Neural Modulation of the Primary Auditory Cortex by Intracortical Microstimulation with a Bio-Inspired Electronic System
Abstract
1. Introduction
2. Materials and Methods
2.1. Mapping of the Primary Auditory Cortex
2.1.1. Animal Preparation and Neural Activity Recording
2.1.2. Data Analysis
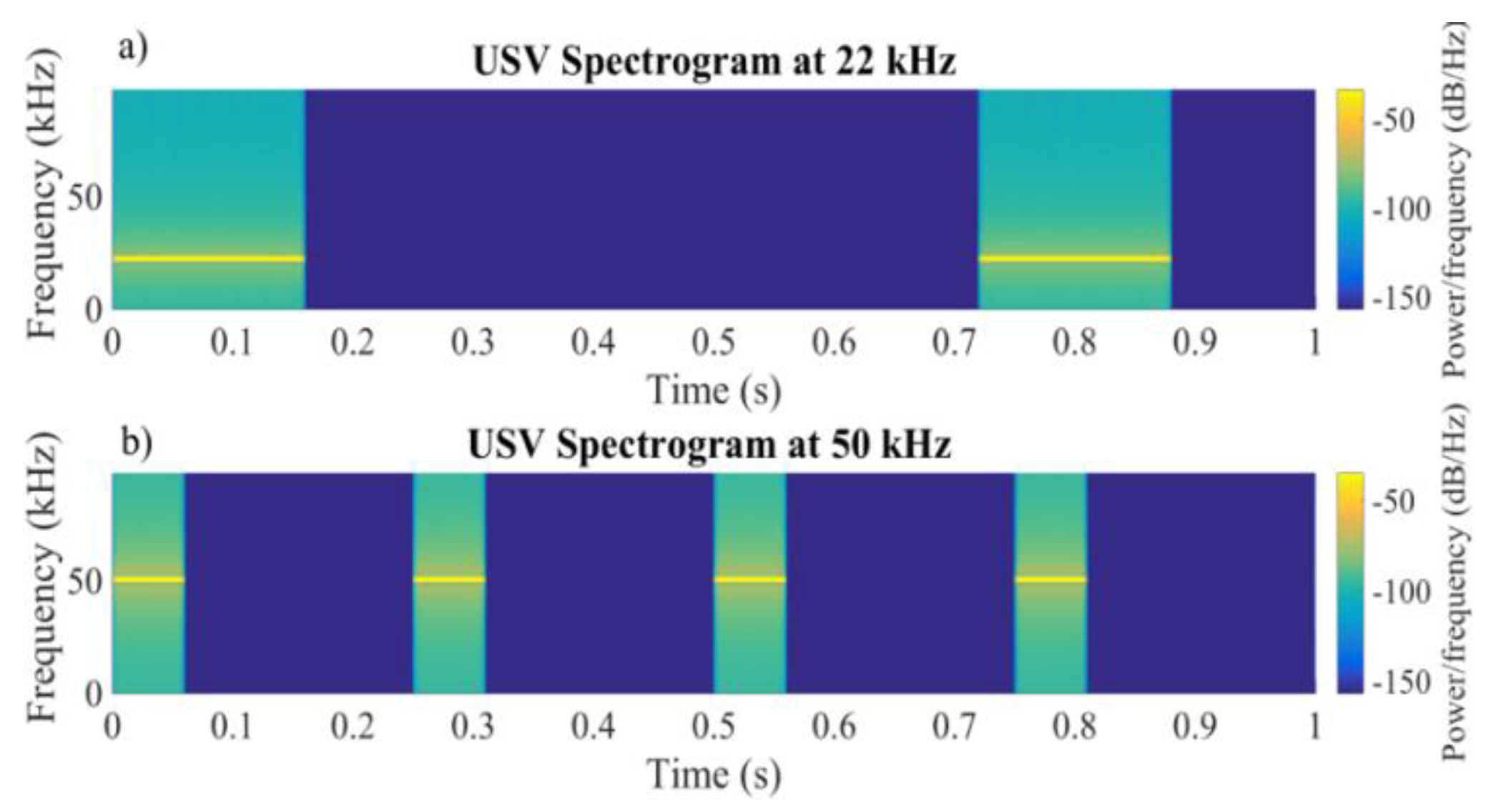
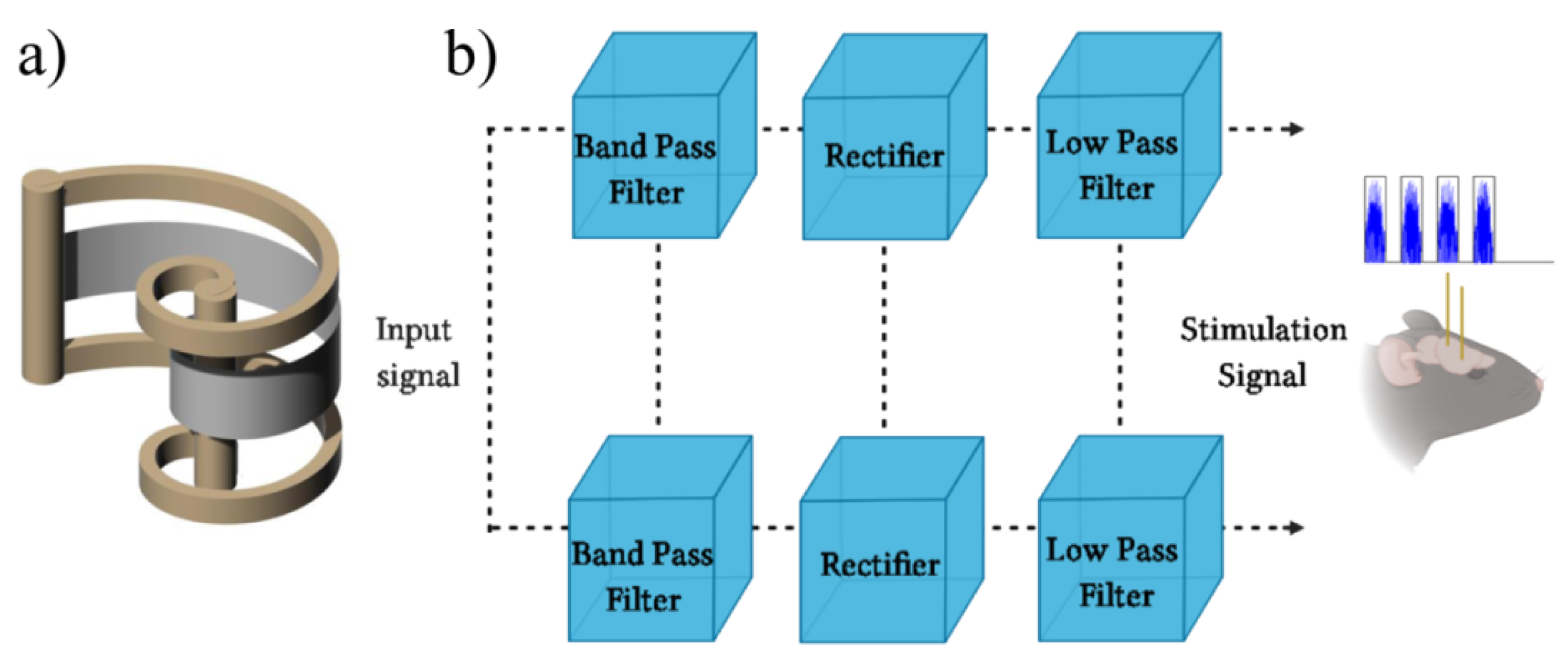
2.2. Electrical Stimulation with a Biomimetic Sonar System
2.2.1. The Biomimetic Sonar Interface
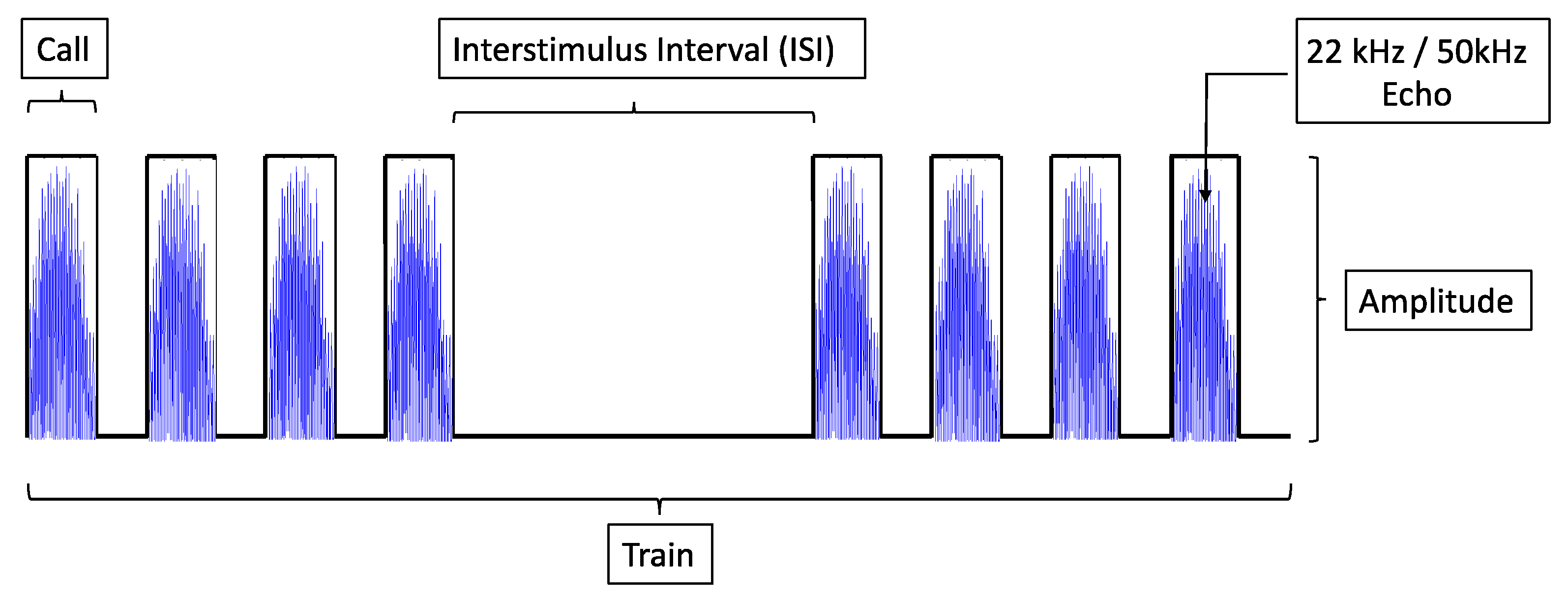
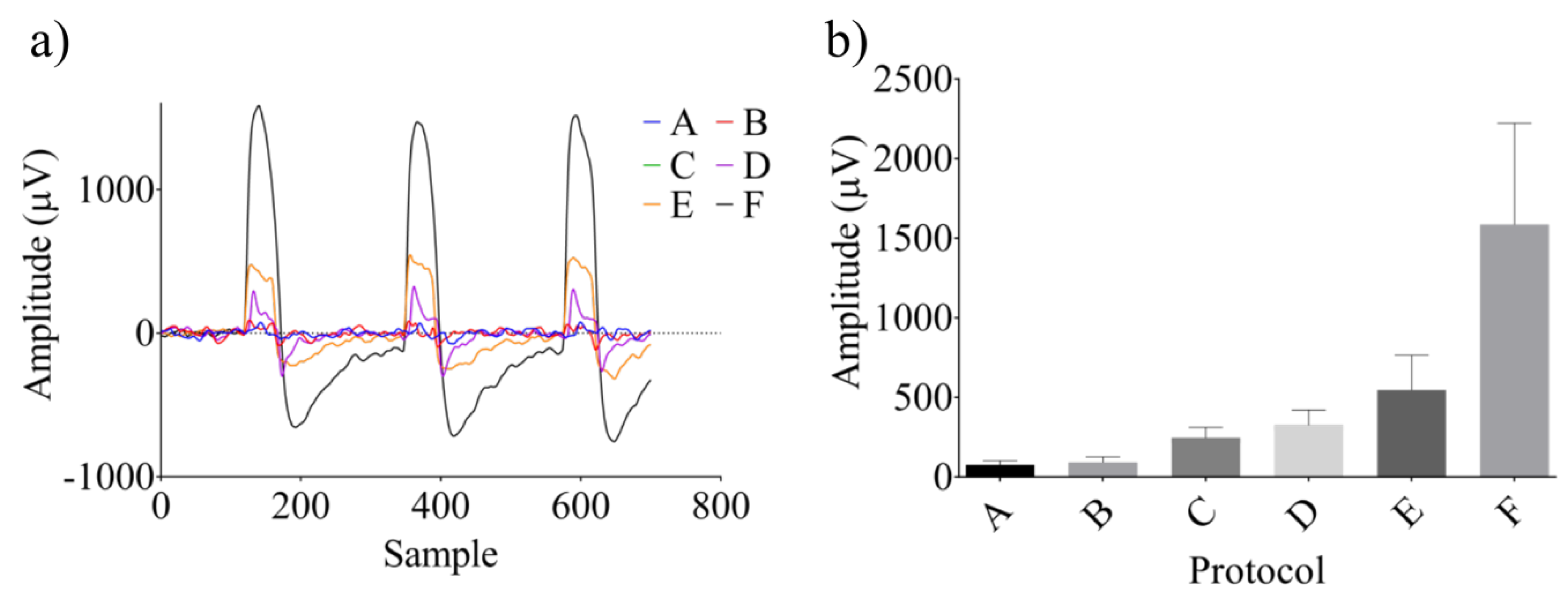
2.2.2. Electrical Stimulation
2.2.3. Control Experiment with Acoustic Stimulation
2.2.4. ECoG Recording
2.2.5. Data Analysis
3. Results
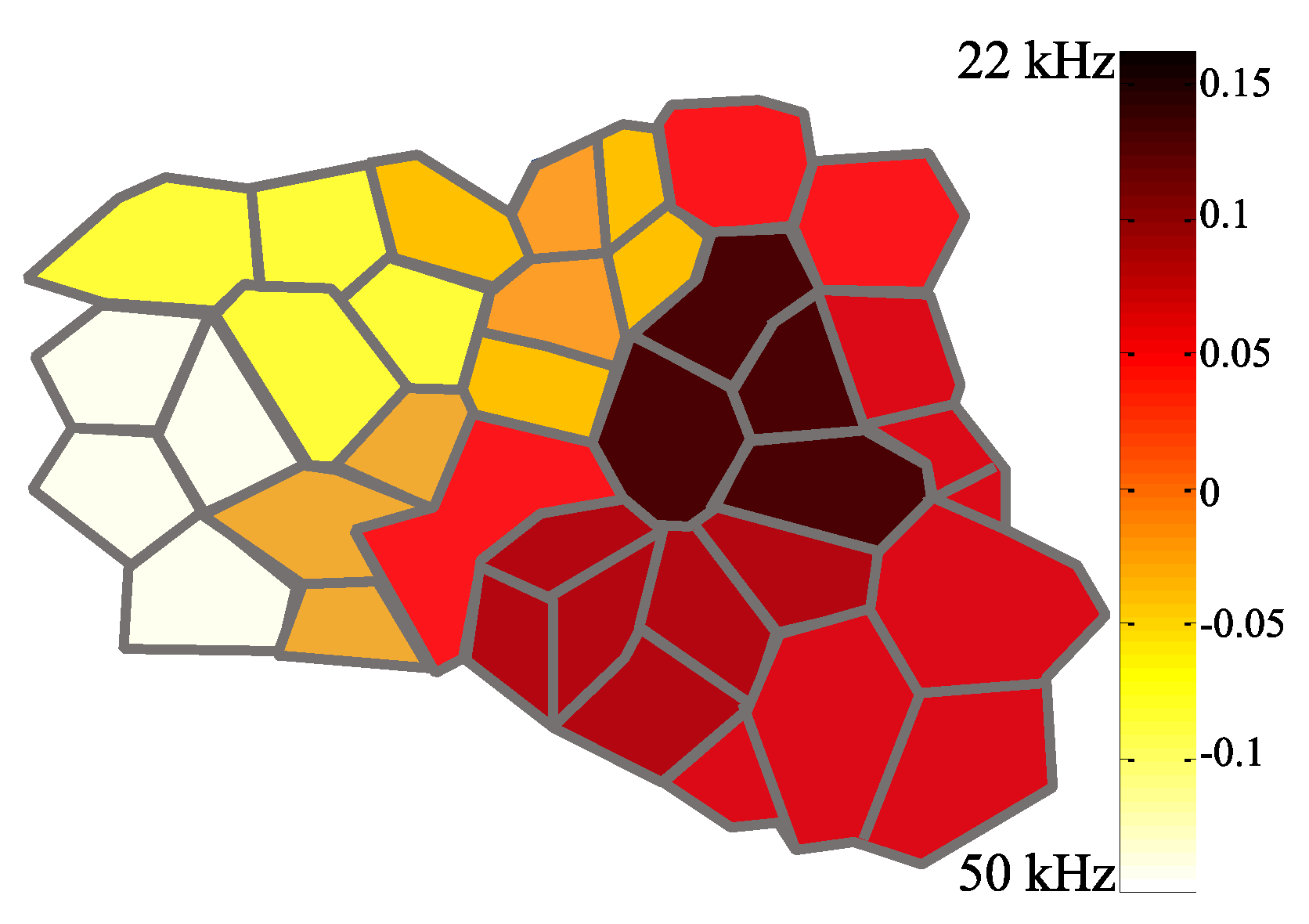
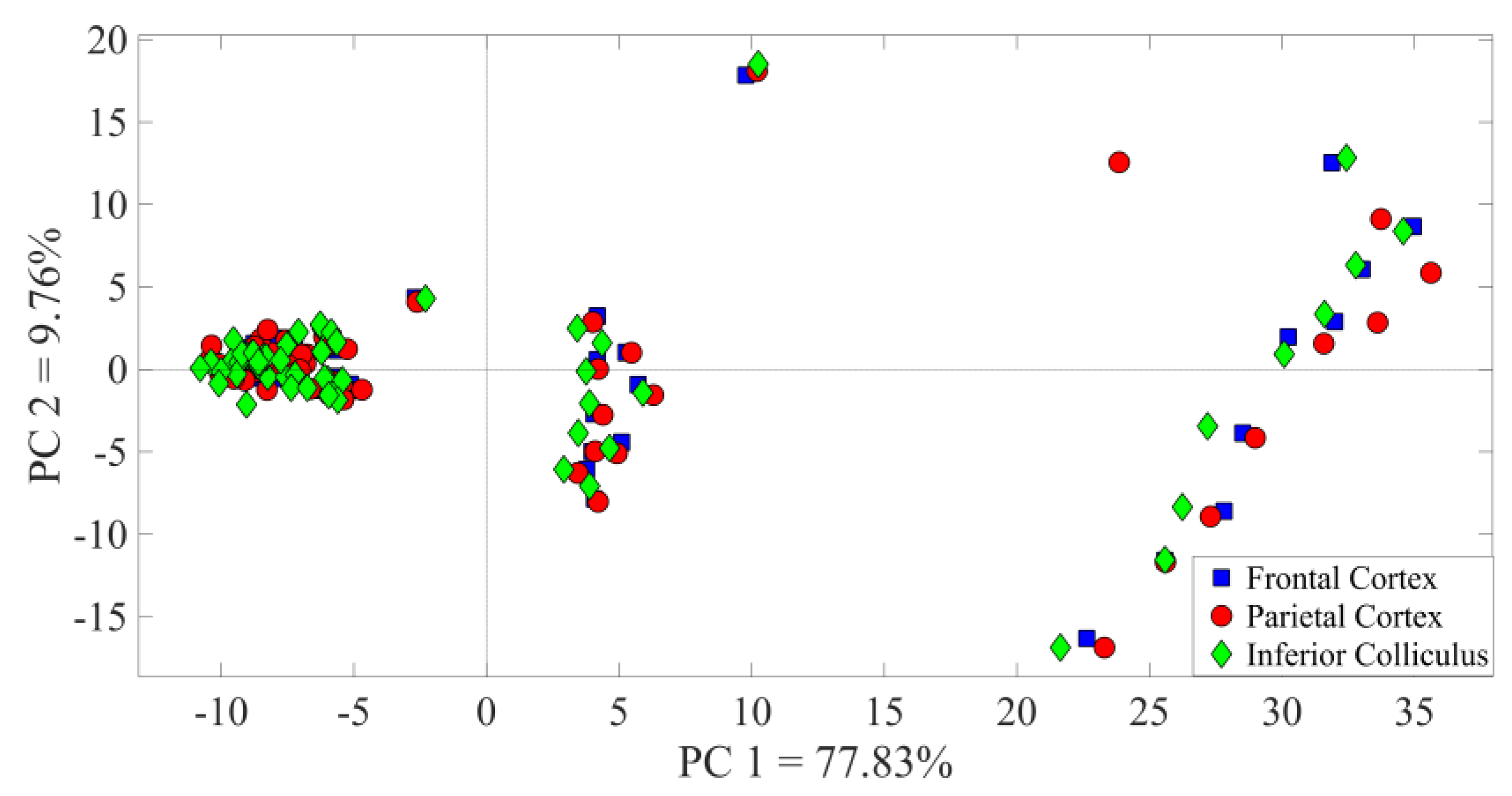
3.1. Identification of the A1 Receptive Fields
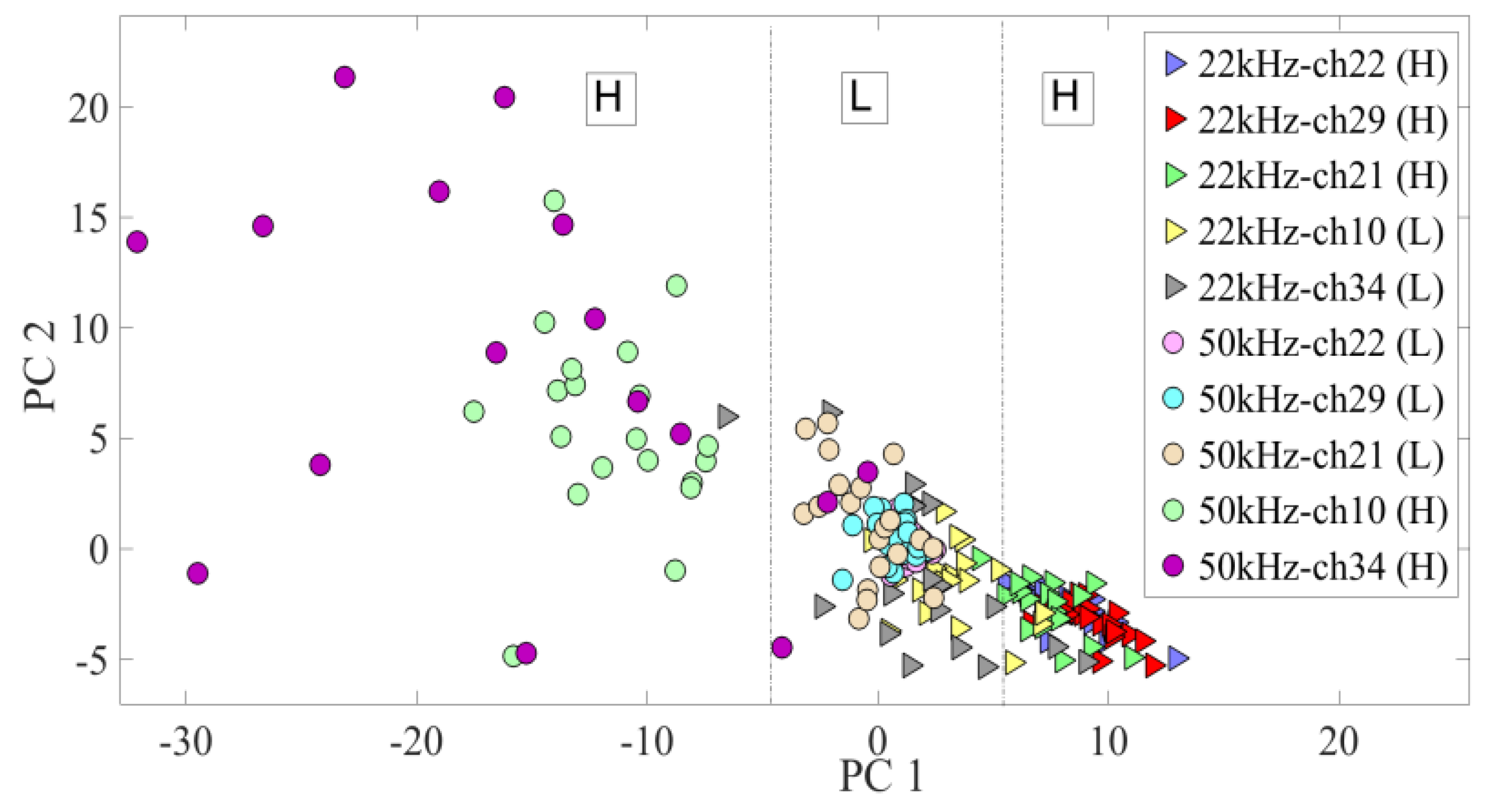
3.2. Neural Modulation with the Biomimetic Sonar System
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Loeb, G.E. Neural Prosthetics: A Review of Empirical vs. Systems Engineering Strategies. Appl. Bionics. Biomech. 2018, 2018, 1435030. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.G.; Pullano, S.A.; Menniti, D.; Critello, C.D.; Citraro, R.; Russo, E.; De Sarro, G.; Fiorillo, A.S. Bioinspired Electric Stimulation: Comparison of ECoG Spectrum in the Main Auditory Structures. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications, Istanbul, Turkey, 11–13 June 2019. [Google Scholar]
- Silva, G.A. A New Frontier: The Convergence of Nanotechnology, Brain Machine Interfaces, and Artificial Intelligence. Front. Neurosci. 2018, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, M.A.; Nicolelis, M.A.L. Brain-machine interfaces: From basic science to neuroprostheses and neurorehabilitation. Physiol. Rev. 2017, 97, 767–837. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.H.; Lenarz, T. Auditory midbrain implant: Research and development towards a second clinical trial. Hear Res. 2015, 322, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Adewole, D.O.; Serruya, M.D.; Harris, J.P.; Burrell, J.C.; Petrov, D.; Chen, H.I.; Wolf, J.A.; Cullen, D.K. The Evolution of Neuroprosthetic Interfaces. Crit. Rev. Biomed. Eng. 2016, 44, 123–152. [Google Scholar]
- O’Doherty, J.E.; Lebedev, M.A.; Ifft, P.J.; Zhuang, K.Z.; Shokur, S.; Bleuler, H.; Nicolelis, M.A. Active tactile exploration using a brain-machine-brain interface. Nature 2011, 479, 228–231. [Google Scholar] [CrossRef]
- Tabot, G.A.; Dammann, J.F.; Berg, J.A.; Tenore, F.V.; Boback, J.L.; Vogelstein, R.J.; Bensmaia, S.J. Restoring the sense of touch with a prosthetic hand through a brain interface. Proc. Natl. Acad. Sci. USA 2013, 110, 18279–18284. [Google Scholar] [CrossRef]
- Chapin, J.K.; Moxon, K.A.; Markowitz, R.S.; Nicolelis, M.A. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat. Neurosci. 1999, 2, 664–670. [Google Scholar] [CrossRef]
- Hartmann, K.; Thomson, E.E.; Zeal, I.; Yun, R.; Mullen, P.; Canarick, J.; Huh, A.; Nicolelis, M.A. Embedding a Panoramic Representation of Infrared Light in the Adult Rat Somatosensory Cortex through a Sensory Neuroprosthesis. J. Neurosci. 2016, 36, 2406–2424. [Google Scholar] [CrossRef]
- Norimoto, H.; Ikegaya, Y. Visual cortical prosthesis with a geomagnetic compass restores spatial navigation in blind rats. Curr. Biol. 2015, 25, 1091–1095. [Google Scholar] [CrossRef]
