Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review
Abstract
1. Introduction
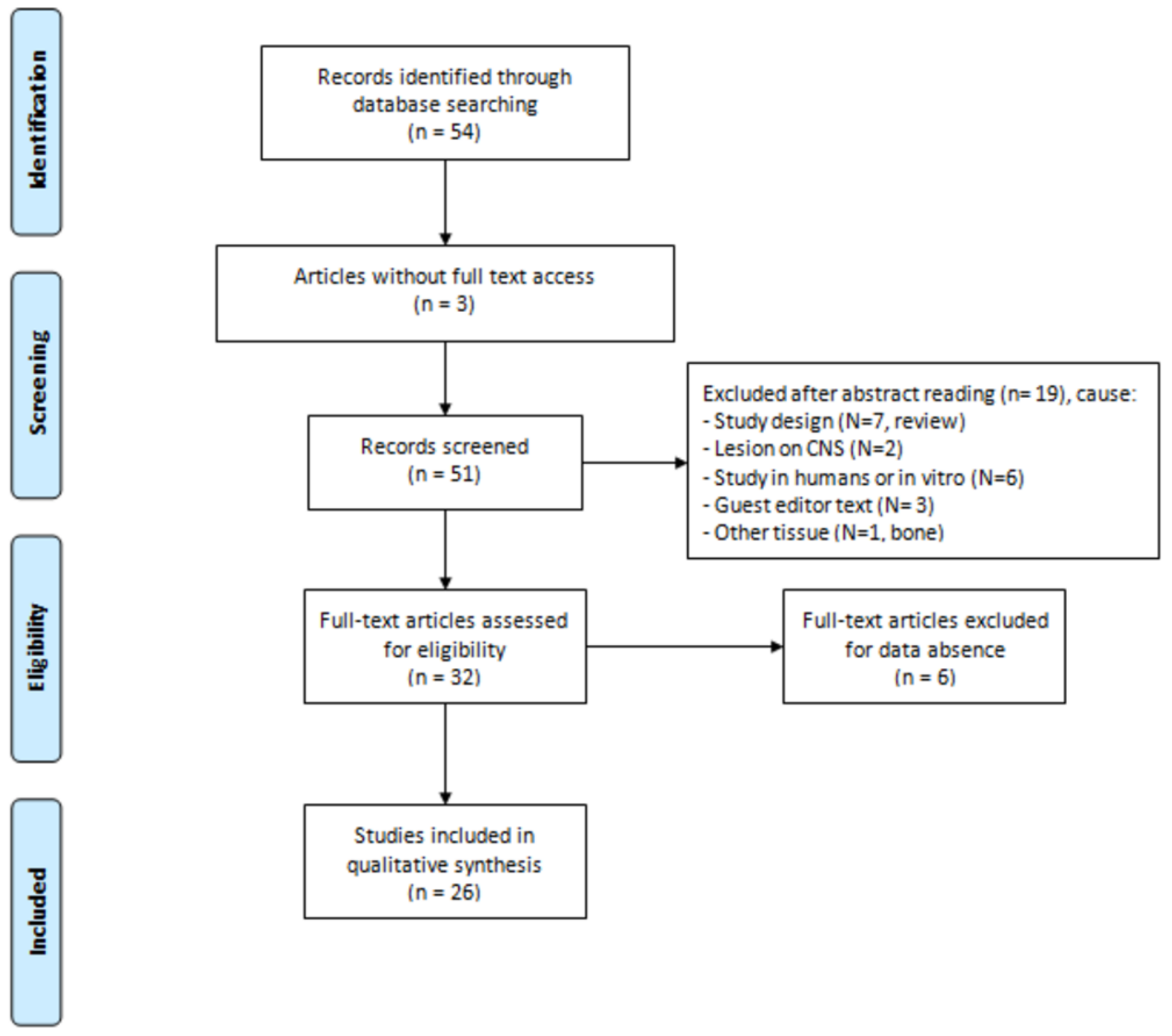
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Conflicts of Interest
References
- Angeletti, P.; Pereira, M.D.; Gomes, H.C.; Hino, C.T.; Ferreira, L.M. Effect of low-level laser therapy (GaAlAs) on bone regeneration in midpalatal anterior suture after surgically assisted rapid maxillary expansion. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, e38–e46. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, R.L.; Andreo, J.C.; Barraviera, B.; Ferreira Junior, R.S.; Buchaim, D.V.; Rosa Junior, G.M.; de Oliveira, A.L.; de Castro Rodrigues, A. Effect of low-level laser therapy (LLLT) on peripheral nerve regeneration using fibrin glue derived from snake venom. Injury 2015, 46, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, D.V.; de Castro Rodrigues, A.; Buchaim, R.L.; Barraviera, B.; Junior, R.S.; Junior, G.M.; Bueno, C.R.; Roque, D.D.; Dias, D.V.; Dare, L.R.; et al. The new heterologous fibrina sealant in combination with low-level laser therapy (LLLT) in the repair of the buccal branch of the facial nerve. Lasers Med. Sci. 2016, 31, 965–972. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Gonçalves, J.B.; Buchaim, D.V.; de Souza Bueno, C.R.; Pomini, K.T.; Barraviera, B.; Júnior, R.S.F.; Andreo, J.C.; de Castro Rodrigues, A.; Cestari, T.M.; Buchaim, R.L. Effects of low-level laser therapy on autogenous bone graft stabilized with a new heterologous fibrin sealant. J. Photochem. Photobiol. B 2016, 162, 663–668. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcellos, L.M.; Barbara, M.A.; Rovai, E.S.; de Oliveira França, M.; Ebrahim, Z.F.; de Vasconcellos, L.G.; Porto, C.D.; Cairo, C.A. Titanium scaffold osteogenesis in healthy and osteoporotic rats is improved by the use of low-level laser therapy (GaAlAs). Lasers Med. Sci. 2016, 31, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, D.V.; Andreo, J.C.; Ferreira Junior, R.S.; Barraviera, B.; Rodrigues, A.C.; Macedo, M.C.; Rosa Junior, G.M.; Shinohara, A.L.; Santos German, I.J.; Pomini, K.T.; et al. Efficacy of Laser Photobiomodulation on Morphological and Functional Repair of the Facial Nerve. Photomed. Laser Surg. 2017, 35, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.P.O.; Rosa Júnior, G.M.; Buchaim, D.V.; German, I.J.S.; Pomini, K.T.; de Souza, R.G.; Pereira, M.; Favaretto Júnior, I.A.; Bueno, C.R.S.; Gonçalves, J.B.O.; et al. Stimulation of morphofunctional repair of the facial nerve with photobiomodulation, using the end-to-side technique or a new heterologous fibrin sealant. J. Photochem. Photobiol. B 2017, 175, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Sulewski, J.G. Historical survey of laser dentistry. Dent. Clin. N. Am. 2000, 44, 717–752. [Google Scholar] [PubMed]
- Ginani, F.; Soares, D.M.; Barreto, M.P.; Barboza, C.A. Effect of low-level laser therapy on mesenchymal stem cell proliferation: A systematic review. Lasers Med. Sci. 2015, 30, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Morries, L.D.; Cassano, P.; Henderson, T.A. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr. Dis. Treat. 2015, 20, 2159–2175. [Google Scholar] [CrossRef]
