Abstract
The late neurocognitive and psychosocial effects of treatment for pediatric brain tumor (PBT) represent important areas of clinical focus and ongoing research. Neurocognitive sequelae and associated problems with learning and socioemotional development negatively impact PBT survivors’ overall health-related quality of life, educational attainment and employment rates. Multiple factors including tumor features and associated complications, treatment methods, individual protective and vulnerability factors and accessibility of environmental supports contribute to the neurocognitive and psychosocial outcomes in PBT survivors. Declines in overall measured intelligence are common and may persist years after treatment. Core deficits in attention, processing speed and working memory are postulated to underlie problems with overall intellectual development, academic achievement and career attainment. Additionally, psychological problems after PBT can include depression, anxiety and psychosocial adjustment issues. Several intervention paradigms are briefly described, though to date research on innovative, specific and effective interventions for neurocognitive late effects is still in its early stages. This article reviews the existing research for understanding PBT late effects and highlights the need for innovative research to enhance neurocognitive and psychosocial outcomes in PBT survivors.
Keywords:
pediatric brain tumor; late effects; neurocognitive; cognitive; psychosocial; survivorship 1. Introduction
Approximately 2970 children and 1170 adolescents are diagnosed with brain and central nervous system tumors in the United States annually [1]. As survival rates following pediatric brain tumor (PBT) increase with improvements in detection and intervention, focus has increased on monitoring and managing the late effects of both disease and treatment (i.e., delayed emergence of neurocognitive, emotional and socioemotional sequelae). It is estimated that as many as 40 to 100% of survivors demonstrate impairment in at least one neurocognitive domain [2] and adult survivors of PBT report the poorest health-related quality of life among all childhood cancers [3]. Neurocognitive and psychosocial late effects are associated with lower high school and college graduation rates and increased likelihood of unemployment [4,5,6,7,8,9], all of which may adversely impact quality of life.
Late effects of treatment for PBT typically emerge in the first few years following treatment and clinically may range from mild performance difficulties that are easily accommodated to severe deficits in functioning that result in the ongoing need for support into adulthood. Here we provide a broad overview of neurocognitive and psychosocial late effects of treatment for PBT, including discussion of significant risk factors and pathophysiology of late effects, a summary of intervention paradigms and discussion of future opportunities to improve outcomes for survivors.
2. Factors Related to Expression of Late Effects
To understand the mechanisms of late effects, a host of factors must be considered. It is important to recognize that isolating the influence of any one variable among the multiple confounding and interacting variables is a consistent challenge in late effects research. With that caveat, factors with documented relationships to outcomes include tumor variables, treatment paradigms and potential moderating variables related to individual patient characteristics and environmental factors.
2.1. Tumor Variables
Tumor size has been associated with lower overall intelligence [1]. Higher risk pathology, such as medulloblastoma, has been associated with poorer neurocognitive outcomes, evident on measures of intelligence, aspects of attention, working memory and processing speed [2,3]. Tumor location is integral, in part due to associated complications. For example, 70–80% of children with posterior fossa tumors develop obstructive hydrocephalus, with approximately 30% requiring cerebrospinal fluid diversion via ventriculoperitoneal shunt or endoscopic third ventriculostomy [4]. Hydrocephalus has been shown to independently correlate with neurocognitive deficits even with otherwise uniform chemotherapy and radiation treatment [5] and is associated with poorer long term intellectual outcomes, regardless of tumor type [6]. Some evidence suggests that children with infratentorial tumors have greater neurocognitive burden than those with supratentorial tumors [7].
2.2. Treatment Variables
Advances in neurosurgical techniques over the last few decades have led to improved histologic diagnosis and decreased morbidity and mortality. Some tumors require only neurosurgical intervention. Still, studies suggest at least some short-term risk for neurocognitive deficits within the first year post surgery [8,9,10]. For example, even with refined neurosurgical practice, the post-surgical complication of posterior fossa syndrome (also known as cerebellar mutism) still occurs in up to 31% of children with infratentorial tumors [11]. This poorly understood entity has been attributed to disruption of cerebello-thalamo-cerebral pathways and is characterized by a unique constellation of symptoms that emerge approximately 24–48 h after surgery, including diminished speech, ataxia, emotional/behavioral lability and apathy. Although the speech and neurologic sequelae often improve with time and rehabilitation, recent evidence suggests worse overall neurocognitive outcomes for PBT survivors who experienced posterior fossa syndrome relative to those who did not [12].
Cranial radiation therapy (CRT) is often considered the most significant treatment-related risk factor for development of neurocognitive late effects [13]. CRT has been associated with significant declines in multiple neurocognitive domains that may continue for years post treatment [14]. Changes to white matter have received much attention as a mechanism of neurocognitive decline following radiation therapy, including decreased normal appearing white matter [15,16,17]. Cranial radiation also affects the growth of new neurons in the hippocampus [18] and decreased hippocampal volume has been associated with specific memory deficits [19] in PBT survivors. Further, working memory performance has been specifically associated with white matter integrity within cerebello-thalamo-cerebral pathways [20].
Effects of chemotherapy alone are difficult to isolate in the context of other treatment paradigms such as surgery and CRT, as well as in the presence of other tumor related variables and complications as reviewed above. While chemotherapy is thought to be less toxic than radiation therapy, specific chemotherapy agents are known to carry direct risk for cognitive impairment [21,22,23] as well as an indirect risk related to ototoxicity [24,25]. Further, concomitant chemotherapy and radiation appears to result in greater cognitive and educational burden compared to CRT alone [26,27].
2.3. Individual Patient and Environmental Characteristics
Age at diagnosis and treatment, as well as time since treatment, moderate neurocognitive outcomes in PBT survivors. Specifically, younger age at diagnosis and treatment has been associated with lower intellectual ability, processing speed, working memory, aspects of attention and academic performance [2,3,28]. In fact, a recent meta-analysis found time since treatment more predictive of overall intelligence than treatment modality in PBT survivors [13]. Further, higher levels of cognitive ability prior to treatment have been associated with greater declines in functioning after PBT treatment [2,28].
Additionally, environmental factors including low socioeconomic status and high stress levels may increase risk for poor neurocognitive and psychosocial outcomes [29,30,31,32,33]. It is well established that survivors of childhood cancer miss a considerable amount of school even after treatment is complete [34], though there is a paucity of research investigating the impact of this and other patient-specific experiential factors on neurocognitive outcome. There have been mixed research findings regarding the impact of gender on cognitive outcomes. While some studies have suggested female medulloblastoma survivors are at higher risk for poorer neurocognitive outcomes than males [28,35,36], others have failed to replicate this finding [5,37,38].
Multiple variables contribute to—and moderate—neurocognitive outcomes in PBT survivors as depicted in Figure 1 adapted from Dennis [39] and Baum, et al. [40]. Moreover, with increasing survivorship and risk-mitigating modifications to treatment regimens, it has become increasingly important to assess neurocognitive and psychological outcomes on an individual level. Several organizations have published psychosocial standards of care for long-term PBT survivors, including the Children’s Oncology Group [41] and the Psychosocial Standards of Care Project for Childhood Cancer [42]. Proposed clinical services range from clinical surveillance to comprehensive neuropsychological evaluations [40].