- Histed, M.H.; Ni, A.M.; Maunsell, J.H.R. Insights into cortical mechanisms of behavior from microstimulation experiments. Prog. Neurobiol. 2013, 103, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.G.; Pullano, S.A.; Critello, C.D.; Menniti, M.; Citraro, R.; Russo, E.; De Sarro, G.; Fiorillo, A.S. Direct Modulation of High–Frequency Neural Oscillations in the Auditory Cortex Through Electrical Microstimulation Generated by PVDF Sensors. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications, Rome, Italy, 11–13 June 2018. [Google Scholar]
- Bianco, M.G.; Pullano, S.A.; Menniti, D.; Fiorillo, A.S.; de Villers-Sidani, E. Evaluation of auditory cortex tonotopical organization in rats Through Principal Component Analysis. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications, Rochester, NY, USA, 7–10 May 2017. [Google Scholar]
- Fiorillo, A.S.; Pullano, S.A.; Bianco, M.G.; Critello, C.D. Bioinspired US sensor for broadband applications. Sens. Actuators A: Phys. 2019, 294, 148–153. [Google Scholar] [CrossRef]
- Wohr, M.; Seffer, D.; Schwarting, R.K.W. Studying socio–affective communication in rats through playback of ultrasonic vocalizations. Curr. Protoc. Neurosci. 2016, 75, 8.35.1–8.35.17. [Google Scholar] [PubMed]
- Fiorillo, A.S.; Pullano, S. Ferroelectric Polymer for Bio-Sonar Replica. Ferroelectr. Appl. 2011, 4, 75–94. [Google Scholar]
- Carruthers, I.M.; Natan, R.G.; Geffen, M.N. Encoding of ultrasonic vocalizations in the auditory cortex. J. Neurophysiol. 2013, 109, 1912–1927. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bao, S. Experience–dependent overrepresentation of ultrasonic vocalization frequencies in the rat primary auditory cortex. J. Neurophysiol. 2013, 110, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, J.; Kroes, R.A.; Moskal, J.R.; Pfaus, J.G.; Brudzynski, S.M.; Pankseep, J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self–administration of playback. J. Comp. Psychol. 2008, 122, 357–367. [Google Scholar] [CrossRef]