- Chang, W.D.; Wu, J.H.; Wang, H.J.; Jiang, J.A. Therapeutic outcomes of low-level laser therapy for closed bone fracture in the human wrist and hand. Photomed. Laser Surg. 2014, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Gladsjo, J.A.; Jiang, S.I. Treatment of surgical scars using a 595-nm pulsed dye laser using purpuric and nonpurpuric parameters: A comparative study. Dermatol. Surg. 2014, 40, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Keaney, T.C.; Tanzi, E.; Alster, T. Comparison of 532 nm potassium titanyl phosphate laser and 595 nm pulsed dye laser in the treatment of erythematous surgical scars: A randomized, controlled, Open-label study. Dermatol. Surg. 2016, 42, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Li, W.T.; Chen, H.L.; Wang, C.T. Effect of light emitting diode irradiation on proliferation of human bone marrow mesenchymal stem cells. J. Med. Biol. Eng. 2006, 26, 35–42. [Google Scholar]
- Wu, Y.H.; Wang, J.; Gong, D.X.; Gu, H.Y.; Hu, S.S.; Zhang, H. Effects of low-level laser irradiation on mesenchymal stem cell proliferation: A microarray analysis. Lasers Med. Sci. 2012, 27, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, J.T.; Huang, Y.Y.; Osmani, B.Z.; Sharma, S.K.; Naeser, M.A.; Hamblin, M.R. Role of low-level laser therapy in neurorehabilitation. PM&R 2010, 2, 292–305. [Google Scholar] [CrossRef]
- Yazdani, S.O.; Golestaneh, A.F.; Shafiee, A.; Hafizi, M.; Omrani, H.A.; Soleimani, M. Effects of low level laser therapy on proliferation and neurotrophic factor gene expression of human schwann cells in vitro. J. Photochem. Photobiol. B 2012, 107, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Albornoz, P.; Delgado, P.J.; Forriol, F.; Maffulli, N. Non-surgical therapies for peripheral nerve injury. Br. Med. Bull. 2011, 100, 73–100. [Google Scholar] [CrossRef] [PubMed]
- Ziago, E.K.; Fazan, V.P.; Iyomasa, M.M.; Sousa, L.G.; Yamauchi, P.Y.; da Silva, E.A.; Borie, E.; Fuentes, R.; Dias, F.J. Analysis of the variation in low-level laser energy density on the crushed sciatic nerves of rats: A morphological, quantitative, and morphometric study. Lasers Med. Sci. 2017, 32, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Gigo-Benato, D.; Geuna, S.; Rochkind, S. Phototherapy for enhancing peripheral nerve repair: A review of the literature. Muscle Nerve 2005, 31, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.P.O.; Buchaim, D.V.; Rosa Junior, G.M.; Andreo, J.C.; Pomini, K.T.; Buchaim, R.L. Low-Level Laser Therapy (LLLT) Improves the Repair Process of Peripheral Nerve Injuries: A Mini Review. Int. J. Neurorehabilit. 2017, 4, 260. [Google Scholar] [CrossRef]
- Gigo-Benato, D.; Geuna, S.; de Castro Rodrigues, A.; Tos, P.; Fornaro, M.; Boux, E.; Battiston, B.; Giacobini-Robecchi, M.G. Low-power laser biostimulation enhances nerve repair after end-to-side neurorrhaphy: A double-blind randomized study in the rat median nerve model. Lasers Med. Sci. 2004, 19, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, S.; Drory, V.; Alon, M.; Nissan, M.; Ouaknine, G.E. Laser phototherapy (780 nm), a new modality in treatment of long-term incomplete peripheral nerve injury: A randomized double-blind placebo-controlled study. Photomed. Laser Surg. 2007, 25, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Khullar, S.M.; Brodin, P.; Messelt, E.B.; Haanaes, H.R. The effects of low level laser treatment on recovery of nerve conduction and motor function after compression injury in the rat sciatic nerve. Eur. J. Oral Sci. 1995, 103, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Stainki, D.R.; Raiser, A.G.; Graça, D.L.; Becker, C.; Fernandez, G.M.S. Gallium arsenide (GaAs) laser radiation in the radial nerve regeneration submitted secondary to surgical repair. Braz. J. Vet. Res. Anim. Sci. 1999, 35, 37–40. [Google Scholar]