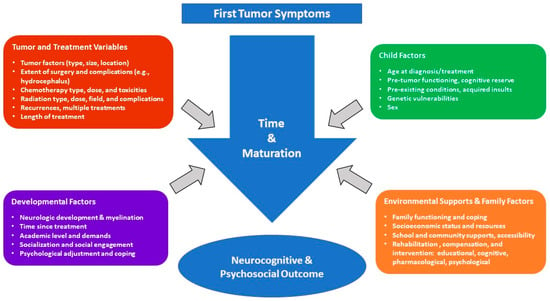
Figure 1.
Factors affecting outcome in PBT survivors.
3. Late Effects of PBT
3.1. Neurocognitive Outcomes
Early studies of neurocognitive outcomes for survivors of PBT focused on global cognitive dysfunction, typically investigating the impact of brain tumors and their treatment on IQ scores and their trajectory over time. More recently, studies have identified specific cognitive functions at greatest risk, believed to represent core deficits that contribute to broader difficulties.
3.1.1. Intellectual Functioning
Declines in IQ are evident in PBT survivors as early as the first year following diagnosis and treatment [43], with potential gradual progression over the next 5 to 7 years [44,45,46]. Few studies have followed survivors longer; however, a handful of studies exploring adult survivors have reported IQs approximately one full standard deviation below healthy controls or the population mean [47,48,49]. Overall intelligence, as well as language-based abilities and non-language abilities, have all been shown to be impacted, with greatest effect sizes for nonverbal functions [13,48,50]. This may be related to the demands commonly administered nonverbal tasks place on visual attention, spatial processing and timed performance [13].
Notably, the decline in IQ scores evident in PBT survivors is related to a failure to make age-appropriate gains over time rather than an actual loss of skill. This was demonstrated in a seminal study [51] in which survivors of medulloblastoma achieved gains in raw scores but only at 49% to 62% the rate of their healthy same-age peers.
The impact of pediatric brain tumors on IQ appears mediated by age, disease and treatment variables. Children diagnosed and treated at a young age (<7) are at greatest risk [52,53], with a potentially more rapid initial decline that plateaus compared to older children, who display a slower, more protracted course [14,52]. Multiple studies have established CRT to carry substantial risk to IQ, mediated by dose, delivery and target [44,45,54,55]. Proton beam radiation (PBRT) has been proposed to carry less neurocognitive risk relative to traditional CRT [56]. Preliminary evidence suggests potential sparing of cognitive and academic functions [57], particularly with focal PBRT [58,59].
3.1.2. Core Deficits—Attention, Processing Speed, & Working Memory
Problems with attention, working memory and processing speed are some of the most commonly reported findings in studies of neurocognitive late effects [2,46,48,60,61]. In a recent meta-analysis, Robinson et al. [50] reported medium to large negative effect sizes for survivors of posterior fossa tumors in multiple cognitive domains, with the largest in attention (Hedges’ g = −1.69) and processing speed (g = −1.40). Moreover, numerous studies have demonstrated a pattern of declining processing speed and working memory scores over time [2,14,45,51,62].
Notably, slowed processing speed is very common in long-term PBT survivors, regardless of tumor type [62]. Processing speed is also suggested to be the most significantly impacted cognitive domain subsequent to treatment for medulloblastoma [2]. Within developmental models, processing speed is conceptualized as a foundational capacity upon which other more complex cognitive abilities are dependent [49,63,64]. For example, analyses have demonstrated age-related gains in processing speed to account for the vast majority of age-related gains in working memory [64]; in addition, these tandem gains in processing speed and working memory occur with a corresponding improvement in intellectual ability.
Given this, it has been proposed that treatment-related deficits in processing speed, attention and working memory are the driving force behind the slowed rate of cognitive development and academic achievement observed in PBT survivors [2,31,49,51,65,66]. Some work has been done developing and evaluating specific interventions targeting these core deficits, although this remains an area of need for continued investigation.
3.1.3. Other Cognitive Functions
Meta-analyses of cognitive late effects in pediatric brain tumor survivors [48,50] have revealed large effect sizes in visually-based tasks, including nonverbal IQ and visual-spatial processing (Hedges’ g = −0.88 to −1.29), as well as medium effect sizes in visual memory (g = −0.68). Studies in other cancers suggest exposure to cranial radiation carries risk for visual-spatial deficits [63]. However, a recent study looking explicitly at children with cerebellar low-grade gliomas requiring surgery alone suggests cerebellar involvement may be sufficient to cause visual-spatial impairment [67].
Executive functions refer to cognitive processes necessary for self-regulation and self-management of thinking, emotions and behavior, ranging from basic attentional and inhibitory control to more complex cognitive flexibility, set shifting and planning. Related deficits have been shown in PBT survivors relative to typically developing peers, both in terms of performance on clinical measures [68,69] and per standardized parent report [70,71]. Interestingly, PBT survivors may have limited awareness of their deficits, characterized by poor metacognition and unrealistic expectations of their abilities [46]. Executive deficits have been specifically associated with poorer long-term outcomes including lower rates of high school graduation and full employment [47,72].
Although less studied than other cognitive domains, memory problems have been demonstrated in PBT survivors across tumor type [73,74,75,76,77,78]. The majority of children with medulloblastoma demonstrate some level of memory impairment; survivors of astrocytoma may be comparatively less impaired but still perform below normal control groups [74,75]. Memory difficulties persist at least into adolescence and young adulthood following PBT [79]. While verbal memory appears more impaired than visual memory [48,50], longitudinal progressive decline has been observed in visual but not verbal memory [14,80].
Language is another area of known risk following treatment for PBT that has not been extensively researched. Meta-analyses have reported medium to large negative effect sizes in PBT survivors, in both general language abilities (Hedges’ g = −0.93 & −0.8) and verbal reasoning (g = −0.74 & −0.68) [48,50]. Cerebellar tumors in particular present with a range of speech and language deficits, including dysfluencies, slowed speech and reduced verbal abilities [81,82], associated with posterior fossa syndrome as discussed above.
PBT survivors are also at risk for sensory and motor impairments that can have a negative downstream impact on learning, academics, communication and social success. For instance, survivors may display early or delayed onset hearing loss attributed to the ototoxic effects of specific chemotherapy agents, as well as potential radiation-related damage to auditory structures [24,25,83].
3.2. Psychosocial Outcomes
Even though psychological problems after PBT have received less research attention than neurocognitive dysfunction, evidence suggests survivors are at greater risk for depression, anxiety, suicidal ideation and behavior problems relative to the general population [84]. Sense of well-being [85], family functioning [86], parent-child health related communication [87] and social involvement [88] have all been implicated as areas of risk after PBT.
Psychological problems and their prevalence rates are highly variable across samples, which impedes conclusive statements regarding patterns of psychological outcome [89]. Social deficits are well established in this population, including low social competence relative to typically developing children, siblings and survivors of non-CNS cancers [90,91]. Causation is unclear, although potential contributors include level of social skill development, functional or sensorimotor deficits, separation from peers and social networks, temperament and specific neurocognitive deficits such as decreased cognitive ability and attention [92,93].
