Understanding the Impact of Stent and Scaffold Material and Strut Design on Coronary Artery Thrombosis from the Basic and Clinical Points of View
Abstract
1. Introduction
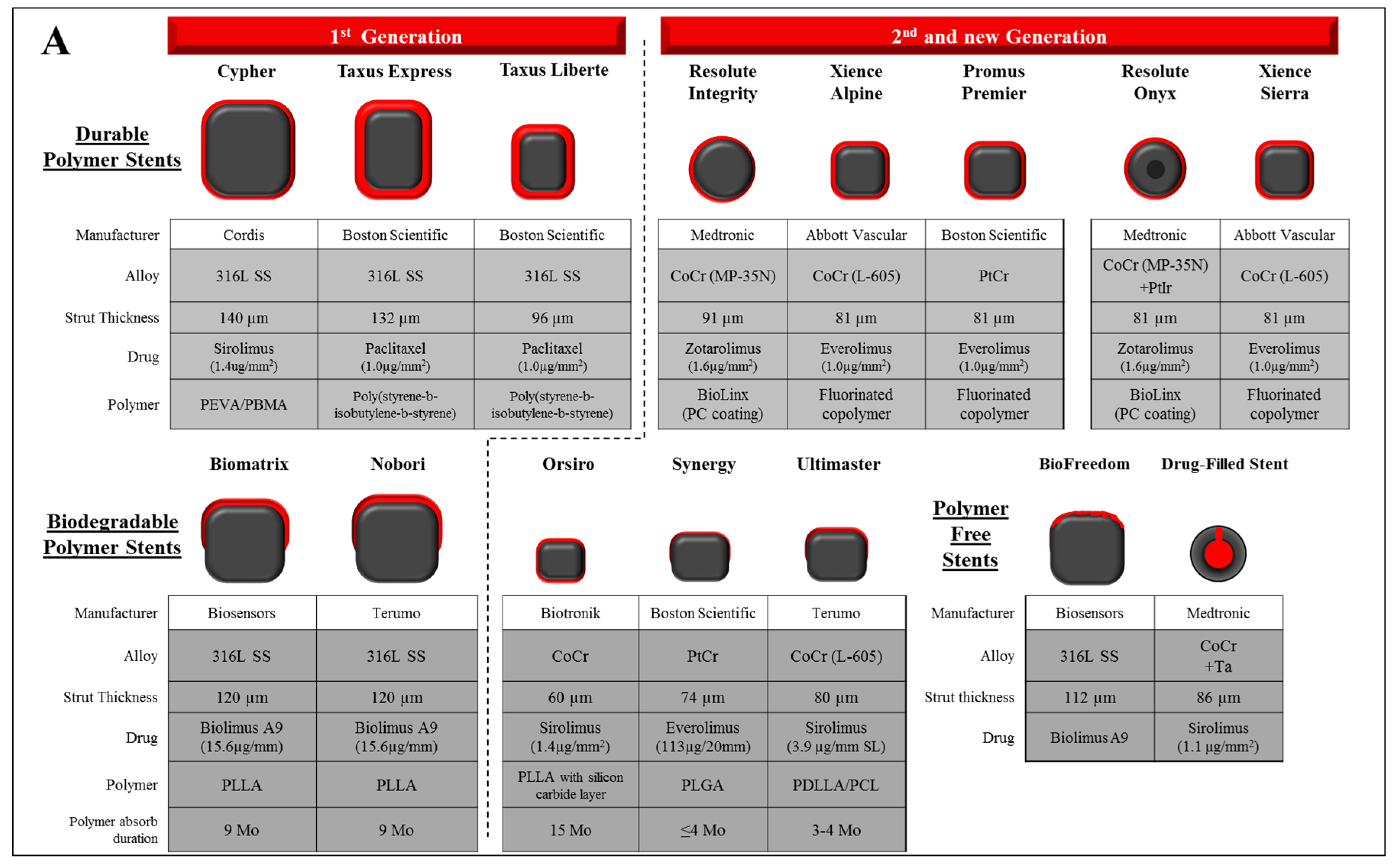
2. Metallic Stents
3. Drug and Polymer
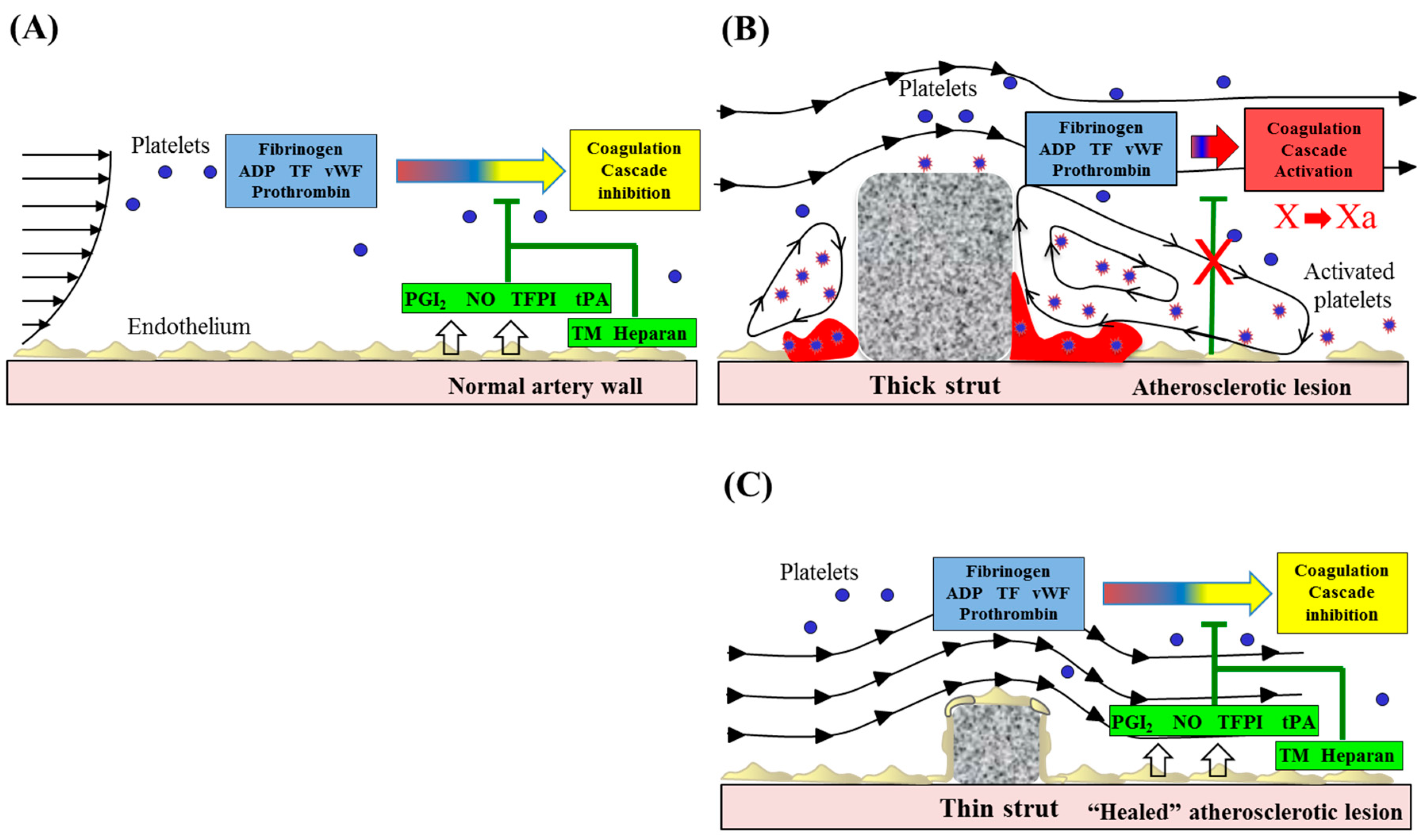
4. Stent Thrombosis in Metallic Stent
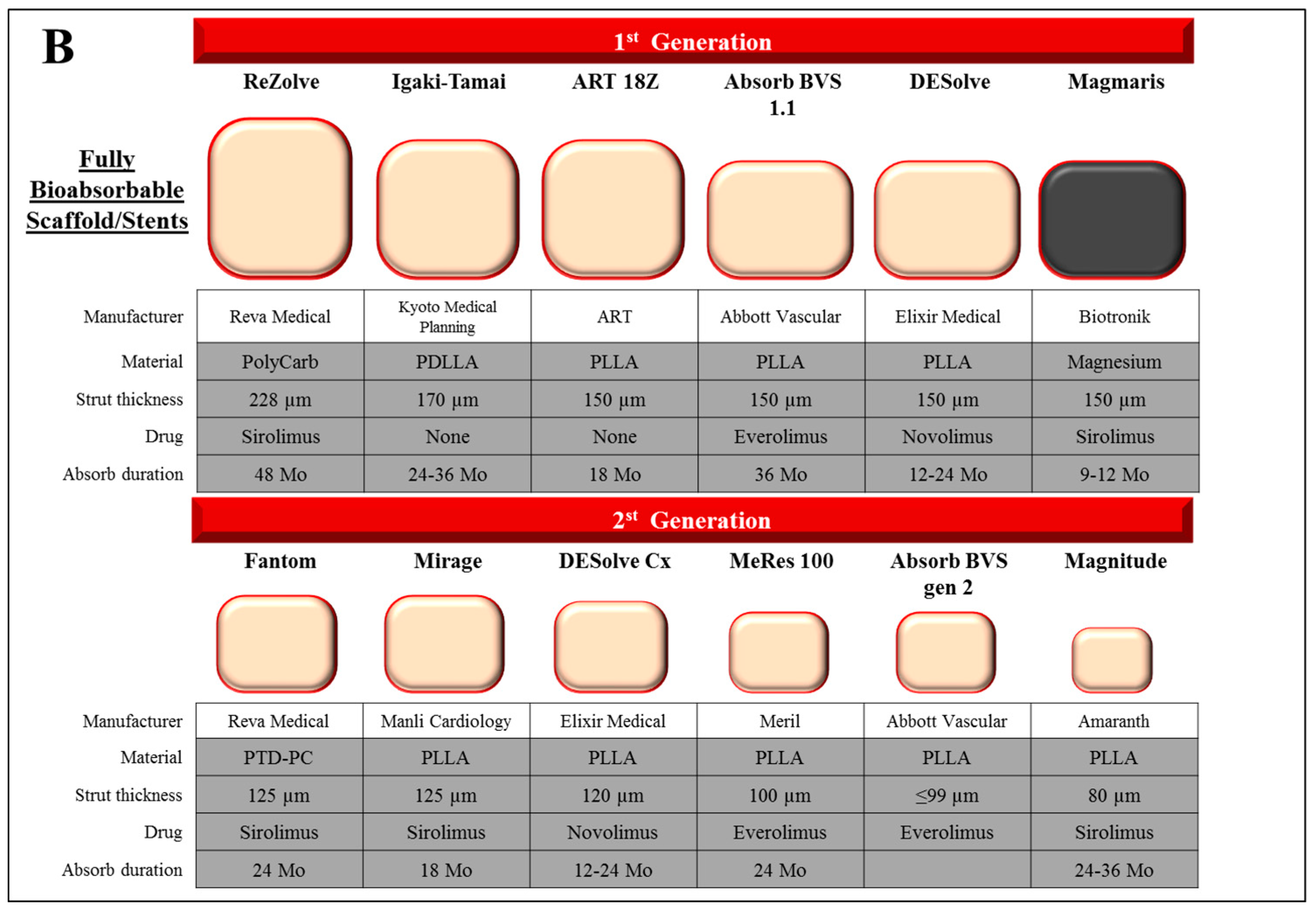
5. Bioabsorbable Scaffold and Stent
6. Polymer Scaffold
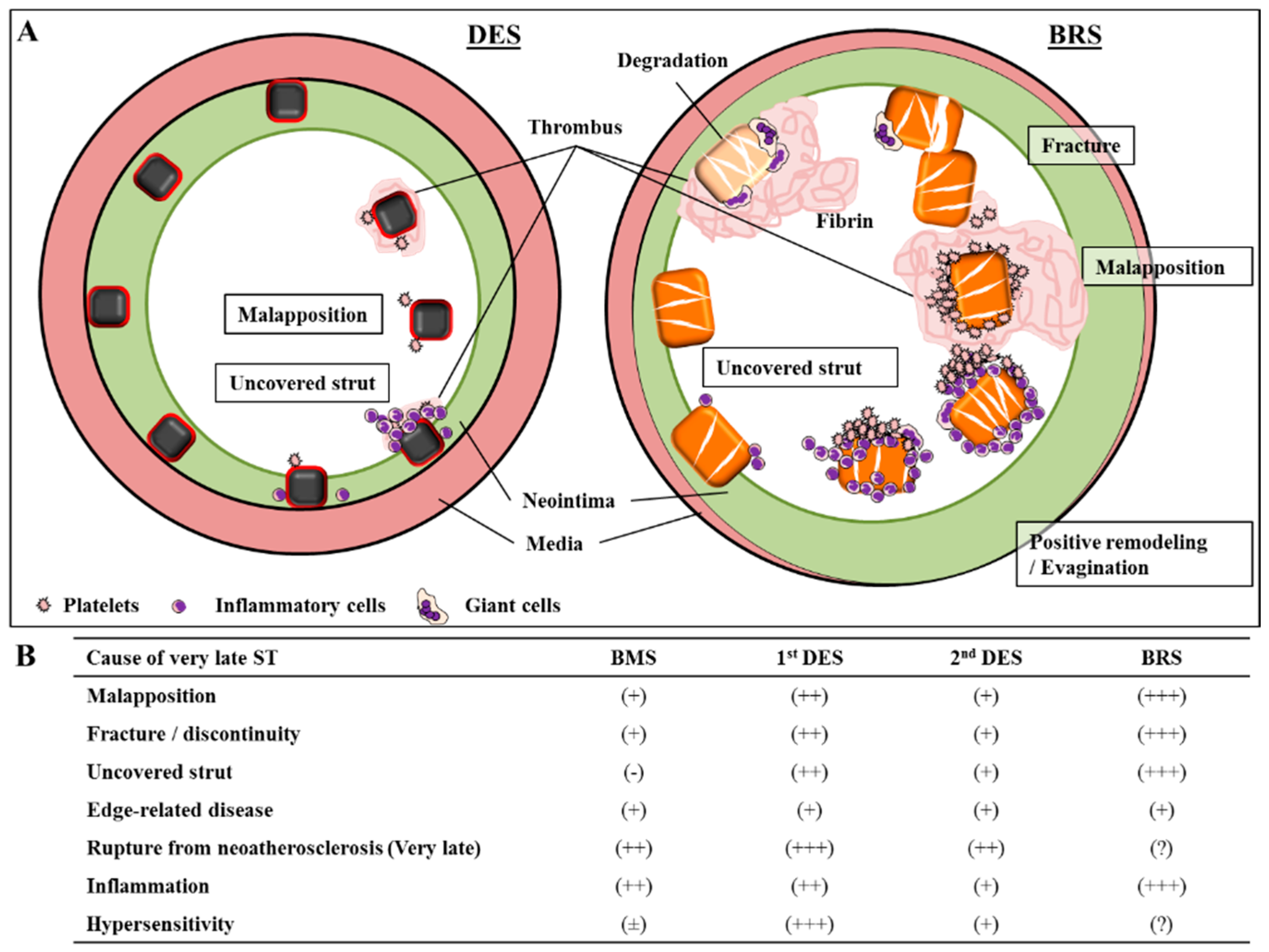
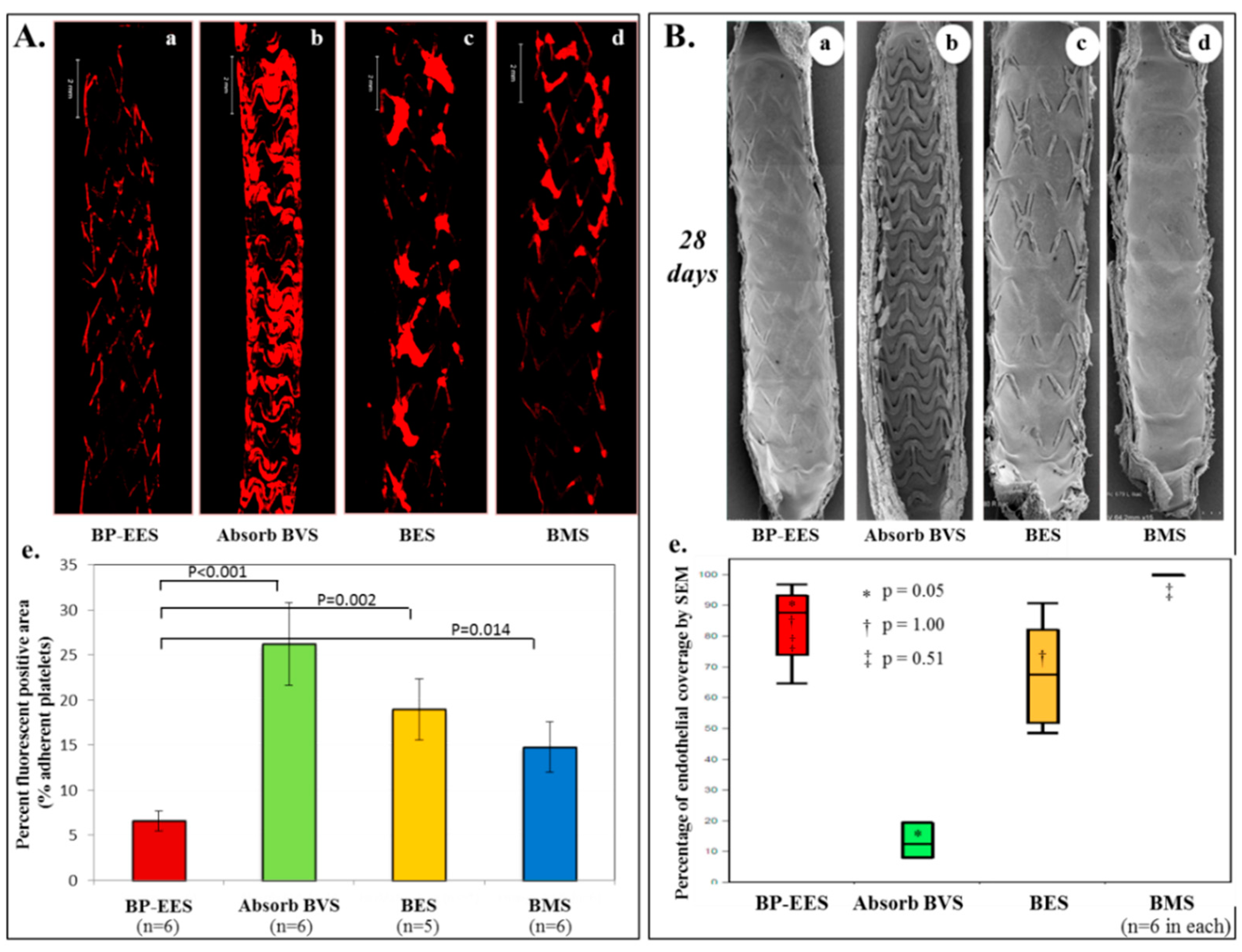
7. Absorb-BVS: A Major Concern of Scaffold Thrombosis from Pathologic Viewpoint
8. Vascular Response to Absorb-BVS
9. Bioerodible Metallic Alloy
10. Future Perspectives
11. Disclosure
Author Contributions
Funding
Conflicts of Interest
References
- Serruys, P.W.; de Jaegere, P.; Kiemeneij, F.; Macaya, C.; Rutsch, W.; Heyndrickx, G.; Emanuelsson, H.; Marco, J.; Legrand, V.; Materne, P.; et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N. Engl. J. Med. 1994, 331, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Fischman, D.L.; Leon, M.B.; Baim, D.S.; Schatz, R.A.; Savage, M.P.; Penn, I.; Detre, K.; Veltri, L.; Ricci, D.; Nobuyoshi, M.; et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N. Engl. J. Med. 1994, 331, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Foin, N.; Lee, R.D.; Torii, R.; Guitierrez-Chico, J.L.; Mattesini, A.; Nijjer, S.; Sen, S.; Petraco, R.; Davies, J.E.; Mario, C.D.; et al. Impact of stent strut design in metallic stents and biodegradable scaffolds. Int. J. Cardiol. 2014, 177, 800–808. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.J.; Stinson, J.S.; Larsen, S.R.; Eppihimer, M.J.; Carroll, W.M. A platinum-chromium steel for cardiovascular stents. Biomaterials 2010, 31, 3755–3761. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Litvack, F.; Dev, V.; Fishbein, M.C.; Forrester, J.S.; Eigler, N. Subacute thrombosis and vascular injury resulting from slotted-tube nitinol and stainless steel stents in a rabbit carotid artery model. Circulation 1996, 94, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Köster, R.; Vieluf, D.; Kiehn, M.; Sommerauer, M.; Kähler, J.; Baldus, S.; Meinertz, T.; Hamm, C.W. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet 2000, 356, 1895–1897. [Google Scholar] [CrossRef]
- Mori, H.; Kutys, R.; Romero, M.; Virmani, R.; Finn, A.V. Metallic coronary stents: Is there a relationship between stent fracture and hypersensitivity? JACC Cardiovasc. Interv. 2017, 10, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Sciahbasi, A.; Pendenza, G.; Golino, L.; Romagnoli, E.; Caferri, G.; Patrizi, R.; Summaria, F.; Serra, F.; Giannico, M.B.; Bruno, E.; et al. Closed versus open cell stent for high-risk percutaneous coronary interventions in ST-elevation acute myocardial infarction: The closed versus open cells stent for high risk percutaneous coronary Interventions in ST-Elevation acute myocardial infarction (COCHISE) pilot study. Am. Heart J. 2013, 165, 415–420. [Google Scholar] [PubMed]