- De Medinaceli, L.; Freed, W.J.; Wyatt, R.J. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol. 1982, 7, 634–643. [Google Scholar] [CrossRef]
- Gasparini, A.L.P.; Barbieri, C.H.; Mazzer, N. Correlation between different methods of gait functional evaluation in rats with ischiatic nerve crushing injuries. Acta Ortop. Bras. 2007, 15, 285–289. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. referred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Alessi Pissulin, C.N.; Henrique Fernandes, A.A.; Sanchez Orellana, A.M.; Rossi, E.; Silva, R.C.; Michelin Matheus, S.M. Low-level laser therapy (LLLT) accelerates the sternomastoid muscle regeneration process after myonecrosis due to bupivacaine. J. Photochem. Photobiol. B 2017, 168, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Takhtfooladi, M.A.; Sharifi, D. A comparative study of red and blue light-emitting diodes and low-level laser in regeneration of the transected sciatic nerve after an end to end neurorrhaphy in rabbits. Lasers Med. Sci. 2015, 30, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Takhtfooladi, M.A.; Jahanbakhsh, F.; Takhtfooladi, H.A.; Yousefi, K.; Allahverdi, A. Effect of low-level laser therapy (685 nm, 3 J/cm2) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med. Sci. 2015, 30, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Chen, Y.J.; Wang, Y.H.; Yeh, M.L.; Huang, M.H.; Ho, M.L.; Liang, J.I.; Chen, C.H. Low-level laser irradiation improves functional recovery and nerve regeneration in sciatic nerve crush rat injury model. PLoS ONE 2014, 13, e103348. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Yang, Y.C.; Liu, B.S. Large-area irradiated low-level laser effect in a biodegradable nerve guide conduit on neural regeneration of peripheral nerve injury in rats. Injury 2011, 42, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Yang, Y.C.; Liu, B.S. Effects of large-area irradiated laser phototherapy on peripheral nerve regeneration across a large gap in a biomaterial conduit. J. Biomed. Mater. Res. 2013, 101, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Medalha, C.C.; Di Gangi, G.C.; Barbosa, C.B.; Fernandes, M.; Aguiar, O.; Faloppa, F.; Leite, V.M.; Renno, A.C. Low-level laser therapy improves repair following complete resection of the sciatic nerve in rats. Lasers Med. Sci. 2012, 27, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Yang, Y.C.; Huang, T.B.; Chan, S.C.; Liu, B.S. Low-Level Laser-Accelerated Peripheral Nerve Regeneration within a Reinforced Nerve Conduit across a Large Gap of the Transected Sciatic Nerve in Rats. Evid. Based Complement. Altern. Med. 2013, 2013, 175629. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Wang, Y.H.; Wang, C.Z.; Ho, M.L.; Kuo, P.L.; Huang, M.H.; Chen, C.H. Effect of low level laser therapy on chronic compression of the dorsal root ganglion. PLoS ONE 2014, 9, e89894. [Google Scholar] [CrossRef] [PubMed]