The examination of individual differences and their impact on PBT survivors’ psychological health has received some attention. As an example, neurocognitive dysfunction has been consistently associated with emotional and behavioral health [94,95,96]. Particular aspects of neurocognitive dysfunction, including executive function problems, present exponentially greater risk for emotional and behavioral health concerns in PBT survivors [97].
A recent conceptualization of the impact of childhood cancer on neurodevelopmental trajectory posits that the experience of childhood cancer is an early threat exposure that impacts psychological functioning and neural development [98] which helps unify the importance of addressing both psychosocial and neurocognitive late effects of PBT.
4. Interventions to Support PBT Survivors
Academic accommodations and modifications remain the primary educational support for academic difficulties resulting from neurocognitive late effects of tumor and treatment [99]. Indeed, special education utilization rates are especially high for this population relative to other types of childhood cancer [100]. Ongoing surveillance for neurocognitive and academic difficulties is considered standard of care and helps to inform academic supports and educational planning [41,42]. Career and vocational counseling may be helpful for PBT survivors who often face difficulties obtaining and maintaining employment when impacted by neurocognitive late effects [101,102].
In addition to educational supports, a number of cognitive training paradigms have targeted aspects of cognitive performance and academic achievement by attempting to enhance commonly affected functions including attention, working memory and processing speed [103]. Among the few studies that exist, results have largely been equivocal in terms of positive impact on brain tumor survivors’ academic performance and outcome [42,103].
One PBT targeted paradigm utilized drill-oriented practice, metacognitive and learning skills acquisition and cognitive behavioral therapy [104,105] focused on improving attention and academic achievement. Statistically significant improvements were observed in a number of clinical measures. However, the relevance of these clinical test and rating improvements to the children’s everyday performance was not established and this is unfortunately a common theme in research intervention programs targeting PBT late effects [103].
Recent attention has been devoted to computer-based training with the Cogmed [106] program to improve working memory using computer exercises along with regular coaching and support. Studies have suggested the intervention is feasible and acceptable in pediatric cancer survivors [107]. Randomized trials have shown performance improvements on clinical testing [108] and such improvements may be durable for months after the intervention [109]. However, studies supporting this program have not demonstrated specificity of computer-based training as the specific agent of improvement while controlling for level of support and coaching offered to participants [110]. More compelling evidence is needed before this intervention should be broadly recommended as efficacious in the PBT survivor population [103].
In addition to efforts focused on remediating specific neurocognitive deficits, other methods have addressed improving outcomes more globally in PBT survivors. For example, researchers have focused on indirect and contextual methods rooted in the premise that improving controllable external variables may hold promise for optimizing performance of brain-based functions that otherwise may not be amenable to direct intervention. For instance, training parents in behavioral modification, cognitive instructional methods and compensatory strategies to allow for ongoing intervention in the child’s natural environment showed some efficacy in improving academic outcomes and warrants further attention [111]. As another example, a recent randomized study isolated improvements in situational motivation as associated with improved academic performance [112]. Situational and intrapersonal factors such as level of intrinsic achievement motivation and responsivity to external incentive may have a role in improving academic performance in PBT survivors. Finally, targeting health-related behaviors such as exercise, has shown promise in neural recovery and neurocognitive improvement and deserves further attention [113].
Pharmacological interventions to address neurocognitive late effects have been used with PBT survivors. Stimulant medications have been shown to improve aspects of attention in survivors but not intellectual functioning or academic skills [114]. In a small pilot study, donepezil—an acetylcholinesterase inhibitor—was shown to be feasible and to improve executive functioning and memory in childhood brain tumor survivors [115], justifying a more rigorous placebo controlled randomized trial. Modafinil has been examined as a possible medication to improve fatigue, cognitive functioning and mood in adult patients with primary brain tumors but its benefits did not exceed that of the placebo control group [116]. Pharmacologic prophylaxis to diminish neurotoxicity and preserve neurocognitive function after PBT treatment has shown preliminary utility in adults undergoing whole brain radiation [117] but no such prophylactic treatments have yet been systematically studied in children.
Finally, psychological interventions have demonstrated efficacy in ameliorating PBT survivors’ behavioral and emotional health problems [118,119]. However, psychological referral standards have yet to be established and there is a clear disconnect in that the number of reported concerns far exceeds the frequency of referral for psychological services [96,120].
Figure 2 provides an overview of the recommended clinical management of neuropsychological late effects of PBT survivors, inclusive of ongoing clinical surveillance through individualized treatment planning. Because of the heterogeneity of factors affecting outcomes and the diversity of outcomes themselves, there currently is not a singular consensus pathway, timeline, or group of identified supports recommended for all patients. Ongoing surveillance for neurocognitive late effects is essential to engaging subsequent clinical neuropsychological assessment and treatment planning to optimize outcomes for PBT survivors.
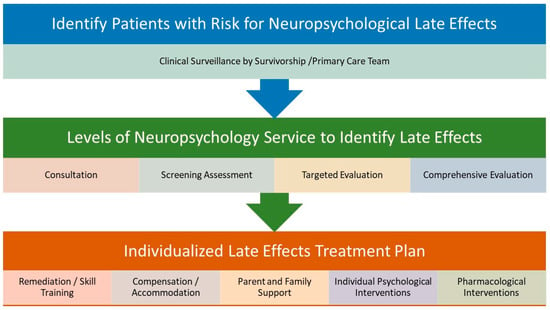
Figure 2.
Clinical management of neuropsychological late effects after PBT.
5. Conclusions
PBT outcomes research is challenging for numerous reasons and it is within this context that the bulk of neurocognitive and psychosocial outcomes research should be understood. Small base rates of specific pediatric tumor types have often resulted in small research samples. A common amelioration of this challenge has been to include mixed types of pediatric cancers and treatment paradigms, though this then contributes to variable rates of reporting of things like psychological difficulties specific to tumor variables and treatment patterns [121]. Accruing patients over long periods of time is another potential remedy, though changes in treatment-related variables (e.g., changes in chemotherapy and/or radiation protocols) may result in incomparable samples over time. Non-medical and demographic factors known to correlate with cognitive and psychosocial functioning (e.g., family functioning, socioeconomic factors) are often unaccounted for and the unique nature of PBT research complicates identification of an appropriate “control” group. While the majority of PBT outcomes studies are cross-sectional, those that are longitudinal often suffer from a lack of clearly defined or valid baseline to which later results can be compared [122]. Finally, the needs of patient care are sometimes at odds with scientific rigor, as providing clinically useful information to families and providers may not align with consistency of data gathering.
While many challenges exist in studying this population, there have been improvements and innovations over the past several decades in terms of treatment paradigms to spare neurocognitive and psychosocial functioning in PBT survivors that have led to meaningful and even dramatic improvements in long-term outcome. Largely these improvements have resulted from refined treatment protocols that have reduced neurotoxicity of treatments for PBT and unfortunately less progress has been achieved in terms of intervention to improve late effects experienced by PBT survivors.
