3D Cell Migration Studies for Chemotaxis on Microfluidic-Based Chips: A Comparison between Cardiac and Dermal Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
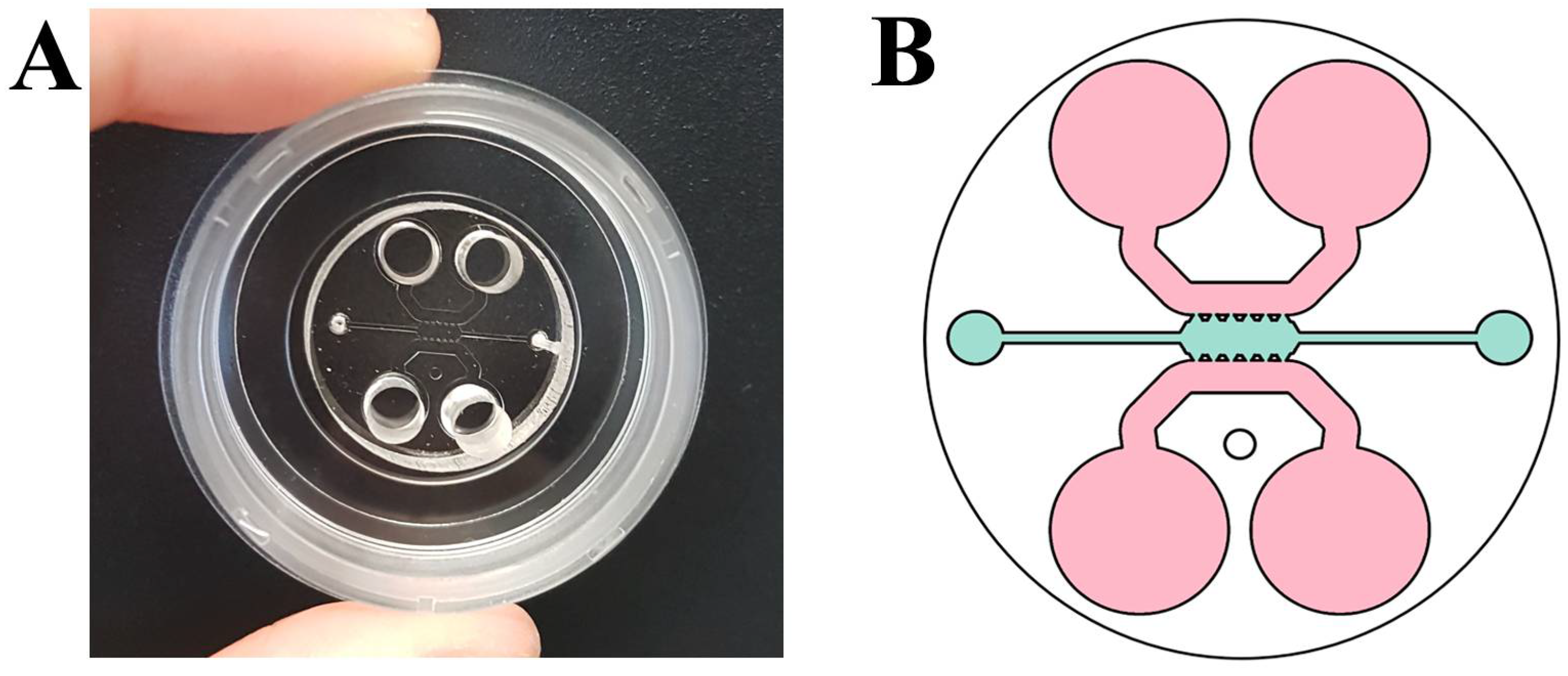
2.2. Fabrication of Microfluidic Devices
2.3. Migration Assay
2.4. Immunofluorescence
3. Results and Discussion
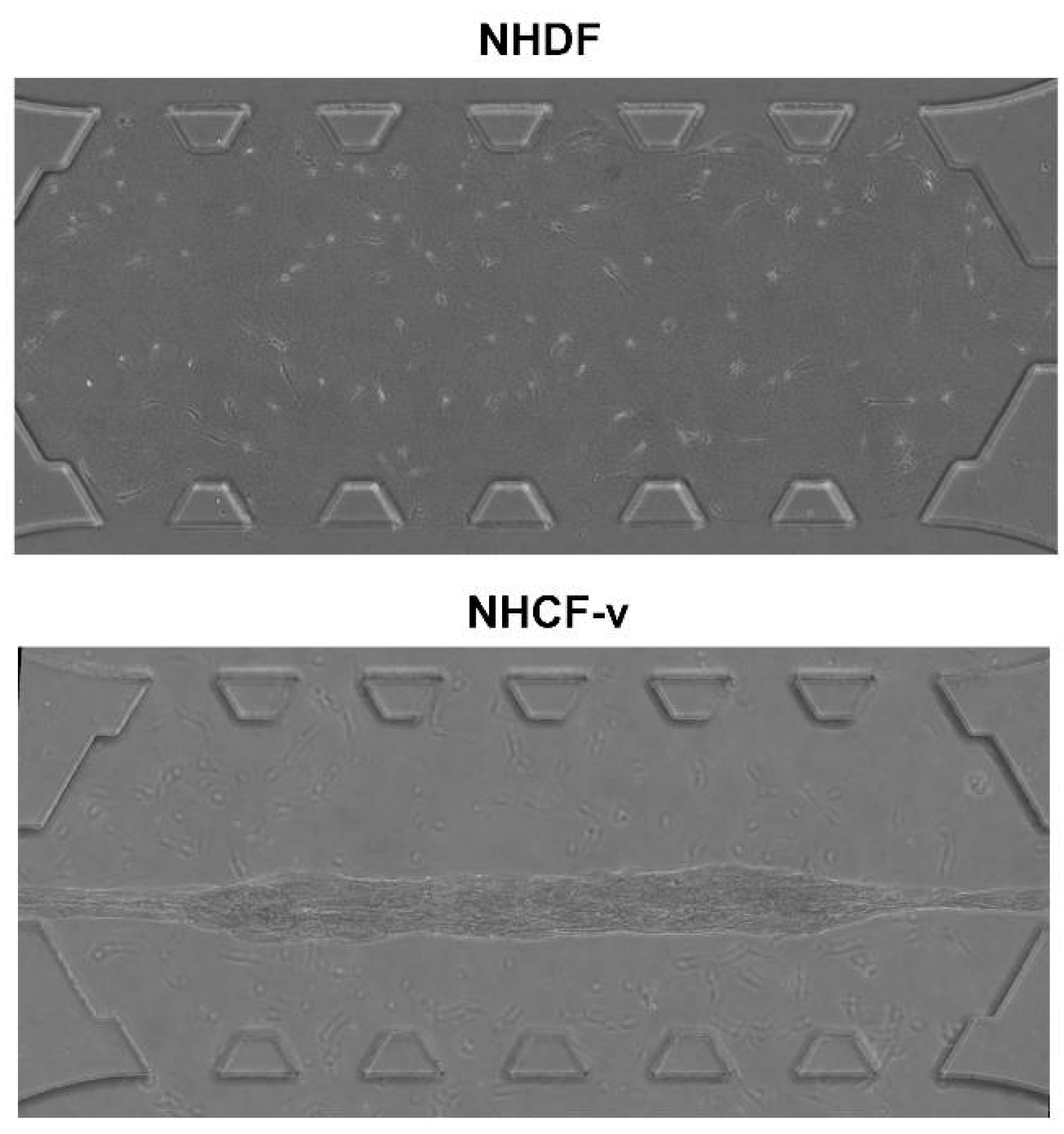
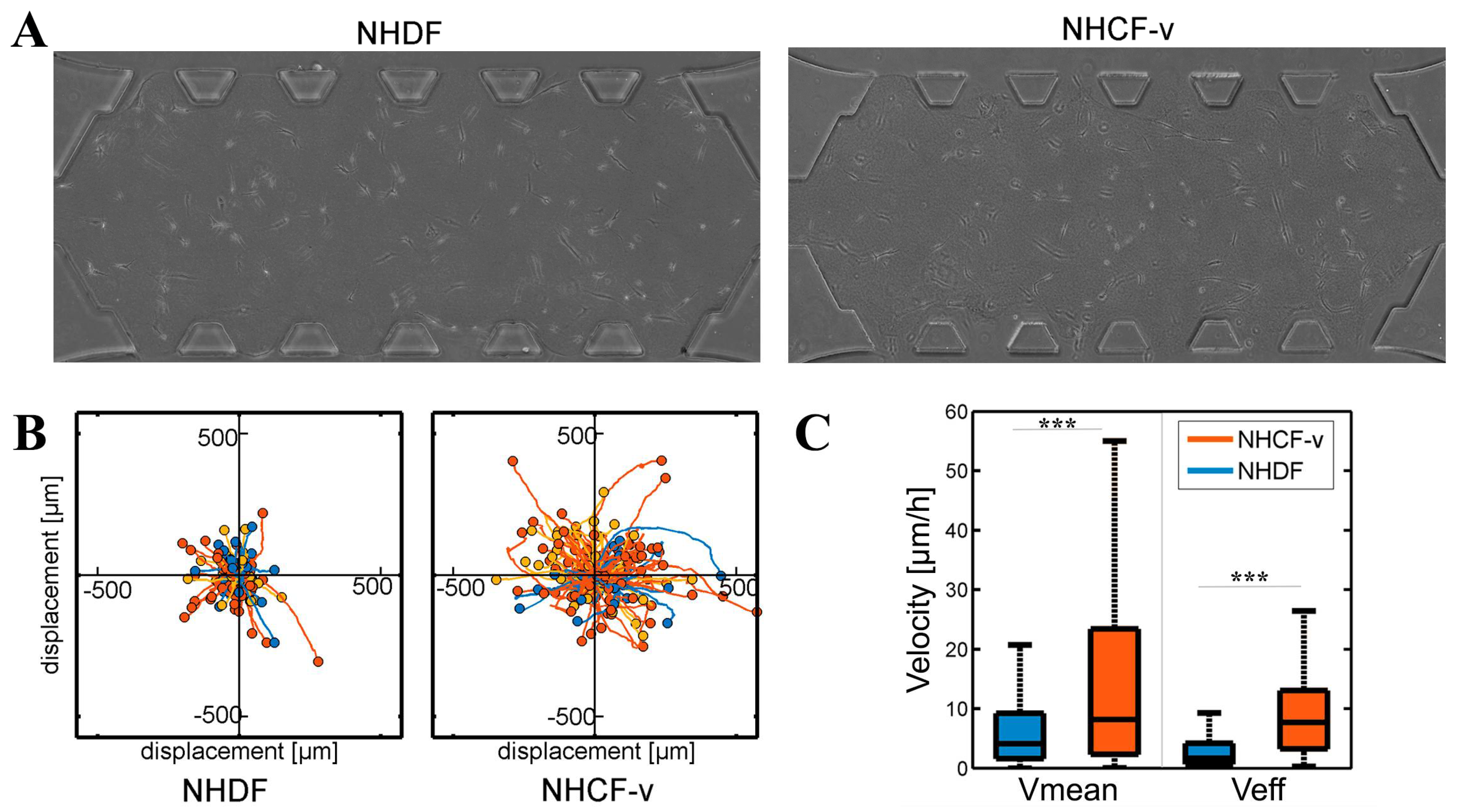
3.1. Cardiac Fibroblasts Migrate at Higher Speeds and a More Directional Pattern than Dermal Fibroblasts