- Bosiers, M.; de Donato, G.; Deloose, K.; Verbist, J.; Peeters, P.; Castriota, F.; Cremonesi, A.; Setacci, C. Does free cell area influence the outcome in carotid artery stenting? Eur. J. Vasc. Endovasc. Surg. 2007, 33, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, A.; Mehilli, J.; Dirschinger, J.; Dotzer, F.; Schühlen, H.; Neumann, F.J.; Fleckenstein, M.; Pfafferott, C.; Seyfarth, M.; Schömig, A. Intracoronary stenting and angiographic results: Strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 2001, 103, 2816–2821. [Google Scholar] [CrossRef] [PubMed]
- Farb, A.; Weber, D.K.; Kolodgie, F.D.; Burke, A.P.; Virmani, R. Morphological predictors of restenosis after coronary stenting in humans. Circulation 2002, 105, 2974–2980. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Yahagi, K.; Otsuka, F.; Sakakura, K.; Finn, A.V.; Kutys, R.; Ladich, E.; Fowler, D.R.; Joner, M.; Virmani, R. Causes of early stent thrombosis in patients presenting with acute coronary syndrome: An ex vivo human autopsy study. J. Am. Coll. Cardiol. 2014, 63, 2510–2520. [Google Scholar] [CrossRef] [PubMed]
- Kolandaivelu, K.; Swaminathan, R.; Gibson, W.J.; Kolachalama, V.B.; Nguyen-Ehrenreich, K.L.; Giddings, V.L.; Coleman, L.; Wong, G.K.; Edelman, E.R. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 2011, 123, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.M.; Davies, P.F. Hemodynamically driven stent strut design. Ann. Biomed. Eng. 2009, 37, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Palmaz, J.C.; Bailey, S.; Marton, D.; Sprague, E. Influence of stent design and material composition on procedure outcome. J. Vasc Surg. 2002, 36, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Schömig, A.; Dibra, A.; Windecker, S.; Mehilli, J.; de Lezo, J.S.; Kaiser, C.; Park, S.J.; Goy, J.J.; Lee, J.H.; Lorenzo, E.D.; et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J. Am. Coll. Cardiol. 2007, 50, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Rizas, K.D.; Mehilli, J. Stent polymers: Do they make a difference? Circ. Cardiovasc. Interv. 2016, 9, e002943. [Google Scholar] [CrossRef] [PubMed]
- Curcio, A.; Torella, D.; Cuda, G.; Coppola, C.; Faniello, M.C.; Achille, F.; Russo, V.G.; Chiariello, M.; Indolfi, C. Effect of stent coating alone on in vitro vascular smooth muscle cell proliferation and apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Otsuka, F.; Yahagi, K.; Sakakura, K.; Kutys, R.; Ladich, E.R.; Finn, A.V.; Kolodgie, F.D.; Virmani1, R. Human autopsy study of drug-eluting stents restenosis: Histomorphological predictors and neointimal characteristics. Eur. Heart J. 2013, 34, 3304–3313. [Google Scholar] [CrossRef] [PubMed]
- Camenzind, E.; Steg, P.G.; Wijns, W. Stent thrombosis late after implantation of first-generation drug-eluting stents: A cause for concern. Circulation 2007, 115, 1440–1455. [Google Scholar] [CrossRef] [PubMed]