Nonetheless, as summarized above, clinicians and researchers alike should note the development of several promising domains that warrant more attention and provide potentially fruitful topics for future clinical research. While educational support through schools is considered standard of care, there is significant opportunity to improve educational programming and support to optimize academic outcomes. Further, novel intervention paradigms have shown some promise in recent years including direct training of neurocognitive functions affected by treatment for PBT. Individual factors such as intrinsic motivation and resilience are now being considered in terms of their relationship to neuropsychological late effects. Recent work has demonstrated potential efficacy of parent and family support as a way to ameliorate late effects. Pharmacological interventions have only recently been explored and clearly there are opportunities for collaborative clinical research to investigate efficacy of medications to improve neurocognitive function after treatment for PBT. Efforts investigating the impact of health-related behaviors such as nutrition and exercise on outcomes from PBT and its treatment are in their infancy and additional research in this area may help identify cost effective and readily accessible ways to improve neurocognitive functions in the PBT population. Finally, the role of preventative methods to reduce late effects burden is only now being explored and may represent a significant opportunity to improve outcomes for PBT survivors.
Optimal clinical management of neuropsychological late effects after treatment for PBT begins with awareness of the need to monitor this population at high risk for neuropsychological deficits. Unfortunately access to appropriate neuropsychological surveillance, evaluation and intervention remains inconsistent for PBT survivors [41,42]. Moreover, we are still in the beginning stages of determining effective strategies for implementing proposed standards of neuropsychological care, meeting patient need within the current healthcare climate and resource constraints common in various clinical settings. Advocacy for improved access to surveillance and care for neuropsychological late effects is the shared responsibility of researchers and clinicians working with this population to bring to the fore the needs of this vulnerable population as well as to establish efficacy of new and innovative interventions to improve outcomes.
Finally, in addition to efforts to improve surveillance and intervention paradigms and access to care, innovations in the basic conceptualization of mechanisms of cognitive impairment in pediatric cancer, such as examining structural connectome organization implicated in efficiency of information processing [123], may further refine our understanding and detection of late effects of PBT and its treatment. What is clear is that preventing, managing and remediating late neurocognitive and psychosocial late effects for PBT survivors is going to require innovation and problem-solving among numerous basic and applied scientific disciplines.
Author Contributions
Conceptualization, P.L.S., M.A.A., S.K.P., N.P.S., R.S.R.; Writing-Original Draft Preparation, P.L.S., M.A.A., S.K.P., N.P.S., R.S.R.; Writing-Review & Editing, P.L.S., M.A.A., S.K.P., N.P.S., R.S.R.
Funding
This review received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Olsson, I.T.; Perrin, S.; Lundgren, J.; Hjorth, L.; Johanson, A. Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr. Neurol. 2014, 51, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.L.; Armstrong, C.; Onar-Thomas, A.; Wu, S.; Wallace, D.; Bonner, M.J.; Schreiber, J.; Swain, M.; Chapieski, L.; Mabbott, D.; et al. Processing speed, attention, and working memory after treatment for medulloblastoma: An international, prospective, and longitudinal study. J. Clin. Oncol. 2013, 31, 3494–3500. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, J.E.; Gurney, J.G.; Palmer, S.L.; Bass, J.K.; Wang, M.; Chen, S.; Zhang, H.; Swain, M.; Chapieski, M.L.; Bonner, M.J. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro-Oncology 2014, 16, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, F.; Jucá, C.E.; Zerah, M.; Sainte-Rose, C. Endoscopic third ventriculostomy and posterior fossa tumors. World Neurosurg. 2013, 79, S18.e15–S18.e19. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.K.; Bonner, M.J.; Willard, V.W.; Watral, M.A.; Gururangan, S. Hydrocephalus as a possible additional contributor to cognitive outcome in survivors of pediatric medulloblastoma. Psycho-Oncol. J. Psychol. Soc. Behav. Dimens. Cancer 2008, 17, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Duffner, P.K. Risk factors for cognitive decline in children treated for brain tumors. Eur. J. Paediatr. Neurol. 2010, 14, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Mullins, W.; O’neil, S.; Wilson, K. Neuropsychological differences between survivors of supratentorial and infratentorial brain tumours. J. Intell. Disabil. Res. 2011, 55, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.W.; Ris, M.D.; Armstrong, F.D.; Fontanesi, J.; Mulhern, R.; Holmes, E.; Wisoff, J.H. Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: Evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130). J. Clin. Oncol. 2005, 23, 5198–5204. [Google Scholar] [CrossRef] [PubMed]
- Jalali, R.; Mallick, I.; Dutta, D.; Goswami, S.; Gupta, T.; Munshi, A.; Deshpande, D.; Sarin, R. Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Ris, M.D.; Beebe, D.W.; Armstrong, F.D.; Fontanesi, J.; Holmes, E.; Sanford, R.A.; Wisoff, J.H. Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: A report from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 4765. [Google Scholar] [CrossRef] [PubMed]
- Avula, S.; Spiteri, M.; Kumar, R.; Lewis, E.; Harave, S.; Windridge, D.; Ong, C.; Pizer, B. Post-operative pediatric cerebellar mutism syndrome and its association with hypertrophic olivary degeneration. Quant. Imaging Med. Surg. 2016, 6, 535. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, J.E.; Palmer, S.L.; Conklin, H.M.; Mabbott, D.J.; Swain, M.A.; Bonner, M.J.; Chapieski, M.L.; Huang, L.; Zhang, H.; Gajjar, A. Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional, prospective study. Neuro-Oncology 2017, 19, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, M.A.; van Mourik, R.; Schouten-van Meeteren, A.Y.; Grootenhuis, M.A.; Oosterlaan, J. Neurocognitive consequences of a paediatric brain tumour and its treatment: A meta-analysis. Dev. Med. Child. Neurol. 2013, 55, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, B.J.; Bouffet, E.; Greenberg, M.L.; Rutka, J.T.; Mabbott, D.J. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J. Clin. Oncol. 2004, 22, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, M.; Chintagumpala, M.; Laningham, F.H.; Ashley, D.; Kellie, S.J.; Langston, J.W.; McCluggage, C.W.; Woo, S.; Kocak, M.; Krull, K. White matter lesions detected by magnetic resonance imaging after radiotherapy and high-dose chemotherapy in children with medulloblastoma or primitive neuroectodermal tumor. J. Clin. Oncol. 2004, 22, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Jacola, L.M.; Ashford, J.M.; Reddick, W.E.; Glass, J.O.; Ogg, R.J.; Merchant, T.E.; Conklin, H.M. The relationship between working memory and cerebral white matter volume in survivors of childhood brain tumors treated with conformal radiation therapy. J. Neuro-Oncol. 