.G. Purification and characterization of antioxidant peptides from soy protein hydrolysate. J. Food Biochem. 2010, 34, 120–132. [Google Scholar] [CrossRef]
- Saha, B.C.; Hayashi, K. Debittering of protein hydrolyzates. Biotechnol. Adv. 2001, 19, 355–370. [Google Scholar] [CrossRef]
- Ishibashi, N.; Ono, I.; Kato, K.; Shigenaga, T.; Shinoda, I.; Okai, H.; Fukui, S. Role of the Hydrophobic Amino Acid Residue in the Bitterness of Peptides. Agric. Biol. Chem. 1988, 52, 91–94. [Google Scholar] [CrossRef]
- Acquah, C.; Stefano, E.D.; Udenigwe, C.C. Role of hydrophobicity in food peptide functionality and bioactivity. J. Food Bioact. 2018, 4, 88–98. [Google Scholar] [CrossRef]
- Fernández-Lucas, J.; Castañeda, D.; Hormigo, D. New trends for a classical enzyme: Papain, a biotechnological success story in the food industry. Trends Food Sci. Technol. 2017, 68, 91–101. [Google Scholar] [CrossRef]
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An overview of industrial application and purification strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Sussmann, D.; Schweiggert-Weisz, U.; Eisner, P. Enzymatic treatment of soy protein isolates: Effects on the potential allergenicity, technofunctionality, and sensory properties. Food Sci. Nutr. 2016, 4, 11–23. [Google Scholar] [CrossRef]
- Chiang, W.-D.; Tsou, M.-J.; Tsai, Z.-Y.; Tsai, T.-C. Angiotensin I-converting enzyme inhibitor derived from soy protein hydrolysate and produced by using membrane reactor. Food Chem. 2006, 98, 725–732. [Google Scholar] [CrossRef]
- Chiang, W.D.; Shih, C.J.; Chu, Y.H. Functional properties of soy protein hydrolysate produced from a continuous membrane reactor system. Food Chem. 1999, 65, 189–194. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane bioreactor (Mbr) technology for wastewater treatment and reclamation: Membrane fouling. Membrane 2016, 6, 33. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Kong, X.; Guo, M.; Hua, Y.; Cao, D.; Zhang, C. Enzymatic preparation of immunomodulating hydrolysates from soy proteins. Bioresour. Technol. 2008, 99, 8873–8879. [Google Scholar] [CrossRef] [PubMed]
- ISO 20483. Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method; International Organization for Standardization (ISO): Geneva, Switzerland, 2013. [Google Scholar]
- Rutherfurd, S.M. Methodology for determining degree of hydrolysis of proteins in Hydrolysates: A review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [PubMed]
- Mahdavi-Yekta, M.; Nouri, L.; Azizi, M.H. The effects of hydrolysis condition on antioxidant activity of protein hydrolyzate from quinoa. Food Sci. Nutr. 2019, 7, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, K.; Abraham, T.E. Chemical modification of papain for use in alkaline medium. J. Mol. Catal. B-Enzym. 2006, 38, 171–177. [Google Scholar] [CrossRef]
- Homaei, A.; Samari, F. Investigation of activity and stability of papain by adsorption on multi-wall carbon nanotubes. Int. J. Biol. Macromol. 2017, 105, 1630–1635. [Google Scholar] [CrossRef]
- Nath, A.; Chakraborty, S.; Bhattacharjee, C.; Chowdhury, R. Studies on the separation of proteins and lactose from casein whey by cross-flow ultrafiltration. Desalin. Water Treat. 2015, 54, 481–501. [Google Scholar] [CrossRef]