- Belchior, A.C.; dos Reis, F.A.; Nicolau, R.A.; Silva, I.S.; Perreira, D.M.; de Carvalho, P.D.T.C. Influence of laser (660 nm) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med. Sci. 2009, 24, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.I.; Marcolino, A.M.; de Jesus Guirro, R.R.; Mazzer, N.; Barbieri, C.H.; de Cássia Registro Fonseca, M. Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med. Sci. 2010, 25, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Marcolino, A.M.; Barbosa, R.I.; das Neves, L.M.; Mazzer, N.; de Jesus Guirro, R.R.; de Cássia Registro Fonseca, M. Assessment of functional recovery of sciatic nerve in rats submitted to low-level laser therapy with different fluences. An experimental study: Laser in functional recovery in rats. J. Hand Microsurg. 2013, 5, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Akgul, T.; Gulsoy, M.; Gulcur, H.O. Effects of early and delayed laser application on nerve regeneration. Lasers Med. Sci. 2014, 29, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Gigo-Benato, D.; Russo, T.L.; Tanaka, E.H.; Assis, L.; Salvini, T.F.; Parizotto, N.A. Effects of 660 and 780 nm low-level laser therapy on neuromuscular recovery after crush injury in rat sciatic nerve. Lasers Surg. Med. 2010, 42, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, F.A.; Belchior, A.C.G.; de Carvalho, P.T.C.; da Silva, B.A.; Pereira, D.M.; Silva, I.S.; Nicolau, R.A. Effect of laser therapy (660 nm) on recovery of the sciatic nerve in rats after injury through neurotmesis followed by epineural anastomosis. Lasers Med. Sci. 2009, 24, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Wang, J.; Chen, S.C.; Hsieh, Y.L. Synergistic effects of low-level laser and mesenchymal stem cells on functional recovery in rats with crushed sciatic nerves. J. Tissue Eng. Regener. Med. 2016, 10, 120–131. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Martins, D.; Martinez dos Santos, F.; Evany de Oliveira, M.; de Britto, L.R.; Benedito Dias Lemos, J.; Chacur, M. Laser therapy and pain-related behavior after injury of the inferior alveolar nerve: Possible involvement of neurotrophins. J. Neurotrauma 2013, 30, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.E.; Dalmarco, E.M.; André, E.S. The brain-derived neurotrophic factor, nerve growth factor, neurotrophin-3, and induced nitric oxide synthase expressions after low-level laser therapy in an axonotmesis experimental model. Photomed. Laser Surg. 2012, 30, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.L.; Chou, L.W.; Chang, P.L.; Yang, C.C.; Kao, M.J.; Hong, C.Z. Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: Possible involvements in hypoxia-inducible factor 1α (HIF-1α). J. Comp. Neurol. 2012, 520, 2903–2916. [Google Scholar] [CrossRef] [PubMed]
- Sene, G.A.; Sousa, F.F.; Fazan, V.S.; Barbieri, C.H. Effects of laser therapy in peripheral nerve regeneration. Acta Ortop. Bras. 2013, 21, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.J.; Issa, J.P.; Coutinho-Netto, J.; Fazan, V.P.; Sousa, L.G.; Iyomasa, M.M.; Papa, P.C.; Watanabe, I.S. Morphometric and high-resolution scanning electron microscopy analysis of low-level laser therapy and latex protein (Hevea brasiliensis) administration following a crush injury of the sciatic nerve in rats. J. Neurol. Sci. 2015, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.; Ridgway, T.D.; Higbee, R.G.; Howard, E.W.; Lucroy, M.D. Effect of wavelength on low-intensity laser irradiation-stimulated cell proliferation in vitro. Lasers Surg. Med. 2005, 36, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Kim, H.Y.; Lee, H. Optogenetic and Chemogenetic Approaches for Studying Astrocytes and Gliotransmitters. Exp. Neurobiol. 2016, 25, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.M.; Vesuna, S.; Ramakrishnan, C.; Huynh, K.; Young, S.; Berndt, A.; Lee, S.Y.; Gorini, C.J.; Deisseroth, K.; Delp, S.L. Optogenetic and chemogenetic strategies for sustained inhibition of pain. Sci. Rep. 2016, 6, 30570. [Google Scholar] [CrossRef] [PubMed]