2014, 119, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Reddick, W.E.; Glass, J.O.; Palmer, S.L.; Wu, S.; Gajjar, A.; Langston, J.W.; Kun, L.E.; Xiong, X.; Mulhern, R.K. Atypical white matter volume development in children following craniospinal irradiation. Neuro-Oncology 2005, 7, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Vogel, H.; Masek, M.; Ligon, K.L.; Fisher, P.G.; Palmer, T.D. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2007, 62, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Nagel, B.J.; Delis, D.C.; Palmer, S.L.; Reeves, C.; Gajjar, A.; Mulhern, R.K. Early patterns of verbal memory impairment in children treated for medulloblastoma. Neuropsychology 2006, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Law, N.; Bouffet, E.; Laughlin, S.; Laperriere, N.; Brière, M.-E.; Strother, D.; McConnell, D.; Hukin, J.; Fryer, C.; Rockel, C. Cerebello–thalamo–cerebral connections in pediatric brain tumor patients: Impact on working memory. Neuroimage 2011, 56, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Taylor, O.A.; Hockenberry, M.J.; McCarthy, K.; Gundy, P.; Montgomery, D.; Ross, A.; Scheurer, M.E.; Moore, I.M. Evaluation of biomarkers of oxidative stress and apoptosis in patients with severe methotrexate neurotoxicity: A case series. J. Pediatr. Oncol. Nurs. 2015, 32, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, S.; Bode, U.; Deinlein, F.; Ottensmeier, H.; Warmuth-Metz, M.; Soerensen, N.; Graf, N.; Emser, A.; Pietsch, T.; Wolff, J.E. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N. Engl. J. Med. 2005, 352, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, C.C.; Heimans, J.J.; Hoekman, K.; Postma, T.J. Neurotoxic complications of chemotherapy in patients with cancer. Drugs 2003, 63, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- McHaney, V.A.; Thibadoux, G.; Hayes, F.A.; Green, A.A. Hearing loss in children receiving cisplatin chemotherapy. J. Pediatr. 1983, 102, 314–317. [Google Scholar] [CrossRef]
- Warrier, R.; Chauhan, A.; Davluri, M.; Tedesco, S.L.; Nadell, J.; Craver, R. Cisplatin and cranial irradiation-related hearing loss in children. Ochsner J. 2012, 12, 191–196. [Google Scholar] [PubMed]
- Bull, K.S.; Spoudeas, H.A.; Yadegarfar, G.; Kennedy, C.R. Reduction of health status 7 years after addition of chemotherapy to craniospinal irradiation for medulloblastoma: A follow-up study in PNET 3 trial survivors—On behalf of the CCLG (formerly UKCCSG). J. Clin. Oncol. 2007, 25, 4239–4245. [Google Scholar] [CrossRef] [PubMed]
- Di Pinto, M.; Conklin, H.M.; Li, C.; Merchant, T.E. Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e363–e369. [Google Scholar] [CrossRef] [PubMed]
- Ris, M.D.; Packer, R.; Goldwein, J.; Jones-Wallace, D.; Boyett, J.M. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: A Children’s Cancer Group study. J. Clin. Oncol. 2001, 19, 3470–3476. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, C.; Root, J.C.; Ahles, T.A.; McEwen, B.S.; Compas, B.E. Cancer, coping, and cognition: A model for the role of stress reactivity in cancer-related cognitive decline. Psycho-Oncology 2015, 24, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Brouwers, P.; Okcu, M.F.; Cirino, P.T.; Krull, K.R. Sex-specific attention problems in long-term survivors of pediatric acute lymphoblastic leukemia. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2009, 115, 4238–4245. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.L. Neurodevelopmental impact on children treated for medulloblastoma: A review and proposed conceptual model. Dev. Disabil. Res. Rev. 2008, 14, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Fernandez, N.; Dekel, N.; Turk, A.; Meier, A.; Ross, P.; Rosenthal, J. Socioeconomic status as a possible moderator of neurocognitive outcomes in children with cancer. Psycho-Oncology 2016, 25, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.E.; Wolfe, K.R.; Yeates, K.O.; Mahone, E.M.; Cecil, K.M.; Ris, M.D. Predictors of adaptive functioning and psychosocial adjustment in children with pediatric brain tumor: A report from the brain radiation investigative study consortium. Pediatr. Blood Cancer 2015, 62, 509–516. [Google Scholar] [CrossRef] [PubMed]
- French, A.E.; Tsangaris, E.; Barrera, M.; Guger, S.; Brown, R.; Urbach, S.; Stephens, D.; Nathan, P.C. School attendance in childhood cancer survivors and their siblings. J. Pediatr. 2013, 162, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.W.; Haser, J.K. Neurocognitive effects of treatment for childhood cancer. Ment. Retard. Dev. Disabil. Res. Rev. 2006, 12, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.L.; Reddick, W.E.; Gajjar, A. Understanding the cognitive impact on children who are treated for medulloblastoma. J. Pediatr. Psychol. 2007, 32, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, D.J.; Spiegler, B.J.; Greenberg, M.L.; Rutka, J.T.; Hyder, D.J.; Bouffet, E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J. Clin. Oncol. 2005, 23, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, D.J.; Penkman, L.; Witol, A.; Strother, D.; Bouffet, E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology 2008, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M. Childhood medical disorders and cognitive impairment: Biological risk, time, development, and reserve. In Pediatric Neuropsychology: Research, Theory, and Practice; Yeates, K.O., Ris, M.D., Taylor, H.G., Eds.; Guilford Press: New York, NY, USA, 2000; pp. 3–22. [Google Scholar]
- Baum, K.T.; Powell, S.K.; Jacobson, L.A.; Gragert, M.N.; Janzen, L.A.; Paltin, I.; Rey-Casserly, C.M.; Wilkening, G.N. Implementing guidelines: Proposed definitions of neuropsychology services in pediatric oncology. Pediatr. Blood Cancer 2017, 64, e26446. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.C.; Patel, S.K.; Dilley, K.; Goldsby, R.; Harvey, J.; Jacobsen, C.; Kadan-Lottick, N.; McKinley, K.; Millham, A.K.; Moore, I.; et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: A report from the Children’s Oncology Group. Arch. Pediatr. Adolesc. Med. 2007, 161, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Annett, R.D.; Patel, S.K.; Phipps, S. Monitoring and Assessment of Neuropsychological Outcomes as a Standard of Care in Pediatric Oncology. Pediatr. Blood Cancer 2015, 62 (Suppl. 5), S460–S513. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, J.C. Effects of Cranial Radiation on Structural and Functional Brain Development in Pediatric Brain Tumors. J. Pediatr. Neuropsychol. 2016, 2, 3–13. [Google Scholar] [CrossRef]
- Grill, J.; Renaux, V.K.; Bulteau, C.; Viguier, D.; Levy-Piebois, C.; Sainte-Rose, C.; Dellatolas, G.; Raquin, M.A.; Jambaque, I.; Kalifa, C. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 137–145. [Google Scholar] [CrossRef]
- Moxon-Emre, I.; Bouffet, E.; Taylor, M.D.; Laperriere, N.; Scantlebury, N.; Law, N.; Spiegler, B.J.; Malkin, D.; Janzen, L.; Mabbott, D. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J. Clin. Oncol. 2014, 32, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Chevignard, M.; Câmara-Costa, H.; Doz, F.; Dellatolas, G. Core deficits and quality of survival after childhood medulloblastoma: A review. Neuro-Oncol. Pract. 2017, 4, 82–97. [Google Scholar] [CrossRef]
- Brinkman, T.M.; Reddick, W.E.; Luxton, J.; Glass, J.O.; Sabin, N.D.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; Krull, K.R. Cerebral white matter integrity and executive function in adult survivors of childhood medulloblastoma. Neuro-Oncology 2012, 14 (Suppl. 4), iv25–iv36. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.E.; Kuttesch, J.F.; Champion, J.E.; Andreotti, C.F.; Hipp, D.W.; Bettis, A.; Barnwell, A.; Compas, B.E. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr. Blood Cancer 2010, 55, 525–531. [Google Scholar] [CrossRef] [PubMed]