- Gaspar, I.; Koris, A.; Bertalan, Z.; Vatai, G. Comparison of ceramic capillary membrane and ceramic tubular membrane with inserted static mixer. Chem. Pap. 2011, 65, 596–602. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kleyn, D.H.; Lynch, J.M.; Barbano, D.M.; Bloom, M.J.; Mitchell, M.W. Determination of Fat in Raw and Processed Milks by the Gerber Method: Collaborative Study. J. AOAC Int. 2001, 84, 1499–1508. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Csehi, B.; Szerdahelyi, E.; Pásztor-Huszár, K.; Salamon, B.; Tóth, A.; Zeke, I.; Jónás, G.; Friedrich, L. Changes of protein profiles in pork and beef meat caused by high hydrostatic pressure treatment. Acta Aliment. 2016, 45, 565–571. [Google Scholar] [CrossRef]
- Ardô, Y.; Meisel, H. Methods for direct measurement of peptide bond cleavage in cheese. Bull. Int. Dairy. Fed. 1991, 261, 10–13. [Google Scholar]
- Navarrete del Toro, M.A.; García-Carreño, F.L. Evaluation of the Progress of Protein Hydrolysis. Curr. Prot. Food Anal. Chem. 2002, 10, B2.2.1–B2.2.14. [Google Scholar]
- Barlow, I.E.; Lloyd, G.T.; Ramshaw, E.H. The measurement of proteolysis in Cheddar cheese: A comparison of trinitrobenzene sulphonic acid procedures. Aust. J. Dairy Technol. 1986, 41, 79–81. [Google Scholar]
- Fujii, T. Coagulation and rheological behaviors of soy milk colloidal dispersions. Biosci. Biotechnol. Biochem. 2017, 81, 680–686. [Google Scholar] [CrossRef]
- Murata, K.; Kusakabe, I.; Kobayashi, H.; Akaike, M.; Murakami, K. Coagulation of Proteins in Leguminous Milk by Commercial Proteinases. Agric. Biol. Chem. 1988, 52, 1317–1318. [Google Scholar]
- Taski-Ajdukovic, K.; Djordjevic, V.; Vidic, M.; Vujakovic, M. Subunit composition of seed storage proteins in high-protein soybean genotypes. Pesq. Agropec. Bras. 2010, 45, 721–729. [Google Scholar] [CrossRef]
- Catsimpoolas, N.; Kenney, J.A.; Meyer, E.W.; Szuhaj, B.F. Molecular weight and amino acid composition of glycinin subunits. J. Sci. Food Agric. 1971, 22, 448–450. [Google Scholar] [CrossRef]
- Sarmiento, C.; Ross, J.H.; Herman, E.; Murphy, D.J. Expression and subcellular targeting of a soybean oleosin in transgenic rapeseed. Implications for the mechanism of oil-body formation in seeds. Plant J. 1997, 11, 783–796. [Google Scholar] [CrossRef]
- Qi, W.; He, Z. Enzymatic hydrolysis of protein: Mechanism and kinetic model. Front. Chem. China 2006, 1, 308–314. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Sadana, A. Enzyme deactivation. Biotechnol. Adv. 1988, 6, 349–446. [Google Scholar] [CrossRef]
- Naidu, G.S.N.; Panda, T. Studies on pH and thermal inactivation of pectolytic enzymes from Aspergillus niger. Biochem. Eng. J. 2003, 16, 57–67. [Google Scholar] [CrossRef]
- Guo, J.; Yang, X.Q.; He, X.T.; Wu, N.N.; Wang, J.M.; Gu, W.; Zhang, Y.Y. Limited Aggregation Behavior of β-Conglycinin and Its Terminating Effect on Glycinin Aggregation during Heating at pH 7.0. J. Agric. Food Chem. 2012, 60, 3782–3791. [Google Scholar] [CrossRef] [PubMed]
- Shimoyamada, M.; Tsushima, N.; Tsuzuki, K.; Asao, H.; Yamauchu, R. Effect of Heat Treatment on Dispersion Stability of Soymilk and Heat Denaturation of Soymilk Protein. Food Sci. Technol. Res. 2008, 14, 32–38. [Google Scholar] [CrossRef]