- Parsana, A.J.; Li, N.; Brown, T.H. Positive and negative ultrasonic social signals elicit opposing firing patterns in rat amygdala. Behav. Brain Res. 2012, 226, 77–86. [Google Scholar] [CrossRef][Green Version]
- De Villers-Sidani, E.; Chang, E.F.; Merzenich, M.M. Critical Period Window for Spectral Tuning Defined in the Primary Auditory Cortex (A1) in the Rat. J. Neurosci. 2007, 27, 180–189. [Google Scholar] [CrossRef]
- Chapin, J.K.; Nicolelis, M.A. Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations. J. Neurosci. Methods 1999, 94, 121–140. [Google Scholar] [CrossRef]
- Menniti, D.; Pullano, S.A.; Bianco, M.G.; Citraro, R.; Russo, E.; De Sarro, G.; Fiorillo, A.S. Biomimetic Sonar for Electrical Activation of the Auditory Pathway. J. Sens. 2017, 2017, 2632178. [Google Scholar] [CrossRef]
- Jang, J.; Lee, J.W.; Woo, S.; Sly, D.J.; Campbell, L.J.; Cho, J.-H.; O’Leary, S.J.; Park, M.-H.; Han, S.; Choi, J.-W.; et al. A microelectromechanical system artificial basilar membrane based on a piezoelectric cantilever array and its characterization using an animal model. Sci. Rep. 2015, 5, 12447. [Google Scholar] [CrossRef] [PubMed]
- Calero, D.; Paul, S.; Gesing, A.; Alves, F.; Cordioli, J.A. A technical review and evaluation of implantable sensors for hearing devices. Biomed Eng. Online 2018, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, A.S.; Grimaldi, D.; Paolino, D.; Pullano, S.A. Low –Frequency Ultrasound in Medicine: An In Vivo Evaluation. Ieee Trans. Instrum. Meas. 2012, 61, 1658–1663. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, R.F.; Schneider, T.R.2; Rach, S.; Trautmann-Lengsfeld, S.A.; Engel, A.K.; Herrmann, C.S. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr. Biol. 2014, 24, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.P.; Pesaran, B. Analysis of dynamic brain imaging data. Biophys J. 1999, 76, 691–708. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Ethotransmission: Communication of emotional states through ultrasonic vocalization in rats. Neurobiology 2013, 23, 310–317. [Google Scholar] [CrossRef]