- King, T.Z.; Ailion, A.S.; Fox, M.E.; Hufstetler, S.M. Neurodevelopmental model of long-term outcomes of adult survivors of childhood brain tumors. Child Neuropsychol. 2017, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.E.; Fraley, C.E.; Pearson, M.M.; Kuttesch, J.F., Jr.; Compas, B.E. Neurocognitive late effects of pediatric brain tumors of the posterior fossa: A quantitative review. J. Int. Neuropsychol. Soc. 2013, 19, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.L.; Goloubeva, O.; Reddick, W.E.; Glass, J.O.; Gajjar, A.; Kun, L.; Merchant, T.E.; Mulhern, R.K. Patterns of intellectual development among survivors of pediatric medulloblastoma: A longitudinal analysis. J. Clin. Oncol. 2001, 19, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.L.; Gajjar, A.; Reddick, W.E.; Glass, J.O.; Kun, L.E.; Wu, S.; Xiong, X.; Mulhern, R.K. Predicting intellectual outcome among children treated with 35–40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology 2003, 17, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, J.; Bunin, G.R.; Sutton, L.N.; Goldwein, J.W.; Phillips, P.C. Cognitive deficits in long-term survivors of childhood medulloblastoma and other noncortical tumors: Age-dependent effects of whole brain radiation. Int. J. Dev. Neurosci. 1994, 12, 327–334. [Google Scholar] [CrossRef]
- Kieffer-Renaux, V.; Bulteau, C.; Grill, J.; Kalifa, C.; Viguier, D.; Jambaque, I. Patterns of neuropsychological deficits in children with medulloblastoma according to craniospatial irradiation doses. Dev. Med. Child Neurol. 2000, 42, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Mulhern, R.K.; Kepner, J.L.; Thomas, P.R.; Armstrong, F.D.; Friedman, H.S.; Kun, L.E. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: A Pediatric Oncology Group study. J. Clin. Oncol. 1998, 16, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V.C.; McDonough, J.; Tochner, Z. Proton beam irradiation in pediatric oncology: An overview. J. Pediatr. Hematol. Oncol. 2005, 27, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Warren, E.; Child, A.; Cirino, P.; Grosshans, D.; Mahajan, A.; Paulino, A.; Chintagumpala, M.; Okcu, F.; Douglas Ris, M.; Minard, C. QOL-42. Better social, cognitive, and academic outcomes among pediatric brain tumor survivors treated with proton versus photon radiation therapy. Neuro-Oncology 2018, 20, i166. [Google Scholar] [CrossRef]
- Kahalley, L.S.; Ris, M.D.; Grosshans, D.R.; Okcu, M.F.; Paulino, A.C.; Chintagumpala, M.; Moore, B.D.; Guffey, D.; Minard, C.G.; Stancel, H.H.; et al. Comparing Intelligence Quotient Change After Treatment With Proton Versus Photon Radiation Therapy for Pediatric Brain Tumors. J. Clin. Oncol. 2016, 34, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Antonini, T.N.; Ris, M.D.; Grosshans, D.R.; Mahajan, A.; Okcu, M.F.; Chintagumpala, M.; Paulino, A.; Child, A.E.; Orobio, J.; Stancel, H.H.; et al. Attention, processing speed, and executive functioning in pediatric brain tumor survivors treated with proton beam radiation therapy. Radiother. Oncol. 2017, 124, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Conklin, H.M.; Ashford, J.M.; Howarth, R.A.; Merchant, T.E.; Ogg, R.J.; Santana, V.M.; Reddick, W.E.; Wu, S.; Xiong, X. Working memory performance among childhood brain tumor survivors. J. Int. Neuropsychol. Soc. 2012, 18, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Reddick, W.E.; White, H.A.; Glass, J.O.; Wheeler, G.C.; Thompson, S.J.; Gajjar, A.; Leigh, L.; Mulhern, R.K. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer 2003, 97, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Kahalley, L.S.; Conklin, H.M.; Tyc, V.L.; Hudson, M.M.; Wilson, S.J.; Wu, S.; Xiong, X.; Hinds, P.S. Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psychooncology 2013, 22, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Schatz, J.; Kramer, J.H.; Ablin, A.; Matthay, K.K. Processing speed, working memory, and IQ: A developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology 2000, 14, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.F.; Hale, S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol. Psychol. 2000, 54, 1–34. [Google Scholar] [CrossRef]
- Holland, A.A.; Hughes, C.W.; Stavinoha, P.L. School competence and fluent academic performance: Informing assessment of educational outcomes in survivors of pediatric medulloblastoma. Appl. Neuropsychol. Child 2015, 4, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Roddy, E.; Mueller, S. Late effects of treatment of pediatric central nervous system tumors. J. Child Neurol. 2016, 31, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Starowicz-Filip, A.; Chrobak, A.A.; Milczarek, O.; Kwiatkowski, S. The visuospatial functions in children after cerebellar low-grade astrocytoma surgery: A contribution to the pediatric neuropsychology of the cerebellum. J. Neuropsychol. 2017, 11, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Koustenis, E.; Driever, P.H.; de Sonneville, L.; Rueckriegel, S.M. Executive function deficits in pediatric cerebellar tumor survivors. Eur. J. Paediatr. Neurol. 2016, 20, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.L.; Hudson, M.M. Survivors of childhood and adolescent cancer: Life-long risks and responsibilities. Nat. Rev. Cancer 2014, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Wochos, G.; Semerjian, C.; Walsh, K.S. Differences in parent and teacher rating of everyday executive function in pediatric brain tumor survivors. Clin. Neuropsychol. 2014, 28, 1243–1257. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.R.; Walsh, K.S.; Reynolds, N.C.; Mitchell, F.; Reddy, A.T.; Paltin, I.; Madan-Swain, A. Executive functions and social skills in survivors of pediatric brain tumor. Child Neuropsychol. 2013, 19, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Liu, Q.; Yasui, Y.; Huang, S.; Ness, K.K.; Leisenring, W.; Hudson, M.M.; Donaldson, S.S.; King, A.A.; Stovall, M.; et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2009, 101, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Ribi, K.; Relly, C.; Landolt, M.A.; Alber, F.D.; Boltshauser, E.; Grotzer, M.A. Outcome of medulloblastoma in children: Long-term complications and quality of life. Neuropediatrics 2005, 36, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Ronning, C.; Sundet, K.; Due-Tonnessen, B.; Lundar, T.; Helseth, E. Persistent cognitive dysfunction secondary to cerebellar injury in patients treated for posterior fossa tumors in childhood. Pediatr. Neurosurg. 2005, 41, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Roncadin, C.; Dennis, M.; Greenberg, M.L.; Spiegler, B.J. Adverse medical events associated with childhood cerebellar astrocytomas and medulloblastomas: Natural history and relation to very long-term neurobehavioral outcome. Childs Nerv. Syst. 2008, 24, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Maddrey, A.M.; Bergeron, J.A.; Lombardo, E.R.; McDonald, N.K.; Mulne, A.F.; Barenberg, P.D.; Bowers, D.C. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J. Neurooncol. 