| Authors | Type of Laser (Manufacturer) | Wavelength (nm)/Spot Beam | Energy (mW) | Energy Density (J/cm2) | Radiation Amount | Variables | Irradiation Site | Evaluation Time | Main Results |
|---|---|---|---|---|---|---|---|---|---|
| Buchaim et al. [2] | GaAlAs (Laserpulse IBRAMED, Brazil) | 660/0.116 | 30 | 4 | 16 s per point; 3 points | Sural nerve graft was coapted to the vagus nerve using the fibrin glue. | Right side of the neck. | Application on the 1st day post-operatory, 5 weeks, 3 times a week. Evaluation 30 days after irradiation. | LLLT improved the nerve regeneration. |
| Buchaim et al. [3] | GaAlAs (Laserpulse IBRAMED, Brazil) | 830/0.116 | 30 | 6 | 24 s per point; 3 points | Neurotmeses of buccal branch of facial nerve, followed by end-to-end suture or coaptation with heterologous fibrin sealant derived from snake venom | On the surgical site, on both sides of the face | Application 1st day post-operatory, 3 times/week for 5 weeks. Evaluation 5 and 10 weeks after the surgery. | LLLT showed satisfactory results on facial nerve regeneration. |
| Buchaim et al. [6] | GaAlAs (Laserpulse IBRAMED®, Brazil) | 830/0.116 | 30 | 6.2 | 24 s per point; 3 points | Neurotmeses of buccal branch of facial nerve, end-to-end anastomosis. Use of epineural suture or coaptation with heterologous fibrin sealant derived from snake venom. | On the surgical site, on both sides of the face | Application on the 1st day post-operatory, 3 times a week, for 5 weeks. | Laser stimulated axonal regeneration accelerated the process of functional recovery of whisker, and the two techniques used allowed the growth of axons. |
| Rosso et al. [7] | GaAlAs (Laserpulse IBRAMED®, Brazil) | 830/0.116 | 30 | 6.2 | 24 s per point; 3 points | Neurotmeses in buccal branch of facial nerve, end-lateral anastomosis in the zygomatic branch of the facial nerve with epineural suture or heterologous sealant of fibrin derived from snake venom. | On the surgical site, on both sides of the face | Application on the 1st day post- operatory, 3 times a week, for 10 weeks. | Laser groups presented faster functional recovery, similar results to the control group. It was observed that PBMT provided accelerated morphological and functional repair in the two techniques used. |
| Ziago et al. [19] | GaAlAs (Twin Laser, MMO, São Carlos, SP, Brazil) | 780/0.04 | 40 | 4 10 50 | 4, 10 e 50 s per point; 3 points | Crushing of the left sciatic nerve. | On the surgical site | Application during 6 sessions on alternate days. | Best morphological quantitative and morphometric results on L10 group after 15 days of nerve lesion. |
