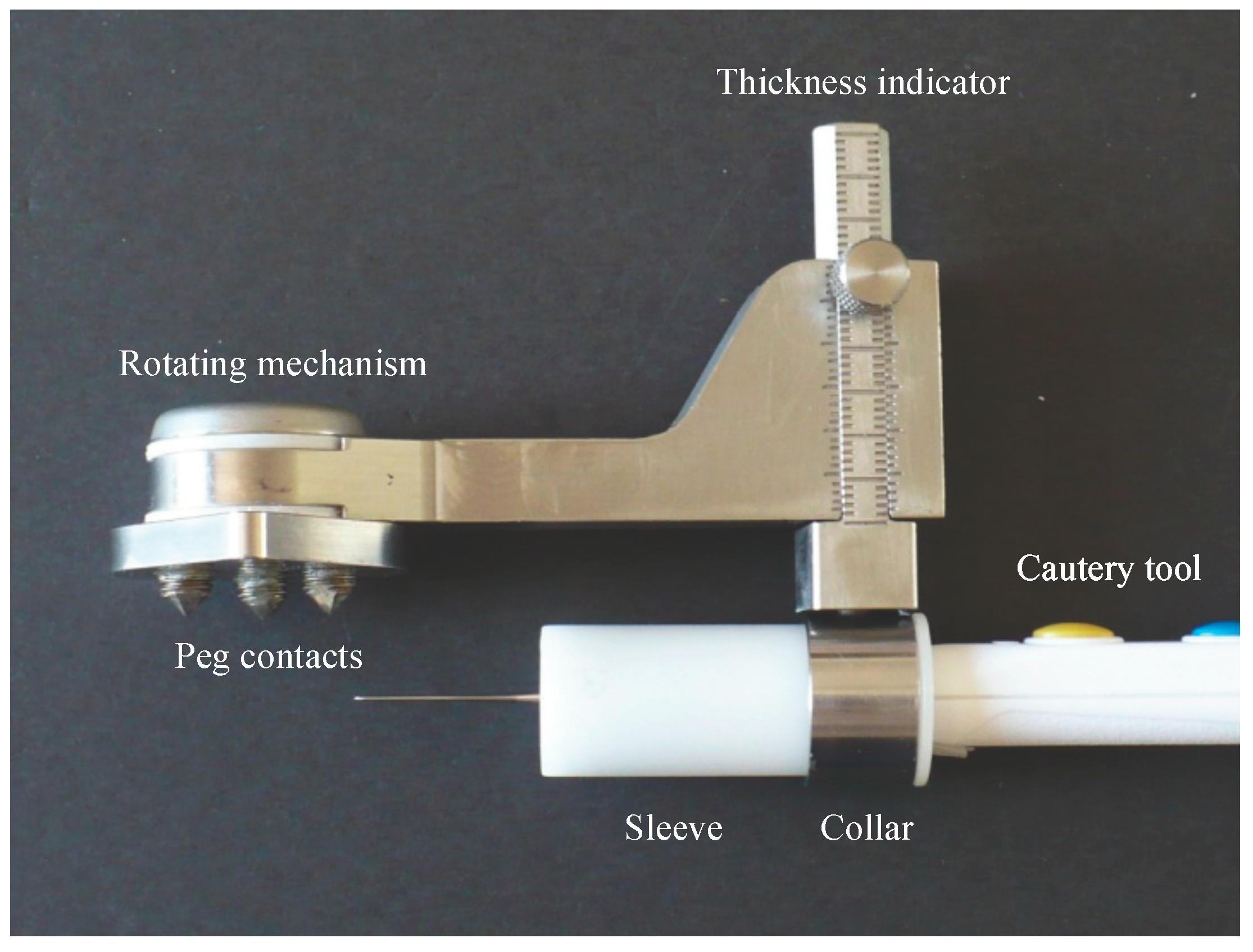
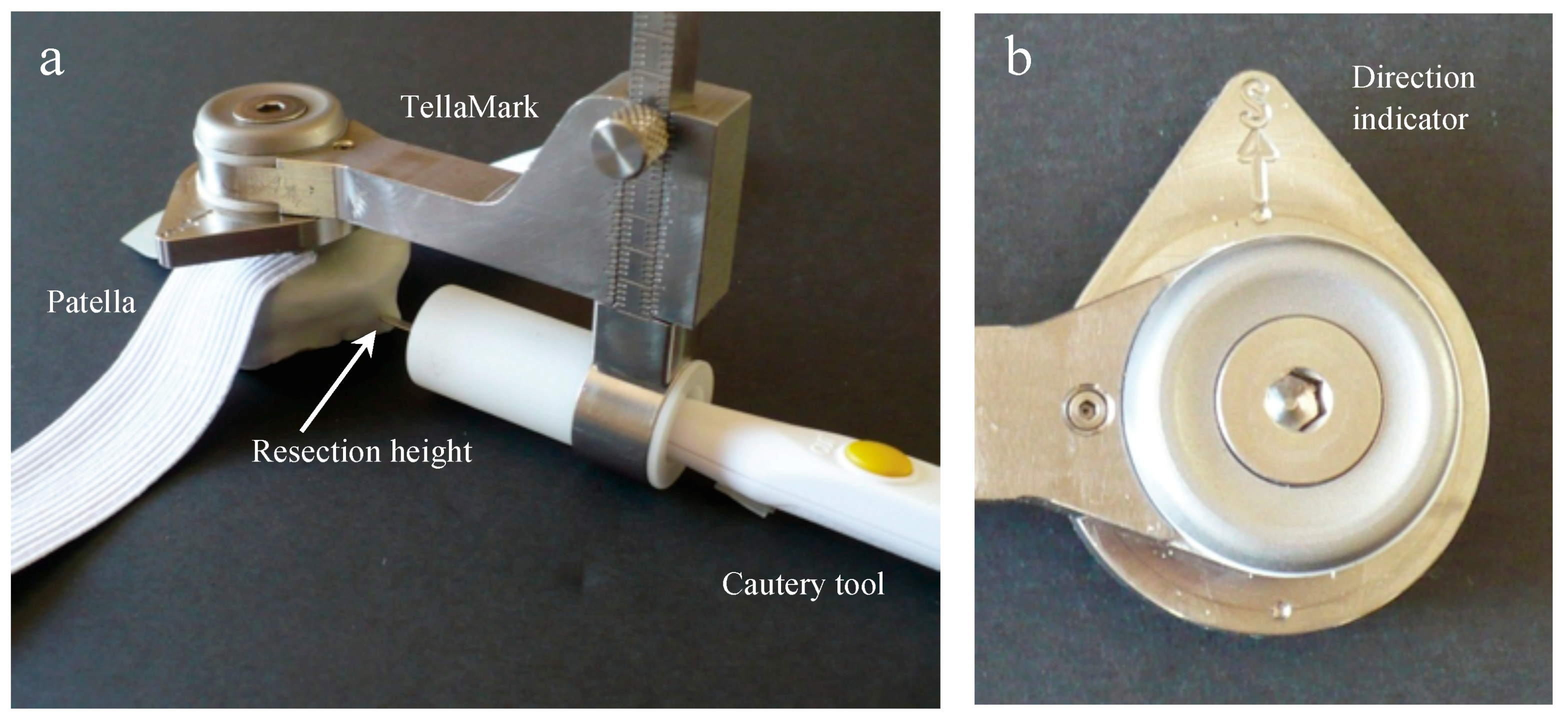
2.1. Device Design
The central concept of the device is to mark a line on the patella parallel to the anterior surface (i.e., around the circumference), using a cautery tool or marker pen, and then remove the device, leaving the marked line. The surgeon then uses their technique of choice (freehand, saw guide, or reamer) to align the saw or reamer with the marked line. In this way, the surgeon can continue to use the device they are most comfortable with, but with greater confidence and accuracy. By not providing a saw slot, the device is lighter, smaller and non-invasive, and leaves the control in the surgeon’s hands. The surgeon or resident can compare the drawn line to what they would have done, learning in the process, and providing a second-thought evaluation of the patellar cut. This is similar to the common practice of drawing several guidelines on the femur (transepicondylar axis, posterior condylar axis, and Whiteside’s line) to see how they compare. Residents may have the opportunity to do the patellar cut earlier in their residency because the surgeon, after checking the drawn line, will feel more confident in the resulting resection. The device is called TellaMark, to describe Marking the paTella (
Figure 1 and
Figure 2).