- Lagerqvist, B.; James, S.K.; Stenestrand, U.; Lindbäck, J.; Nilsson, T.; Wallentin, L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. New Engl. J. Med. 2007, 356, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Galløe, A.M.; Kelbæk, H.; Thuesen, L.; Hansen, H.S.; Ravkilde, J.; Hansen, P.R.; Christiansen, E.H.; Abildgaard, U.; Stephansen, G.B.; Lassen, J.F.; et al. 10-Year Clinical Outcome After Randomization to Treatment by Sirolimus- or Paclitaxel-Eluting Coronary Stents. J. Am. Coll. Cardiol. 2017, 69, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kopia, G.; Hayashi, S.; Bailey, L.R.; Llanos, G.; Wilensky, R.; Klugherz, B.D.; Papandreou, G.; Narayan, P.; Leon, M.B.; et al. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation 2001, 104, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Heldman, A.W.; Cheng, L.; Jenkins, G.M.; Heller, P.F.; Kim, D.W.; Ware, M.J.; Nater, C.; Hruban, R.H.; Rezai, B.; Abella, B.S.; et al. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation 2001, 103, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Farb, A.; Heller, P.F.; Shroff, S.; Cheng, L.; Kolodgie, F.D.; Carter, A.J.; Scott, D.S.; Froehlich, J.; Virmani, R. Pathological analysis of local delivery of paclitaxel via a polymer-coated stent. Circulation 2001, 104, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Drachman, D.E.; Edelman, E.R.; Seifert, P.; Groothuis, A.R.; Bornstein, D.A.; Kamath, K.R.; Palasis, M.; Yang, D.; Nott, S.H.; Rogers, C. Neointimal thickening after stent delivery of paclitaxel: Change in composition and arrest of growth over six months. J. Am. Coll. Cardiol. 2000, 36, 2325–2332. [Google Scholar] [CrossRef]
- Nakazawa, G.; Finn, A.V.; Vorpahl, M.; Ladich, E.R.; Kolodgie, F.D.; Virmani, R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J. Am. Coll. Cardiol. 2011, 57, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, G.; Finn, A.V.; Ladich, E.; Ribichini, F.; Coleman, L.; Kolodgie, F.D.; Virmani, R. Drug-eluting stent safety: Findings from preclinical studies. Expert Rev. Cardiovasc. Ther. 2008, 6, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Joner, M.; Nakazawa, G.; Kolodgie, F.; Newell, J.; John, M.C.; Gold, H.K.; Virmani, R. Pathological correlates of late drug-eluting stent thrombosis: Strut coverage as a marker of endothelialization. Circulation 2007, 115, 2435–2441. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Byrne, R.A.; Simunovic, I.; King, L.A.; Cassese, S.; Joner, M.; Fusaro, M.; Schneider, S.; Schulz, S.; Ibrahim, T.; et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: Results from a registry of 18,334 patients. JACC Cardiovasc. Interv. 2013, 6, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Dundon, B.K.; Puri, R.; Yeend, R.A.S. Very late stent fracture associated with a sirolimus-eluting stent. Heart Lung Circ. 2008, 17, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Shite, J.; Matsumoto, D.; Yokoyama, M. Sirolimus-eluting stent fracture with thrombus, visualization by optical coherence tomography. Eur. Heart J. 2006, 27, 1389. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, G.; Finn, A.V.; Vorpahl, M.; Ladich, E.; Kutys, R.; Balazs, I.; Kolodgie, F.D.; Virmani, R. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J. Am. Coll. Cardiol. 2009, 54, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, G.; Vorpahl, M.; Finn, A.V.; Narula, J.; Virmani, R. One step forward and two steps back with drug-eluting-stents: From preventing restenosis to causing late thrombosis and nouveau atherosclerosis. JACC Cardiovasc. Imaging 2009, 2, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.; Karmali, V.; Polavarapu, R.; Akahori, H.; Cheng, Q.; Pachura, K.; Kolodgie, F.D.; Finn, A.V. Sirolimus-FKBP12.6 impairs endothelial barrier function through protein kinase C-alpha activation and disruption of the p120-vascular endothelial cadherin interaction. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2425–2431. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Orsenigo, F.; Lampugnani, M.G. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008, 121, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Harari, E.; Guo, L.; Smith, S.L.; Paek, K.H.; Fernandez, R.; Sakamoto, A.; Mori, H.; Kutyna, M.D.; Habib, A.; Torii, S.; et al. Direct targeting of the mTOR (mammalian target of rapamycin) kinase improves endothelial permeability in drug-eluting stents. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Cheng, Q.; Lutter, C.; Smith, S.; Guo, L.; Kutyna, M.; Torii, S.; Harari, E.; Acampado, E.; Joner, M.; et al. Endothelial barrier protein expression in biodegradable polymer sirolimus-eluting versus durable polymer everolimus-eluting metallic stents. JACC Cardiovasc. Interv. 2017, 10, 2375–2387. [Google Scholar] [CrossRef] [PubMed]
- El-Hayek, G.; Bangalore, S.; Casso Dominguez, A.; Devireddy, C.; Jaber, W.; Kumar, G.; Mavromatis, K.; Tamis-Holland, J.; Samady, H. Meta-Analysis of Randomized Clinical Trials Comparing Biodegradable Polymer Drug-Eluting Stent to Second-Generation Durable Polymer Drug-Eluting Stents. JACC Cardiovasc. Interv. 2017, 10, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Kok, M.M.; Zocca, P.; Buiten, R.A.; Danse, P.W.; Schotborgh, C.E.; Scholte, M.; Hartmann, M.; Stoel, M.G.; van Houwelingen, G.; Linssen, G.C.M.; et al. Two-year clinical outcome of all-comers treated with three highly dissimilar contemporary coronary drug-eluting stents in the randomised BIO-RESORT trial. EuroIntervention 2018. [Google Scholar] [CrossRef]
- Lefèvre, T.; Haude, M.; Neumann, F.J.; Stangl, K.; Skurk, C.; Slagboom, T.; Sabaté, M.; Goicolea, J.; Barragan, P.; Cook, S.; et al. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: 5-Year outcomes of the randomized BIOFLOW-II trial. JACC Cardiovasc. Interv. 2018, 11, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Bønaa, K.H.; Mannsverk, J.; Wiseth, R.; Aaberge, L.; Myreng, Y.; Nygård, O.; Nilsen, D.W.; Kløw, N.E.; Uchto, M.; Trovik, T.; et al. Drug-eluting or bare-metal stents for coronary artery disease. New Engl. J. Med. 2016, 375, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yaszemski, M.J.; Yasko, A.W.; Engel, P.S.; Mikos, A.G. Injectable biodegradable polymer composites based on poly(propylene fumarate) crosslinked with poly(ethylene glycol)-dimethacrylate. Biomaterials 2000, 21, 2389–2394. [Google Scholar] [CrossRef]