| Alessi Pissulin et al. [29] | GaAs (Endophoton, KLD Biosystems, Amparo, Brazil) | 904/0.035 | 50 | 69 | 48 s per point | 0.5% bupivacaine injection to the right and 0.9% sodium chloride injection to the left on sternocleidomastoid muscle and accessory nerve exposed in surgery. | Ventral side of the neck | Application 1st day post-operatory, during 5 successive days. | LLLT reduced the aggressive effects of bupivacaine on the nerve and the muscle, of muscular degeneration, of myonecrosis and fibrosis, kept the morphology of the axon and the myelin sheath. |
| Takhtfooladi; Sharifi [30] | GaAlAs (pulsed) LED (red and blue) (-----) | 680/0.04 650/1.5 red 450/1.5 blue | 10 | 10 | 200 s per point; 3 points | Neurotmeses of right sciatic nerve followed by epineural neurorrhaphy. | On the surgical site, sciatic nerve | Application 1st day post-operatory, during 14 successive days | LLLT increased Schwann cells on the great myelinic axons and on neurons, sped up and potentialized nerve regeneration. |
| Takhtfooladi et al. [31] | InGaAlP (Teralaser; DMC® São Carlos, SP, Brazil) | 685/0.028 | 15 | 3 | 10 s per point | Crushing of the left sciatic nerve. | On the surgery site on sciatic nerve. | Application on the 1st day post-operatory, during 21 successive days. | LLLT accelerated and improved the nerve function after crushing lesion. |
| Wang et al. [32] | GaAlAs (Transverse IND. CO., LTD., Taipei, Taiwan) | 808/3.8 | 170 | 3 8 15 | 67.2 s 179 s 335.6 s | Crushing of the right sciatic nerve. | On lesion on sciatic nerve. | Application during 20 successive days. | LLLT (3 and 8 J/cm2) accelerated functional and morphologic recovery of the nerve, increased the expression of the marker GAP43. |
| Shen; Yang; Liu [33] | AlGaInP (Megalas1-AM-800, Konftec Co., Taipei, Taiwan, ROC) | 660/----- | 0.0032 | 3.84 | 5 min per day | Neurotmeses of the left sciatic nerve, 10 mm gap and use of biodegradable tube containing genipin-cross-linked gelatin annexed with β-tricalcium phosphate ceramic particles (genipin-gelatin-tricalcium phosphate, GGT) | Applied to the surgical site. | Application 1st day post-operatory, during 20 successive days. Euthanasia after 8 weeks. | LLLT obtained better functional, electrophysiological and histomorphometric results and assisted on neural repair. |
| Shen; Yang; Liu [34] | AlGaInP (MegalasVR -AM-800; Konftec, Taipei, Taiwan) | 660/---- | 50 | Immediate post-surgery (5.76) 9 following days (0.96) | Immediate post-surgery (30 min) 9 successive consecutive (5 min) | Neurotmeses of the left sciatic nerve, 15 mm gap and the use of 1-ethyl-3-(3-dimethylaminopro-pyl) carbodiimide (EDC) cross-linked gelatin, annexed with β-tricalcium phosphate (TCP) ceramic particles (EDC-Gelatin-TCP, EGT). | On the surgery site. | Application immediately after the lesion, during 9 successive days. Euthanasia after 12 weeks. | LLLT showed better results on the functional index, on development, on electrophysiology, on nerve regeneration, larger neural tissue area, larger axon, and myelin sheath diameter. |
| Medalha et al. [35] | GaAlAs (Teralaser, DMC São Carlos, São Paulo, Brazil) | 660/0.028 808/0.028 | 30 30 | 10 e 50 10 e 50 | 9 s and 47 s; 3 points 9 s and 47 s; 3 points | Neurotmeses of the sciatic nerve, approximately 3 mm distal to the tendon of the internal obturator. Anastomosis with 3 sutures using nylon monofilament 10-0. | Applied to the surgical site. | Application 1st day post-operatory during 5 successive days and 2 days interval until completing 15 days. | LLLT 808 nm on 50 J/cm2 obtained higher fiber density. LLLT 660 nm on 50 J/cm2 presented larger diameters of axons and of fibers of gait functional recovery. |