Essential to the device is accurately defining the anterior surface to achieve the desired resection. The contact configuration was determined by first having four surgeons draw lines on 18 axial and sagittal X-ray images created from computed tomography (CT) images of patellae, marking the estimated anterior surface and desired resection line, and then testing different contact configurations virtually on the 3D CT images to achieve the desired resection plane. The best configuration was determined to be a 16 mm equilateral triangle with two points superiorly and one inferiorly, centred on the patella (
Figure 1). The teardrop shape, with ‘S’ marked for superior, can be easily rotated to suit a right or left patella (
Figure 2). The prototype design used for testing had cone-point set screws as the contact points to allow their depth to be adjusted during the initial stages of testing. The length was chosen to be short enough to promote stability while being long enough to allow visibility when applying the device to the anterior surface. The size and sharpness were tested to grab onto the bone without digging in excessively.
Rotating the cautery tool around the patella is achieved using a swing arm, a strong rare earth magnetic coupling, and a custom Delrin sleeve that fits around the cautery tool, sliding in and out of the metal collar (
Figure 1). This sleeve could have a different inner profile for cautery tools with a different shape. The device could also be used with a marker pen, but the surgeons and residents preferred the cautery tool as it is more reliable and leaves a finer line. By pushing the cautery tool in and out of the metal collar while rotating it around the patella, the line can be drawn more than 180° around the patella, posterior to the tendon attachments, providing guidance in both the ML and SI directions.
The desired depth is set on the sliding dovetail mechanism (
Figure 1 and
Figure 2), allowing the surgeon continuous depth adjustment. It was originally intended to be set exactly at the desired depth, but through this testing we discovered that it is advantageous to set the depth slightly thinner, resecting posterior to the line instead of on it, so that alignment with the marked line can be checked following resection. This could be part of the instruction procedure or could be incorporated directly into the device.
The device is held onto the patella with the thumb and forefingers, to avoid using an invasive bone screw or bulky clamping device, and to provide haptic feedback to the surgeon when applying the device to help avoid tilting the contacts off the anterior surface. Using the thumb and fingers works because the device is only used to mark the line rather than to create the saw cut, and is only held on for a short duration of time. The resulting profile provides good visibility of the patella while marking the line. The device is suitable for all patellar shapes and sizes, medial or lateral approaches, with right- or left-handed surgeons.
To our knowledge, no other device exists in which the desired cut line is drawn on the bone surface, for this or any other joint.
2.2. Artificial Bone Testing