- Coleman, M.M.; Painter, P.C. Fundamentals of Polymer Science: An Introductory Text, 2nd ed.; Technomic: Lancaster, PA, USA, 2000. [Google Scholar]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Alexy, R.D.; Levi, D.S. Materials and manufacturing technologies available for production of a pediatric bioabsorbable stent. BioMed Res. Int. 2013, 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Serruys, P.W. Bioresorbable scaffold: The advent of a new era in percutaneous coronary and peripheral revascularization? Circulation 2011, 123, 779–797. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.G.; Kereiakes, D.J.; Christopher Metzger, D.; Caputo, R.P.; Rizik, D.G.; Teirstein, P.S.; Litt, M.R.; Kini, A.; Kabour, A.; Marx, S.O.; et al. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. New Engl. J. Med. 2015, 373, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Wykrzykowska, J.J.; Kraak, R.P.; Hofma, S.H.; van der Schaaf, R.J.; Arkenbout, E.K.; IJsselmuiden, A.J.; Elias, J.; van Dongen, I.M.; Tijssen, R.Y.G.; Koch, K.T.; et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. New Engl. J. Med. 2017, 376, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; Serruys, P.W.; Kimura, T.; Gao, R.; Ellis, S.G.; Kereiakes, D.J.; Onuma, Y.; Simonton, C.; Zhang, Z.; Stone, G.W.; et al. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: A systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet 2017, 390, 760–772. [Google Scholar] [CrossRef]
- Puricel, S.; Cuculi, F.; Weissner, M.; Schmermund, A.; Jamshidi, P.; Nyffenegger, T.; Binder, H.; Eggebrecht, H.; Münzel, T.; Cook, S.; et al. Bioresorbable Coronary Scaffold Thrombosis: Multicenter Comprehensive Analysis of Clinical Presentation, Mechanisms, and Predictors. J. Am. Coll. Cardiol. 2016, 67, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.G.; Steffenino, G.; Kereiakes, D.J.; Stone, G.W.; van Geuns, R.J.; Abizaid, A.; Nef, H.; Cortese, B.; Testa, L.; Menichelli, M.; et al. Clinical, angiographic, and procedural correlates of acute, subacute, and late absorb scaffold thrombosis. JACC Cardiovasc. Interv. 2017, 10, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Ueki, Y.; Souteyrand, G.; Daemen, J.; Wiebe, J.; Nef, H.; Adriaenssens, T.; Loh, J.P.; Lattuca, B.; Wykrzykowska, J.J.; et al. Mechanisms of very late bioresorbable scaffold thrombosis: The INVEST registry. J. Am. Coll. Cardiol. 2017, 70, 2330–2344. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Virmani, R.; Finn, A.V. Bioresorbable vascular scaffolds: Implication of very late scaffold thrombosis. Coron. Artery Dis. 2017, 28, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Serruys, P.W.; Muramatsu, T.; Nakatani, S.; van Geuns, R.J.; de Bruyne, B.; Dudek, D.; Christiansen, E.; Smits, P.C.; Chevalier, B.; McClean, D.; Koolen, J.; Windecker, S.; et al. Incidence and imaging outcomes of acute scaffold disruption and late structural discontinuity after implantation of the absorb everolimus-eluting fully bioresorbable vascular scaffold: Optical coherence tomography assessment in the ABSORB cohort B trial (A clinical evaluation of the bioabsorbable everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc. Interv. 2014, 7, 1400–1411. [Google Scholar] [PubMed]