| Shen et al. [36] | GaAlAsP (Aculas-AM-100A, Konftec Co., Taipei, Taiwan) | 660/0.1 | 50 | 2 | 2 min per day; 2 points at the same time | A biodegradable nerve conduit containing genipin-cross-linked gelatin was annexed using beta-tricalcium phosphate (TCP) ceramic particles (genipin-gelatin-TCP, GGT) with a 15 mm sciatic nerve transection gap. | On the sciatic nerve. | Application 1st day post-operatory during 10 successive days. | LLLT accelerated the nerve regeneration due to the larger neural tissue, larger diameter and thicker myelin sheath, motor function, electrophysiology and muscular innervation. |
| Chen et al. [37] | GaAlAs (Transverse IND. CO., LTD., Taipei, Taiwan) | 808 ± 5/≤0.5 | 190 | 8 | 207 s | Chronic compression on dorsal root ganglion. A thin L shaped needle (0,6 mm of diameter) was inserted 4 mm in the L4 and L5 intervertebral foramen. | On the dorsal root of L4 and L5. | Application 1st day post-operatory, per 8 successive days. Euthanasia 4 e 8 days. | LLLT decreased the levels of inflammatory cytokines and of pain, facilitating the nerve regeneration, demonstrated by levels of TNF-a, IL-1b e GAP-43. |
| Belchior et al. [38] | GaAlAs (KLD® Endophoton model) | 660/0.63 | 26.3 | 4 | 96.7 s; 3 points | Crushing of the right sciatic nerve. | On the surgical site. | Application 1st day post-operatory, during 20 successive days. | LLLT was positive on the functional index after the 21st day. |
| Barbosa et al. [39] | GaAlAs (Ibramed® Equipamentos Médicos) | 660/0.06 830/0.116 | 30 | 10 10 | 20 s 38.66 s | Crushing of the right sciatic nerve. | On the surgical site. | Application 1st day post-operatory, during 20 successive days. | LLLT 660 nm promoted functional recovery in a faster manner. |
| Marcolino et al. [40] | AlGaAs (Laser Diode, Ibramed) | 830/0.116 | 30 | 10 40 80 | 38.66 s 154.66 s 309.33 s | Crushing of the right fibular nerve. | On the right sciatic nerve. | Application immediately after surgery and during the 21 successive days. | 40 J/cm2 and 80 J/cm2 LLLT influenced the functional recovery of the nerve. |
| Akgul; Gulsoy; Gulcur [41] | Laser diode (model: DH650-24-3(5), Huanic, China) | 650/≈0.14 | 25 | 10 | 57 s on 3 points | Crushing of the sciatic nerve. | On the sciatic nerve. | Early group: Application after surgery, up to the 14th day. Delayed group: Application on the 7th day post-operatory and up to the 21st day. | LLLT accelerated nervous recovery. The group with delayed application showed better functional results. |
| Gigo-Benato et al. [42] | GaAlAs (TWIN LASER; MM Optics, São Carlos, SP, Brazil) | 660/0.04 780/0.04 | 40 40 | 10, 60 and 120 10, 60 and 120 | 0.3 s, 1 min and 2 min 0.3 s, 1 min and 2 min; 2 points | Crushing of the left sciatic nerve. | Applied to the surgical site. | Application 1st day post-operatory, during 10 successive days. | LLLT (660 nm, 10 J/cm2 or 60 J/cm2) accelerated the neuromuscular recuperation. |
| dos Reis et al. [43] | AlGaAs (KLD®; Endophoton model) | 660/0.63 | 26.3 | 4 | 96.7 s per point; 3 points | Neurotmeses and epineural anastomosis on the right sciatic nerve. | On the surgical site. | Application 1st day post-operatory, 20 successive days. | LLLT significantly changed the morphometry (myelin sheath), but did not interfere on functionality. |