- Burgdorf, J.; Pankseep, J.; Moskal, J.R. Rat 22-kHz Ultrasonic Vocalizations as a Measure of Emotional Set Point During Social Interactions. Handb. Behav. Neurosci. 2008, 25, 261–265. [Google Scholar]
- Burgdorf, J.; Knutson, B.; Pankseep, J.; Shippenbeg, T.S. Evaluation of rat ultrasonic vocalizations as predictors of the conditioned aversive effects of drugs. Psychopharmacology 2001, 155, 25–42. [Google Scholar] [CrossRef]
- Joachimsthaler, B.; Uhlmann, M.; Miller, F.; Ehret, G.; Kurt, S. Quantitative analysis of neuronal response properties in primary and higher–order auditory cortical fields of awake house mice (Mus musculus). Eur. J. Neurosci. 2014, 39, 904–918. [Google Scholar] [CrossRef]
- Scholes, C.; Palmer, A.R.; Sumner, C.J. Forward suppression in the auditory cortex is frequency–specific. Eur. J. Neurosci. 2011, 33, 1240–1251. [Google Scholar] [CrossRef][Green Version]
- Beetz, J.M.; Hechavarría, J.C.; Kössl, M. Cortical neurons of bats respond best to echoes from nearest targets when listening to natural biosonar multi–echo streams. Sci. Rep. 2016, 6, 35991. [Google Scholar] [CrossRef]
- Hechavarría, J.C.; Beetz, M.J.; Macias, S.; Kössl, M. Distress vocalization sequences broadcasted by bats carry redundant information. J. Comp. Physiol. 2016, 202, 503–515. [Google Scholar] [CrossRef]
- Beetz, M.J.; Hechavarría, J.C.; Kössl, M. Temporal tuning in the bat auditory cortex is sharper when studied with natural echolocation sequences. Sci. Rep. 2016, 6, 29102. [Google Scholar] [CrossRef]