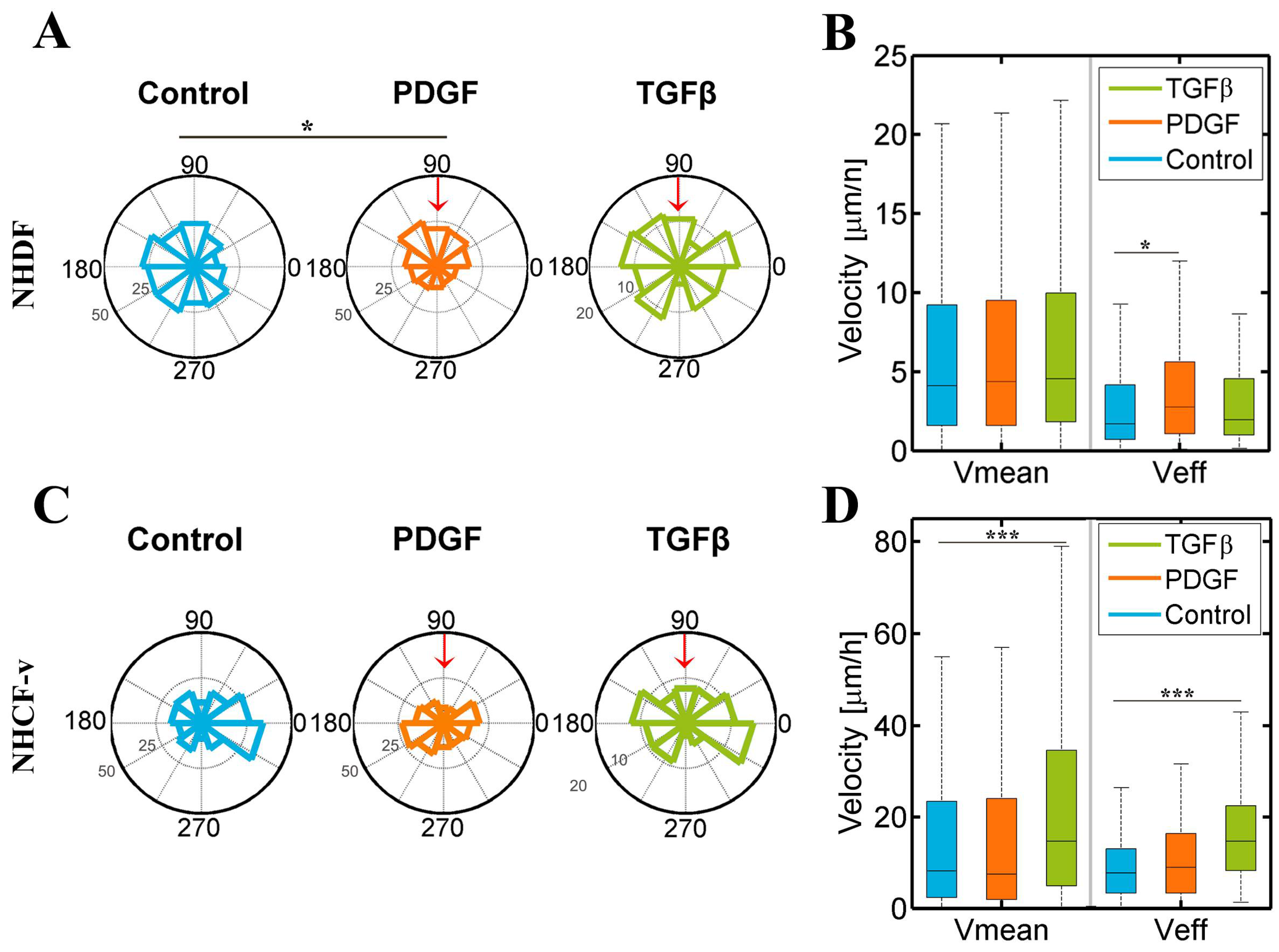
3.2. Dermal Fibroblasts Are Chemoattracted by PDGF-BB, Whereas Cardiac Fibroblasts Respond to TGF-β1, Increasing Their Mean and Effective Velocities
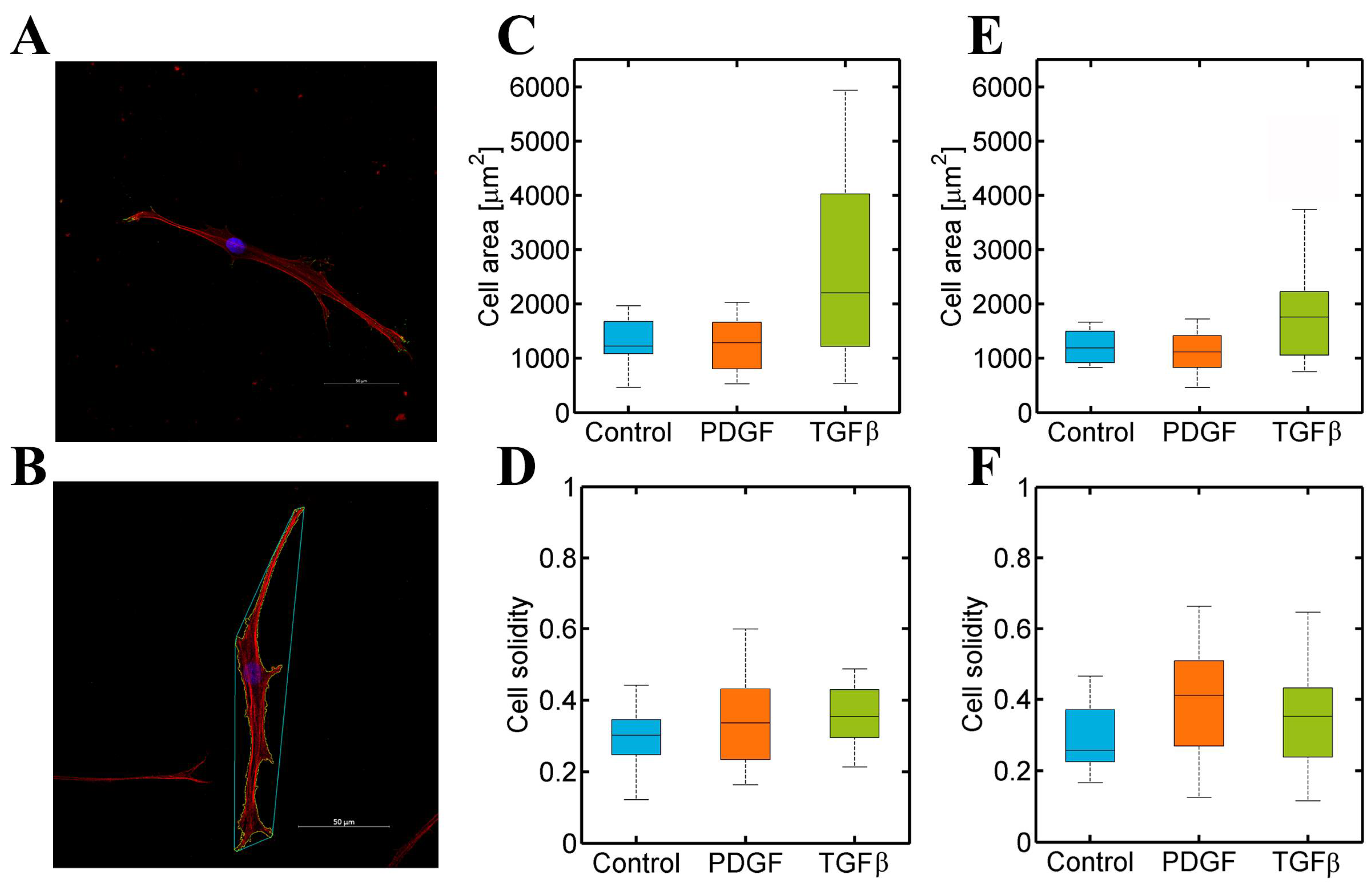
3.3. PDGF-BB and TGF-β1 Do Not Modify Dermal or Cardiac Fibroblast Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| 2D | two dimensions |
| 3D | three dimensions |
| BSA | bovine serum albumin |
| DAPI | 4′,6-diamino-2-fenilindol |
| FBS | fetal bovine serum |
| GA-1000 | gentaminicin sulfate/Amphotericin |