2005, 72, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, K.; Spiegler, B.J.; Fung, S.; Panzarella, T.; Mabbott, D.J.; Jewitt, N.; D’Agostino, N.M.; Mason, W.P.; Bouffet, E.; Tabori, U.; et al. Early aging in adult survivors of childhood medulloblastoma: Long-term neurocognitive, functional, and physical outcomes. Neuro-Oncology 2011, 13, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Mulhern, R.K.; Palmer, S.L.; Reddick, W.E.; Glass, J.O.; Kun, L.E.; Taylor, J.; Langston, J.; Gajjar, A. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J. Clin. Oncol. 2001, 19, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Hocking, M.C.; Hobbie, W.L.; Deatrick, J.A.; Hardie, T.L.; Barakat, L.P. Family functioning mediates the association between neurocognitive functioning and health-related quality of life in young adult survivors of childhood brain tumors. J. Adolesc. Young Adult Oncol. 2015, 4, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Redmond, K.J.; Mahone, E.M.; Terezakis, S.; Ishaq, O.; Ford, E.; McNutt, T.; Kleinberg, L.; Cohen, K.J.; Wharam, M.; Horska, A. Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: A prospective study. Neuro-Oncology 2013, 15, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.F.; Bradley, K.; Spiegler, B.; Dennis, M. Long-term neuromotor speech deficits in survivors of childhood posterior fossa tumors: Effects of tumor type, radiation, age at diagnosis, and survival years. J. Child Neurol. 2007, 22, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Catsman-Berrevoets, C.E.; Aarsen, F.K. The spectrum of neurobehavioural deficits in the Posterior Fossa Syndrome in children after cerebellar tumour surgery. Cortex 2010, 46, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Bass, J.K.; Khan, R.; Kun, L.E.; Merchant, T.E. Hearing loss after radiotherapy for pediatric brain tumors: Effect of cochlear dose. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Dellarole, A.; Peterson, E.C.; Bregy, A.; Komotar, R.; Harvey, P.D.; Elhammady, M.S. Long-term psychiatric outcomes in pediatric brain tumor survivors. Child’s Nerv. Syst. 2015, 31, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Memmesheimer, R.M.; Lange, K.; Dölle, M.; Heger, S.; Mueller, I. Psychological well-being and independent living of young adults with childhood-onset craniopharyngioma. Dev. Med. Child Neurol. 2017, 59, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Quast, L.F.; Phillips, P.C.; Li, Y.; Kazak, A.E.; Barakat, L.P.; Hocking, M.C. A prospective study of family predictors of health-related quality of life in pediatric brain tumor survivors. Pediatr. Blood Cancer 2018, 65, e26976. [Google Scholar] [CrossRef] [PubMed]
- Adduci, A.; Jankovic, M.; Strazzer, S.; Massimino, M.; Clerici, C.; Poggi, G. Parent–child communication and psychological adjustment in children with a brain tumor. Pediatr. Blood Cancer 2012, 59, 290–294. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, M.A.; Schouten-van Meeteren, A.Y.N.; van Vuurden, D.G.; Maurice-Stam, H.; Gidding, C.; Beek, L.R.; Granzen, B.; Oosterlaan, J.; Grootenhuis, M.A. Psychosocial profile of pediatric brain tumor survivors with neurocognitive complaints. Qual. Life Res. 2016, 25, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Fuemmeler, B.F.; Elkin, T.D.; Mullins, L.L. Survivors of childhood brain tumors: Behavioral, emotional, and social adjustment. Clin. Psychol. Rev. 2002, 22, 547–585. [Google Scholar] [CrossRef]
- Emond, A.; Edwards, L.; Peacock, S.; Norman, C.; Evangeli, M. Social competence in children and young people treated for a brain tumour. Support. Care Cancer 2016, 24, 4587–4595. [Google Scholar] [CrossRef] [PubMed]
- Schulte, F. Social competence in pediatric brain tumor survivors: Breadth versus depth. Curr. Opin. Oncol. 2015, 27, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.A.; Colaluca, B.; Bailey, L.; Stavinoha, P.L. Impact of attention on social functioning in pediatric medulloblastoma survivors. Pediatr. Hematol. Oncol. 2018, 35, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Sands, S.A.; Pasichow, K.P. Psychological and social impact of being a pediatric brain tumor survivor. In Late Effects of Treatment for Brain Tumors; Springer: New York, NY, USA, 2009; pp. 297–307. [Google Scholar]
- Nassau, J.H.; Drotar, D. Social competence among children with central nervous system-related chronic health conditions: A review. J. Pediatr. Psychol. 1997, 22, 771–793. [Google Scholar] [CrossRef] [PubMed]
- Poggi, G.; Liscio, M.; Galbiati, S.; Adduci, A.; Massimino, M.; Gandola, L.; Spreafico, F.; Clerici, C.A.; Fossati-Bellani, F.; Sommovigo, M. Brain tumors in children and adolescents: Cognitive and psychological disorders at different ages. Psycho-Oncol. J. Psychol. Soc. Behav. Dimens. Cancer 2005, 14, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Kahalley, L.S.; Wilson, S.J.; Tyc, V.L.; Conklin, H.M.; Hudson, M.M.; Wu, S.; Xiong, X.; Stancel, H.H.; Hinds, P.S. Are the psychological needs of adolescent survivors of pediatric cancer adequately identified and treated? Psycho-Oncology 2013, 22, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Ventura, L.M.; Grieco, J.A.; Evans, C.L.; Kuhlthau, K.A.; MacDonald, S.M.; Tarbell, N.J.; Yock, T.I.; Pulsifer, M.B. Executive functioning, academic skills, and quality of life in pediatric patients with brain tumors post-proton radiation therapy. J. Neuro-Oncol. 2018, 137, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Marusak, H.A.; Iadipaolo, A.S.; Harper, F.W.; Elrahal, F.; Taub, J.W.; Goldberg, E.; Rabinak, C.A. Neurodevelopmental consequences of pediatric cancer and its treatment: Applying an early adversity framework to understanding cognitive, behavioral, and emotional outcomes. Neuropsychol. Rev. 2017, 28, 123–175. [Google Scholar] [CrossRef] [PubMed]
- Hay, G.H.; Nabors, M.; Sullivan, A.; Zygmund, A. Students with Pediatric Cancer: A Prescription for School Success. Phys. Disabil. Educ. Relat. Serv. 2015, 34, 1–13. [Google Scholar] [CrossRef]
- Mitby, P.A.; Robison, L.L.; Whitton, J.A.; Zevon, M.A.; Gibbs, I.C.; Tersak, J.M.; Meadows, A.T.; Stovall, M.; Zeltzer, L.K.; Mertens, A.C.; et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2003, 97, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, A.C.; Leisenring, W.; Krull, K.R.; Ness, K.K.; Friedman, D.L.; Armstrong, G.T.; Stovall, M.; Park, E.R.; Oeffinger, K.C.; Hudson, M.M.; et al. Unemployment among adult survivors of childhood cancer: A report from the childhood cancer survivor study. Med. Care 2010, 48, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.L.; Christiansen, H.L.; Elam, M.; Hoag, J.; Irwin, M.K.; Pao, M.; Voll, M.; Noll, R.B.; Kelly, K.P. Academic Continuity and School Reentry Support as a Standard of Care in Pediatric Oncology. Pediatr. Blood Cancer 2015, 62 (Suppl. 5), S805–S817. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.; Sands, S.A. Cognitive training programs for childhood cancer patients and survivors: A critical review and future directions. Child Neuropsychol. 2016, 22, 509–536. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.W.; Copeland, D.R. Attentional processes and their remediation in children treated for cancer: A literature review and the development of a therapeutic approach. J. Int. Neuropsychol. Soc. 2002, 8, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.W.; Copeland, D.R.; Fairclough, D.L.; Mulhern, R.K.; Katz, E.R.; Kazak, A.E.; Noll, R.B.; Patel, S.K.; Sahler, O.J.Z. A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. J. Consult. Clin. Psychol. 2008, 76, 367. [Google Scholar] [CrossRef] [PubMed]
- Pearson Education, Incorporated Cogmed. Available online: https://www.cogmed.com/ (accessed on 20 July 2018).