- Hechavarría, J.C.; Beetz, M.J.; Macias, S.; Kössl, M. Vocal sequences suppress spiking in the bat auditory cortex while evoking concomitant steady –state local field potentials. Sci. Rep. 2016, 6, 39226. [Google Scholar] [CrossRef]
- Doron, N.N.; Ledoux, J.E.; Semple, M.N. Redefining the Tonotopic Core of Rat Auditory Cortex: Physiological Evidence for a Posterior Field. J. Comp. Neurol. 2002, 453, 345–360. [Google Scholar] [CrossRef]
- Tsukano, H.; Horie, M.; Takahashi, K.; Hishida, R.; Takebayashi, H.; Shibuki, K. Independent tonotopy and thalamocortical projection patterns in two adjacent parts of the classical primary auditory cortex in mice. Neurosci. Lett. 2017, 637, 26–30. [Google Scholar] [CrossRef]
- Jakobsen, L.; Brinkløv, S.; Surlykke, A. Intensity and directionality of bat echolocation signals. Front. Physiol. 2013, 4, 89. [Google Scholar] [CrossRef]
- Gaiarsa, J.D. BDNF and the Plasticity of Brain Networks during Maturation. In Cellular Migration and Formation of Neuronal Connections; Rubenstein, J.L.R., Rakic, P., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 895–907. [Google Scholar]
- Oess, T.; Krichmar, J.L.; Röhrbein, F. A Computational Model for Spatial Navigation Based on Reference Frames in the Hippocampus, Retrosplenial Cortex, and Posterior Parietal Cortex. Front. Neur. 2017, 11, 4. [Google Scholar] [CrossRef]
- Nitz, D.A. Spaces within spaces: Rat parietal cortex neurons register position across three reference frames. Nat. Neurosci. 2012, 15, 1365–1367. [Google Scholar] [CrossRef]
- Hok, V.; Save, E.; Lenck-Santini, P.P.; Poucet, B. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc. Natl. Acad. Sci. USA 2005, 102, 4602–4607. [Google Scholar] [CrossRef]