| Yang et al. [44] | GaAlAs (Aculas-Am series, Multi-channel LLLT System, Konftec Corp., Taipei, Taiwan) | 660/≈0.2 | 30 | 9 | 60 s per point; 4 points | Use of Mesenchymal stem cells (MSC) on the lesion by crushing of sciatic nerve. | On the sciatic nerve | 7 successive days. | LLLT+MSC improved the electrophysiologic function, S100 immunoreactivity, less inflammatory cells and less vacuole formation. |
| de Oliveira Martins et al. [45] | GaAs (Laserpulse-Laser, Ibramed Brazil) pulsado | 904/0.1 | 70 Wpk | 6 | 18 s on 5 points | Pulsed LLLT. Lesion on alveolar nerve, by a hemostatic Crile clamp. | On the sciatic nerve. | 10 sessions every 10 days. | LLLT obtained better nociception, higher expression of neural growth factor (NGF) 53% and of expression of neurotrophic factor (BDNF) 40%. |
| Gomes; Dalmarco; André [46] | HeNe (----) | 632.8/0.1 | 5 | 10 | 20 s on 10 points | Crushing of the right sciatic nerve. | On the sciatic nerve. | 1st Application 24 h after surgery; 7, 14 and 21 successive days. | LLLT increased the expression of mRNA and the factors BDNF and NGF after 14 days and maximum expression was observed on the 21st day. |
| Hsieh et al. [47] | GaAlAs (Aculas-Am series, Multi-channel laser system; Konftec, Taipei, Taiwan) | 660/≈0.2 | 30 | 9 | 60 s per point; 4 points | Lesion on the sciatic nerve with 4 ligatures, using chromic suture 4-0. | On the surgery site. | Application 7th post-operatory, during 7 successive days. | LLLT improved functional index, decreased HIF-1a, TNF-a, and IL-1b, increased VEGF, NGF, and S100, reduced tissue ischemia and inflammation, helped the nerve recovery. |
| Sene et al. [48] | GaAsAl (Physiolux Dual, BIOSET, Rio Claro, Brazil) | 830/0.02 | 30 | 5 10 20 | Maximum time of application was 40 s | Crushing of the right fibular nerve. | Application fibular nerve region. | Application immediately after the lesion, during 21 successive days. | LLLT simulation group obtained a larger nerve transverse area; group 10 J/cm2 obtained higher density of the fiber. LLLT did not speed up nerve recovery. |
| Dias et al. [49] | GaAlAs (Mm Twin Laser Optics, São Carlos, Brazil) | 780/0.4 | 30 | 15 | 20 s per point; 3 points | Latex protein (F1) on lesion per crushing of sciatic nerve. | On the surgery site, sciatic nerve. | Application per 6 sessions on alternate days. | LLLT associated to the F1 protein did not present positive results and did not potentialize the effects of this protein. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosso, M.P.d.O.; Buchaim, D.V.; Kawano, N.; Furlanette, G.; Pomini, K.T.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering 2018, 5, 44. https://doi.org/10.3390/bioengineering5020044
Rosso MPdO, Buchaim DV, Kawano N, Furlanette G, Pomini KT, Buchaim RL. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering. 2018; 5(2):44. https://doi.org/10.3390/bioengineering5020044
Chicago/Turabian StyleRosso, Marcelie Priscila de Oliveira, Daniela Vieira Buchaim, Natália Kawano, Gabriela Furlanette, Karina Torres Pomini, and Rogério Leone Buchaim. 2018. "Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review" Bioengineering 5, no. 2: 44. https://doi.org/10.3390/bioengineering5020044
APA StyleRosso, M. P. d. O., Buchaim, D. V., Kawano, N., Furlanette, G., Pomini, K. T., & Buchaim, R. L. (2018). Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering, 5(2), 44. https://doi.org/10.3390/bioengineering5020044