| NHCF-v | normal human cardio fibroblasts from ventricles |
| NHDF | normal human dermal fibroblasts |
| PBS | phosphate-buffered saline |
| PDGF-BB | platelet derived growth factor BB |
| PDL | poly-d-lysine |
| PDMS | polydimethylsiloxane |
| rhFGF-B | Fibroblast Growth Factor basic human recombinant |
| TGF-β1 | transforming growth factor beta 1 |
References
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, P.; Walter, K. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; ISBN 0-8153-3218-1. [Google Scholar]
- Zeisberg, E.; Kalluri, R. Origins of cardiac Fibroblasts. Circ. Res. 2010, 107, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Docherty, G.; Morales, A.; Lopez, J.; Pérez, F. The transition of epithelial cells to fibromioblasts. Mechanisms involved and its possible relationship with renal fibrosis. Nefrologia 2007, 27, 681–688. [Google Scholar] [PubMed]
- Leask, A. TGFβ, cardiac fibroblasts, and the fibrotic response. Cardiovasc. Res. 2007, 74, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, T.; Huang, V.; Chen, Y.; Ahokas, R.; Sun, Y. Platelet-Derived Growth Factor Involvement in Myocardial Remodeling following Infarction. J. Mol. Cell Cardiol. 2011, 51, 830–868. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.; Grobbelaar, A.; Rolfe, K. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burns Trauma 2012, 2, 18–28. [Google Scholar] [PubMed]
- Wound Healing and Growth Factors. Available online: http://emedicine.medscape.com/article/1298196-overview#a3 (accessed on 4 October 2015).