- De Martino, F.; Moerel, M.; van de Moortele, P.F.; Ugurbil, K.; Goebel, R.; Yacoub, E.; Formisano, E. Spatial organization of frequency preference and selectivity in the human inferior colliculus. Nat. Commun. 2013, 4, 1386. [Google Scholar] [CrossRef]
- Suga, N. Neural processing of auditory signals in the time domain: Delay–tuned coincidence detectors in the mustached bat. Hear. Res. 2015, 324, 19–36. [Google Scholar] [CrossRef]
- Gao, E.; Suga, N. Experience–dependent plasticity in the auditory cortex and the inferior colliculus of bats: Role of the corticofugal system. Proc. Natl. Acad. Sci. USA 2000, 97, 8081–8086. [Google Scholar] [CrossRef]
- Klimesch, W. Alpha –band oscillations, attention, and controlled access to stored information. Trend Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef]
- Mazaheri, A.; Jensen, O. Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Haenschel, C.; Baldeweg, T.; Croft, R.J.; Whittington, M.; Gruzelier, J. Gamma and beta frequency oscillations in response to novel auditory stimuli: A comparison of human electroencephalogram (EEG) data with in vitro models. Proc. Natl. Acad. Sci. USA 2000, 97, 7645–7650. [Google Scholar] [CrossRef]
- Fujioka, T.; Ross, B.; Trainor, L.J. Beta–band oscillations represent auditory beat and its metrical hierarchy in perception and imagery. J Neurosci. 2015, 35, 15187–15198. [Google Scholar] [CrossRef]
- Hong, L.E.; Buchanan, R.W.; Thaker, G.K.; Shepard, P.D.; Summerfelt, A. Beta (~16 Hz) frequency neural oscillations mediate auditory sensory gating in humans. Psychophysiology 2008, 45, 197–204. [Google Scholar] [CrossRef]
- Vianney–Rodrigues, P.; Iancu, O.D.; Welsh, J.P. Gamma oscillations in the auditory cortex of awake rats. Eur. J. Neurosci. 2011, 33, 119–129. [Google Scholar] [CrossRef]