- Sotomi, Y.; Suwannasom, P.; Serruys, P.W.; Onuma, Y. Possible mechanical causes of scaffold thrombosis: Insights from case reports with intracoronary imaging. EuroIntervention 2017, 12, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Koppara, T.; Cheng, Q.; Yahagi, K.; Mori, H.; Sanchez, O.D.; Feygin, J.; Wittchow, E.; Kolodgie, F.D.; Virmani, R.; Joner, M. Thrombogenicity and early vascular healing response in metallic biodegradable polymer-based and fully bioabsorbable drug-eluting stents. Circ. Cardiovasc. Interv. 2015, 8, e002427. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Franchini, M.; Targher, G. Arterial thrombus formation in cardiovascular disease. Nat. Rev. Cardiol. 2011, 8, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Toschi, V.; Gallo, R.; Lettino, M.; Fallon, J.T.; Gertz, S.D.; Fernández-Ortiz, A.; Chesebro, J.H.; Badimon, L.; Nemerson, Y.; Fuster, V.; et al. Tissue factor modulates the thrombogenicity of human atherosclerotic plaques. Circulation 1997, 95, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Sotomi, Y.; Shiomi, H.; Ozaki, Y.; Namiki, A.; Yasuda, S.; Ueno, T.; Ando, K.; Furuya, J.; Igarashi, K.; et al. Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent: Insights from the randomised ABSORB Japan trial. EuroIntervention 2016, 12, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Gori, T.; Jansen, T.; Weissner, M.; Foin, N.; Wenzel, P.; Schulz, E.; Cook, S.; Münzel, T. Coronary evaginations and peri-scaffold aneurysms following implantation of bioresorbable scaffolds: Incidence, outcome, and optical coherence tomography analysis of possible mechanisms. Eur. Heart J. 2016, 37, 2040–2049. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, N.; Shishido, K.; Tanaka, Y.; Yokota, S.; Hayashi, T.; Miyashita, H.; Koike, T.; Yokoyama, H.; Takada, T.; Nishimoto, T.; et al. Neoatherosclerosis 5 years after bioresorbable vascular scaffold implantation. J. Am. Coll. Cardiol. 2018, 71, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Halcox, J.P.; Schenke, W.H.; Zalos, G.; Mincemoyer, R.; Prasad, A.; Waclawiw, M.A.; Nour, K.R.; Quyyumi, A.A. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002, 106, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Onuma, Y.; Dudek, D.; Smits, P.C.; Koolen, J.; Chevalier, B.; de Bruyne, B.; Thuesen, L.; McClean, D.; van Geuns, R.J.; et al. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis: 12-month clinical and imaging outcomes. J. Am. Coll. Cardiol. 2011, 58, 1578–1588. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Ormiston, J.A.; Onuma, Y.; Regar, E.; Gonzalo, N.; Garcia-Garcia, H.M.; Nieman, K.; Bruining, N.; Dorange, C.; Miquel-Hébert, K.; et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 2009, 373, 897–910. [Google Scholar] [CrossRef]