- Hardy, K.K.; Willard, V.W.; Allen, T.M.; Bonner, M.J. Working memory training in survivors of pediatric cancer: A randomized pilot study. Psycho-Oncology 2013, 22, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Conklin, H.M.; Ogg, R.J.; Ashford, J.M.; Scoggins, M.A.; Zou, P.; Clark, K.N.; Martin-Elbahesh, K.; Hardy, K.K.; Merchant, T.E.; Jeha, S. Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: A randomized controlled trial. J. Clin. Oncol. 2015, 33, 3894. [Google Scholar] [CrossRef] [PubMed]
- Conklin, H.M.; Ashford, J.M.; Clark, K.N.; Martin-Elbahesh, K.; Hardy, K.K.; Merchant, T.E.; Ogg, R.J.; Jeha, S.; Huang, L.; Zhang, H. Long-term efficacy of computerized cognitive training among survivors of childhood cancer: A single-blind randomized controlled trial. J. Pediatr. Psychol. 2016, 42, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Chacko, A.; Bedard, A.; Marks, D.; Feirsen, N.; Uderman, J.; Chimiklis, A.; Rajwan, E.; Cornwell, M.; Anderson, L.; Zwilling, A. A randomized clinical trial of Cogmed working memory training in school-age children with ADHD: A replication in a diverse sample using a control condition. J. Child Psychol. Psychiatry 2014, 55, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Ross, P.; Cuevas, M.; Turk, A.; Kim, H.; Lo, T.T.; Wong, L.F.; Bhatia, S. Parent-directed intervention for children with cancer-related neurobehavioral late effects: A randomized pilot study. J. Pediatr. Psychol. 2014, 39, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.A.; Hughes, C.W.; Harder, L.; Silver, C.; Bowers, D.C.; Stavinoha, P.L. Effect of motivation on academic fluency performance in survivors of pediatric medulloblastoma. Child Neuropsychol. 2016, 22, 570–586. [Google Scholar] [CrossRef] [PubMed]
- Riggs, L.; Piscione, J.; Laughlin, S.; Cunningham, T.; Timmons, B.W.; Courneya, K.S.; Bartels, U.; Skocic, J.; de Medeiros, C.; Liu, F. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: A controlled clinical trial with crossover of training versus no training. Neuro-Oncology 2016, 19, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Conklin, H.M.; Reddick, W.E.; Ashford, J.; Ogg, S.; Howard, S.C.; Morris, E.B.; Brown, R.; Bonner, M.; Christensen, R.; Wu, S. Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. J. Clin. Oncol. 2010, 28, 4465. [Google Scholar] [CrossRef] [PubMed]
- Castellino, S.M.; Tooze, J.A.; Flowers, L.; Hill, D.F.; McMullen, K.P.; Shaw, E.G.; Parsons, S.K. Toxicity and efficacy of the acetylcholinesterase (AChe) inhibitor donepezil in childhood brain tumor survivors: A pilot study. Pediatr. Blood Cancer 2012, 59, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Boele, F.W.; Douw, L.; de Groot, M.; van Thuijl, H.F.; Cleijne, W.; Heimans, J.J.; Taphoorn, M.J.; Reijneveld, J.C.; Klein, M. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: A multicenter randomized controlled trial. Neuro-Oncology 2013, 15, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Pugh, S.; Laack, N.N.; Wefel, J.S.; Khuntia, D.; Meyers, C.; Choucair, A.; Fox, S.; Suh, J.H.; Roberge, D. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro-Oncology 2013, 15, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Poggi, G.; Liscio, M.; Pastore, V.; Adduci, A.; Galbiati, S.; Spreafico, F.; Gandola, L.; Massimino, M. Psychological intervention in young brain tumor survivors: The efficacy of the cognitive behavioural approach. Disabil. Rehabil. 2009, 31, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Barrera, M.; Atenafu, E.G.; Sung, L.; Bartels, U.; Schulte, F.; Chung, J.; Cataudella, D.; Hancock, K.; Janzen, L.; Saleh, A. A randomized control intervention trial to improve social skills and quality of life in pediatric brain tumor survivors. Psycho-Oncology 2018, 27, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.C.; Enderby, K.; O’Toole, M.; Thomas, S.A.; Ashley, D.; Rosenfeld, J.V.; Simos, E.; Tokatlian, N.; Gedye, R. The role of social support in families coping with childhood brain tumor. J. Psychosoc. Oncol. 2009, 27, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Macartney, G.; Stacey, D.; Harrison, M.B.; VanDenKerkhof, E. Symptoms, coping, and quality of life in pediatric brain tumor survivors: A qualitative study. Oncol. Nurs. Forum 2014, 41, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Ris, M.D.; Grosch, M.; Fletcher, J.M.; Metah, P.; Kahalley, L.S. Measurement of neurodevelopmental changes in children treated with radiation for brain tumors: What is a true ‘baseline?’. Clin. Neuropsychol. 2017, 31, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R.; Gugel, M.; Huston-Warren, E.; Watson, C. Atypical structural connectome organization and cognitive impairment in young survivors of acute lymphoblastic leukemia. Brain Connect. 2016, 6, 273–282. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).