- Medvedev, A.V.; Kanwal, J.S. Communication call–evoked gamma– band activity in the auditory cortex of awake bats is modified by complex acoustic features. Brain Res. 2008, 1188, 76–86. [Google Scholar] [CrossRef]





| Stimulating Electrodes | |||
| Left Auditory Cortex (anterior area: 50 kHz) AP = −3.3; L = 7.5; H = 4.7 | Left Auditory Cortex (posterior area: 22 kHz) AP = −4.8; L = 7.2; H = 3.5 | ||
| Recording Electrodes | |||
| Frontal Cortex AP = −2; L = 2.5 | Parietal Cortex AP = −6; L = 2.5 | Inferior Colliculus AP = −9.3; L = −1.5; H = 4.5 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, M.G.; Pullano, S.A.; Citraro, R.; Russo, E.; De Sarro, G.; de Villers Sidani, E.; Fiorillo, A.S. Neural Modulation of the Primary Auditory Cortex by Intracortical Microstimulation with a Bio-Inspired Electronic System. Bioengineering 2020, 7, 23. https://doi.org/10.3390/bioengineering7010023
Bianco MG, Pullano SA, Citraro R, Russo E, De Sarro G, de Villers Sidani E, Fiorillo AS. Neural Modulation of the Primary Auditory Cortex by Intracortical Microstimulation with a Bio-Inspired Electronic System. Bioengineering. 2020; 7(1):23. https://doi.org/10.3390/bioengineering7010023
Chicago/Turabian StyleBianco, Maria Giovanna, Salvatore Andrea Pullano, Rita Citraro, Emilio Russo, Giovambattista De Sarro, Etienne de Villers Sidani, and Antonino S. Fiorillo. 2020. "Neural Modulation of the Primary Auditory Cortex by Intracortical Microstimulation with a Bio-Inspired Electronic System" Bioengineering 7, no. 1: 23. https://doi.org/10.3390/bioengineering7010023
APA StyleBianco, M. G., Pullano, S. A., Citraro, R., Russo, E., De Sarro, G., de Villers Sidani, E., & Fiorillo, A. S. (2020). Neural Modulation of the Primary Auditory Cortex by Intracortical Microstimulation with a Bio-Inspired Electronic System. Bioengineering, 7(1), 23. https://doi.org/10.3390/bioengineering7010023