- Brugaletta, S.; Heo, J.H.; Garcia-Garcia, H.M.; Farooq, V.; van Geuns, R.J.; de Bruyne, B.; Dudek, D.; Smits, P.C.; Koolen, J.; McClean, D.; et al. Endothelial-dependent vasomotion in a coronary segment treated by ABSORB everolimus-eluting bioresorbable vascular scaffold system is related to plaque composition at the time of bioresorption of the polymer: Indirect finding of vascular reparative therapy? Eur. Heart J. 2012, 33, 1325–1333. [Google Scholar] [PubMed]
- Serruys, P.W.; Chevalier, B.; Sotomi, Y.; Cequier, A.; Carrié, D.; Piek, J.J.; Van Boven, A.J.; Dominici, M.; Dudek, D.; McClean, D.; et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): A 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016, 388, 2479–2491. [Google Scholar] [CrossRef]
- Chevalier, B.; Cequier, A.; Dudek, D.; Haude, M.; Carrie, D.; Sabaté, M.; Windecker, S.; Reith, S.; de Sousa Almeida, M.; Campo, G. Four-year follow-up of the randomised comparison between an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II trial). EuroIntervention 2018, 13, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lara, J.; Brugaletta, S.; Ortega-Paz, L.; Vandeloo, B.; Moscarella, E.; Salas, M.; Romaguera, R.; Roura, G.; Ferreiro, J.L.; Teruel, L.; Gracida, M.; et al. Long-term coronary functional assessment of the infarct-related artery treated with everolimus-eluting bioresorbable scaffolds or everolimus-eluting metallic stents: insights of the TROFI II trial. JACC Cardiovasc. Interv. 2018, 11, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Rapetto, C.; Leoncini, M. Magmaris: A new generation metallic sirolimus-eluting fully bioresorbable scaffold: Present status and future perspectives. J. Thorac. Dis. 2017, 9, S903–S913. [Google Scholar] [CrossRef] [PubMed]
- Joner, M.; Ruppelt, P.; Zumstein, P.; Lapointe-Corriveau, C.; Leclerc, G.; Bulin, A.; Castellanos, M.I.; Wittchow, E.; Haude, M.; Waksman, R. Preclinical evaluation of degradation kinetics and elemental mapping of first and second generation bioresorbable magnesium scaffolds. EuroIntervention 2018. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; von Birgelen, C.; Christiansen, E.H.; Wijns, W.; Neumann, F.J.; Kaiser, C.; et al. Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. Eur. Heart J. 2016, 37, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Lipinski, M.J.; Acampado, E.; Cheng, Q.; Adams, L.; Torii, S.; Gai, J.; Torguson, R.; Hellinga, D.M.; Westman, P.C.; et al. Comparison of acute thrombogenicity for metallic and polymeric bioabsorbable scaffolds: magmaris versus absorb in a porcine arteriovenous shunt model. Circ. Cardiovasc. Interv. 2017, 10, e004762. [Google Scholar] [CrossRef] [PubMed]
| Materials | Fe | Co | Cr | Pt | Ni | W | Mo | Mn | Ti | Mg | Ir |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 316L SS | 63 | - | 18 | - | 14 | - | 2.6 | <2.0 | - | - | - |