- Shukor, Y.; Baharom, N.A.; Rahman, F.A.; Abdullah, M.P.; Shamaan, N.A.; Syed, M.A. Development of a heavy metals enzymatic-based assay using papain. Anal. Chim. Acta 2006, 566, 283–289. [Google Scholar] [CrossRef]
- Krstić, D.M.; Tekić, M.N.; Carić, M.Đ.; Milanović, S.D. The effect of turbulence promoter on cross-flow microfiltration of skim milk. J. Memb. Sci. 2002, 208, 303–314. [Google Scholar] [CrossRef]
- Jokić, A.; Zavargo, Z.; Šereš, Z.; Tekić, M. The effect of turbulence promoter on cross-flow microfiltration of yeast suspensions: A response surface methodology approach. J. Memb. Sci. 2010, 350, 269–278. [Google Scholar] [CrossRef]
- Cstorer, A.; Ménard, R. Catalytic mechanism in papain family of cysteine peptidases. Methods Enzymol. 1994, 244, 486–500. [Google Scholar]
- Malanovic, N.; Lohner, K. Antimicrobial peptides targeting gram-positive bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Travkova, O.G.; Moehwald, H.; Brezesinski, G. The interaction of antimicrobial peptides with membranes. Adv. Colloid Interface Sci. 2017, 247, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Patrzykat, A.; Douglas, S. Antimicrobial Peptides: Cooperative Approaches to Protection. Protein Pept. Lett. 2005, 12, 19–25. [Google Scholar] [CrossRef] [PubMed]

| Characteristics of Tubular Membrane | Characteristics of Twisted Tape Static Turbulence Promoter | ||
|---|---|---|---|
| Pore size | 20 nm | Aspect ratio | 2 |
| Length | 250 mm | Diameter | 6.5 mm |
| Inner diameter | 7 mm | Total length | 241 mm |
| Outer diameter | 10 mm | Pitch length | 13.2 mm |
| Active surface area | 5 × 10−3 m2 | Number of mixing elements | 36 |
| Active layer | Titanium oxide | Thickness | 1.2 mm |
| Support layer | Aluminum oxide | Material | Stainless steel (SS316) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, A.; Kailo, G.G.; Mednyánszky, Z.; Kiskó, G.; Csehi, B.; Pásztorné-Huszár, K.; Gerencsér-Berta, R.; Galambos, I.; Pozsgai, E.; Bánvölgyi, S.; et al. Antioxidant and Antibacterial Peptides from Soybean Milk through Enzymatic- and Membrane-Based Technologies. Bioengineering 2020, 7, 5. https://doi.org/10.3390/bioengineering7010005
Nath A, Kailo GG, Mednyánszky Z, Kiskó G, Csehi B, Pásztorné-Huszár K, Gerencsér-Berta R, Galambos I, Pozsgai E, Bánvölgyi S, et al. Antioxidant and Antibacterial Peptides from Soybean Milk through Enzymatic- and Membrane-Based Technologies. Bioengineering. 2020; 7(1):5. https://doi.org/10.3390/bioengineering7010005
Chicago/Turabian StyleNath, Arijit, Geremew Geidare Kailo, Zsuzsanna Mednyánszky, Gabriella Kiskó, Barbara Csehi, Klára Pásztorné-Huszár, Renáta Gerencsér-Berta, Ildikó Galambos, Emília Pozsgai, Szilvia Bánvölgyi, and et al. 2020. "Antioxidant and Antibacterial Peptides from Soybean Milk through Enzymatic- and Membrane-Based Technologies" Bioengineering 7, no. 1: 5. https://doi.org/10.3390/bioengineering7010005
APA StyleNath, A., Kailo, G. G., Mednyánszky, Z., Kiskó, G., Csehi, B., Pásztorné-Huszár, K., Gerencsér-Berta, R., Galambos, I., Pozsgai, E., Bánvölgyi, S., & Vatai, G. (2020). Antioxidant and Antibacterial Peptides from Soybean Milk through Enzymatic- and Membrane-Based Technologies. Bioengineering, 7(1), 5. https://doi.org/10.3390/bioengineering7010005