- Lindner, D.; Zietsch, C.; Tank, J.; Sossalla, S.; Fluschnik, N.; Hinrichs, S.; Maier, L.; Poller, W.; Blankenberg, S.; Schultheiss, H.; Tschöpe, C.; Westermann, D. Cardiac fibroblasts support cardiac inflammation in heart failure. Basic Res. Cardiol. 2014, 109, 428. [Google Scholar] [CrossRef] [PubMed]
- Midgley, A.; Rogers, M.; Hallett, M.; Claytonn, A.; Bowen, T.; Phillips, A.; Steadman, R. Transforming Growth Factor-β1 (TGF-β1)-stimulated Fibroblast to Myofibroblast Differentiation Is Mediated by Hyaluronan (HA)-facilitated Epidermal Growth Factor Receptor (EGFR) and CD44 Co-localization in Lipid Rafts. J. Biol. Chem. 2013, 288, 14824–14838. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-H.; Giri, A.; Sun, S. X.; Wirtz, D. Three-dimensional cell migration does not follow a random walk. Proc. Natl. Acad. Sci. USA 2014, 111, 3949–3954. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.; Fulton, A.; Beebe, D. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R. Advantages and challenges of microfluidic cell culture in polydimethysiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Movilla, N.; Borau, C.; Valero, C.; García-Aznar, J. Degradation of extracellular matrix regulates osteoblast migration: A microfluidic-based study. Bone 2017, 107, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, C.; Borau, C.; Movilla, N.; Asín, J.; García-Aznar, J. Quantifying 3D chemotaxis in microfluidic-based chips with step gradients of collagen hydrogel concentrations. Integr. Biol. 2017, 9, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arotzena, O.; Mendoza, G.; Cóndor, M.; Rüberg, T.; García-Aznar, J. Inducing chemotactic and haptotactic cues in microfluidic devices for three-dimensional in vitro assays. Biomicrofluidics 2014, 8, 064211. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Han, S.; Jeon, J.; Yamamoto, K.; Zervantonakis, I.; Sudo, R.; Kamm, R.; Chung, S. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat. Protoc. 2012, 7, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Haeberle, S.; Roth, G.; von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chem. Soc. Rev. 2010, 39, 1153–1182. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.; Weaver, V. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Whisler, J.; Fröse, J.; Yu, C.; Shin, Y.; Kamm, R. On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat. Protoc. 2017, 12, 865–880. [Google Scholar] [CrossRef] [PubMed]
- Sigel, A.; Centrella, M.; Eghbali-Webb, M. Regulation of proliferative response of cardiac fibroblasts by transforming growth factor-beta 1. J. Mol. Cell Cardiol. 1996, 28, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arotzena, O.; Borau, C.; Movilla, N.; Vicente-Manzanares, M.; García-Aznar, J. Fibroblast Migration in 3D is Controlled by Haptotaxis in a Non-muscle Myosin II-Dependent Manner. Ann. Biomed. Eng. 2015, 43, 3025–3039. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, C.; Olivares, O.; Cóndor, M.; Blanco, A.; Santolaria, J.; Asín, J.; Borau, C.; García-Aznar, J. Matrix architecture plays a pivotal role in 3D osteoblast migration: The effect of interstitial fluid flow. J. Mech. Behav. Biomed. Mater. 2018, 83, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Rebouças JS, J.D.S.; Santos-Magalhães, N.S.; Formiga, F.R. Cardiac Regeneration using Growth Factors: Advances and Challenges. Arg. Bras Cardiol 2016, 107, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.; Minasian, R.; Caterson, E. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Zeller, P.; Skalak, T.; Ponce, A.; Prince, R. In vivo chemotactic properties and spatial expression of PDGF in developing mesenteric microvascular networks. Am. J. Physiol. Hear. Circ. Physiol. 2001, 280, H2116–H2125. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Rodríguez, S.; Tomás-González, E.; García-Aznar, J.M. 3D Cell Migration Studies for Chemotaxis on Microfluidic-Based Chips: A Comparison between Cardiac and Dermal Fibroblasts. Bioengineering 2018, 5, 45. https://doi.org/10.3390/bioengineering5020045
Pérez-Rodríguez S, Tomás-González E, García-Aznar JM. 3D Cell Migration Studies for Chemotaxis on Microfluidic-Based Chips: A Comparison between Cardiac and Dermal Fibroblasts. Bioengineering. 2018; 5(2):45. https://doi.org/10.3390/bioengineering5020045
Chicago/Turabian StylePérez-Rodríguez, Sandra, Esther Tomás-González, and José Manuel García-Aznar. 2018. "3D Cell Migration Studies for Chemotaxis on Microfluidic-Based Chips: A Comparison between Cardiac and Dermal Fibroblasts" Bioengineering 5, no. 2: 45. https://doi.org/10.3390/bioengineering5020045
APA StylePérez-Rodríguez, S., Tomás-González, E., & García-Aznar, J. M. (2018). 3D Cell Migration Studies for Chemotaxis on Microfluidic-Based Chips: A Comparison between Cardiac and Dermal Fibroblasts. Bioengineering, 5(2), 45. https://doi.org/10.3390/bioengineering5020045