| CoCr (L605) | 3 | 52 | 20 | - | 10 | 15 | - | 1.5 | - | - | - |
| CoCr (MP-35N) | <1.0 | 34 | 20 | - | 35 | - | 9.75 | <0.15 | <1.0 | - | - |
| PtCr | 37 | - | 18 | 33 | 9 | - | 2.6 | <0.05 | - | - | - |
| Titanium | - | - | - | - | - | - | - | - | 90–100 | - | - |
| Nitinol | - | - | - | - | 55 | - | - | - | 45 | - | - |
| Mgalloy | - | - | - | - | - | - | - | - | - | 93.6 | - |
| Pure iron | 99.8 | - | - | - | - | - | - | - | - | - | - |
| PtIr (90Pt/10Ir) | <0.015 | - | - | 90 | - | - | - | - | - | - | 9.5–10.5 |
| Materials | Density (g/cm3) | Elastic Young’s Modulus (Gpa) | Tensile Strength (Mpa) | Elongation at Break (%) | Corrosion Resistance | Visibility | Biocompatibility | Low Recoil | Biodegradability (Months) |
|---|---|---|---|---|---|---|---|---|---|
| 316L SS | 8 | 193 | 670 | 48 | + | + | + | + | − |
| CoCr (L-605) | 9.1 | 243 | >1000 | >50 | + | + | + | + | − |
| CoCr (MP-35N) | 8.43 | 233 | 930 | 45–60 | + | + | + | + | − |
| PtCr | 9.9 | 203 | 834 | 45 | + | + + | + | + | − |
| Nitinol | 6.45 | 40 | 800–1200 | 12–25 | + | + | + | + | − |
| Pure iron | 7.8 | 150 | 210 | 40 | − | + | + − | + | >12 |
| Fe-35MN | 7.6 | 235 | 530 | 32 | − | + | − | n/a | >12 |
| Mg (WE43) | 1.83 | 40–130 | 280 | 6.8 | − | + | + − | + | 1–3 |
| PLLA | 1.2–1.4 | 2.7–4.0 | 40–65 | 2–6 | n/a | − | + | + − | 18–36 |
| PDLA | 1.8 | 1.0–3.5 | 40–55 | 2–6 | n/a | − | + | + − | 12–16 |
| PGA | 1.5 | 6.0–7.0 | 90–110 | 1–2 | n/a | − | + | + − | 4–6 |
| PCL | 1.1 | 0.2–0.4 | 25–35 | >300 | n/a | − | + | + − | 24–36 |
| PLGA (85 L/15 G) | 1.3 | 2.0–4.0 | 40–70 | 2–6 | n/a | − | + | + − | 12–18 |
| PDLGA (50 DL/50G) | 1.2–1.3 | 2.0–4.0 | 40–50 | 1–4 | n/a | − | + | + − | 1–2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, A.; Jinnouchi, H.; Torii, S.; Virmani, R.; Finn, A.V. Understanding the Impact of Stent and Scaffold Material and Strut Design on Coronary Artery Thrombosis from the Basic and Clinical Points of View. Bioengineering 2018, 5, 71. https://doi.org/10.3390/bioengineering5030071
Sakamoto A, Jinnouchi H, Torii S, Virmani R, Finn AV. Understanding the Impact of Stent and Scaffold Material and Strut Design on Coronary Artery Thrombosis from the Basic and Clinical Points of View. Bioengineering. 2018; 5(3):71. https://doi.org/10.3390/bioengineering5030071
Chicago/Turabian StyleSakamoto, Atsushi, Hiroyuki Jinnouchi, Sho Torii, Renu Virmani, and Aloke V. Finn. 2018. "Understanding the Impact of Stent and Scaffold Material and Strut Design on Coronary Artery Thrombosis from the Basic and Clinical Points of View" Bioengineering 5, no. 3: 71. https://doi.org/10.3390/bioengineering5030071
APA StyleSakamoto, A., Jinnouchi, H., Torii, S., Virmani, R., & Finn, A. V. (2018). Understanding the Impact of Stent and Scaffold Material and Strut Design on Coronary Artery Thrombosis from the Basic and Clinical Points of View. Bioengineering, 5(3), 71. https://doi.org/10.3390/bioengineering5030071