To mimic the surgical setup in the artificial bone testing, and to perform pilot testing for design and use iterations before testing on valuable and limited cadaveric specimens, medium-sized right and left legs (Sawbones, Pacific Research Laboratories Inc., Vashon, WA, USA), without patellae, were set up at full extension and anchored onto a table. Custom patellae (see below) were attached to the femur and tibia models using materials simulating the tendons and lateral retinaculum, and covered with material representing skin. A standard incision represented the visibility and access during surgery.
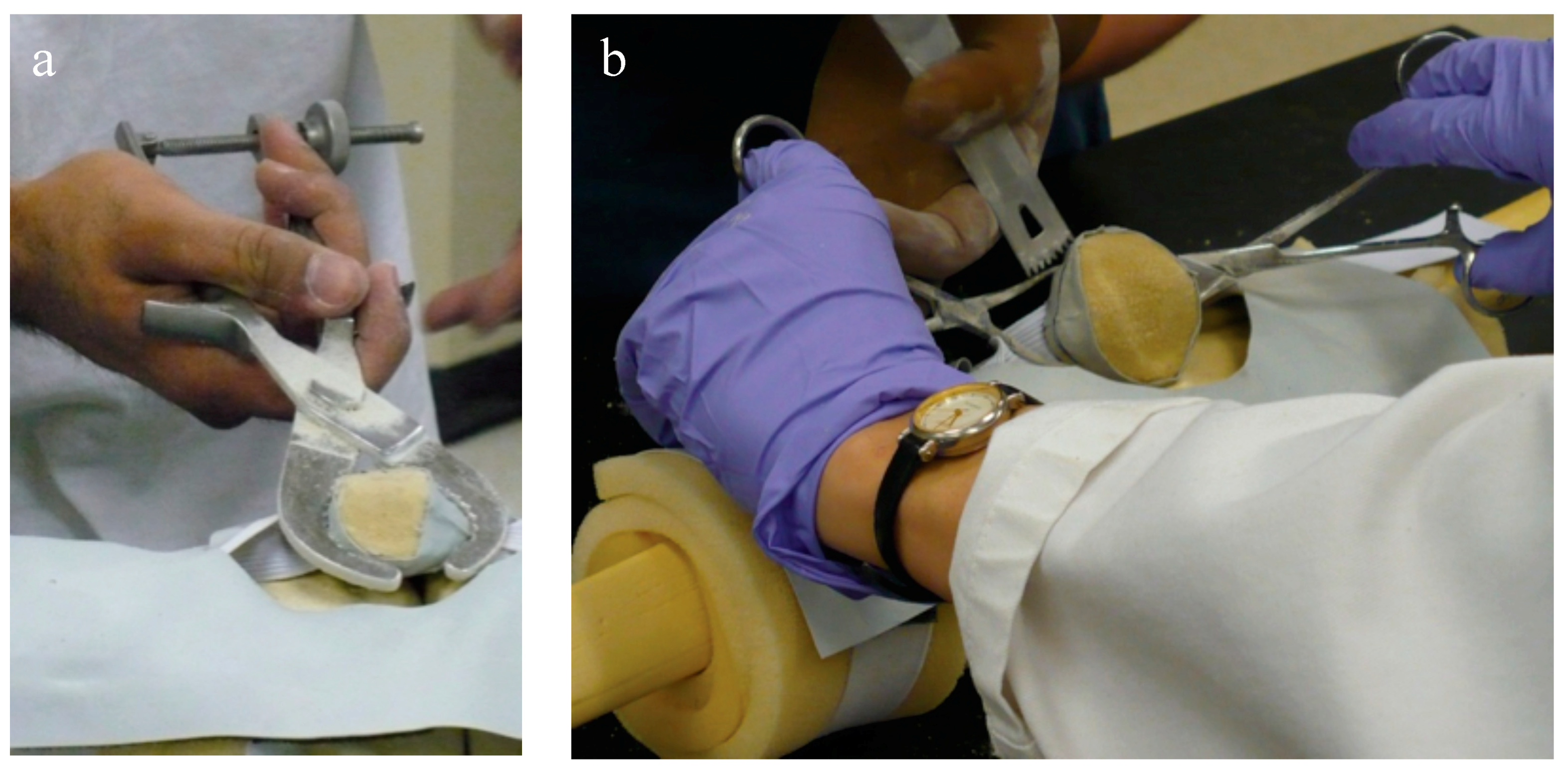
Two custom-molded patellar geometries were created and used in the testing phase, an approach that could be useful to other researchers, as they were more realistic than previously-used Sawbones patellae and were derived from CT scans of cadaveric specimens with which we had done earlier resection analyses and could in turn be used to compare the resulting resections in the present experiments. Geometry 1 was a left patella, smaller, regularly-shaped, and considered the ‘easier’ geometry, based on the most consistent estimated resection lines drawn by surgeons on pseudo-X-rays generated from the CT scans. Geometry 2 was a right patella, larger, irregularly-shaped, and considered the ‘harder’ geometry based on the least consistent estimated resection lines. Patellar bone models were generated from the CT scans and rapid prototyped. A mold made from the rapid-prototyped model was used to generate the patellar bone models (Foam-it 15; SmoothOn Inc., Easton, PA, USA, for which the density is 15 pounds per cubic foot). The anterior surface was covered with a thin layer of Thera-band to provide compliance and to partially obscure the anterior surface. Since the foam is insulating and the cautery tool requires a conduction path, the experimenters instead dipped the cautery tool in calligraphy ink, leaving an ink line on the patella (
Figure 3). Normal use of the cautery tool was verified during the cadaveric testing.
Two orthopaedic surgery residents (4th and 5th year) performed resections using three techniques: (1) using the conventional technique with a standard surgical saw guide (Zimmer; Warsaw, IN, USA); (2) TellaMark with the saw guide; and (3) TellaMark using a freehand technique, in each case with a surgical oscillating saw (
Figure 4).
For the TellaMark resections, the initial resection was left as it was, whereas for the conventional resections, the experimenter measured the thickness and symmetry with calipers and had the option of revising the cut until satisfied. After initial practice with the instruments and experimental setup, each experimenter performed three repetitions of each of the three techniques on the two different geometries, for a total of 18 tests each. Tests were performed in a randomized order. The procedure time was recorded, including a breakdown of the steps.
The TellaMark procedure began by locating the center of the patella; this was done by feeling the height and width with the fingers, and marking the resulting central point with a marker pen or cautery tool (in the future, a dedicated device can be developed). The desired remaining thickness, determined from the patellar thickness minus the prosthesis height, was set on the depth gauge of the device and the device applied to the center of the patella with the arrow pointing superiorly. The line was then drawn with the cautery tool more than 180° around the patella, allowing both the ML and SI planes to be guided. The device was removed and the experimenter either aligned the saw or saw guide with the line to complete the cut.
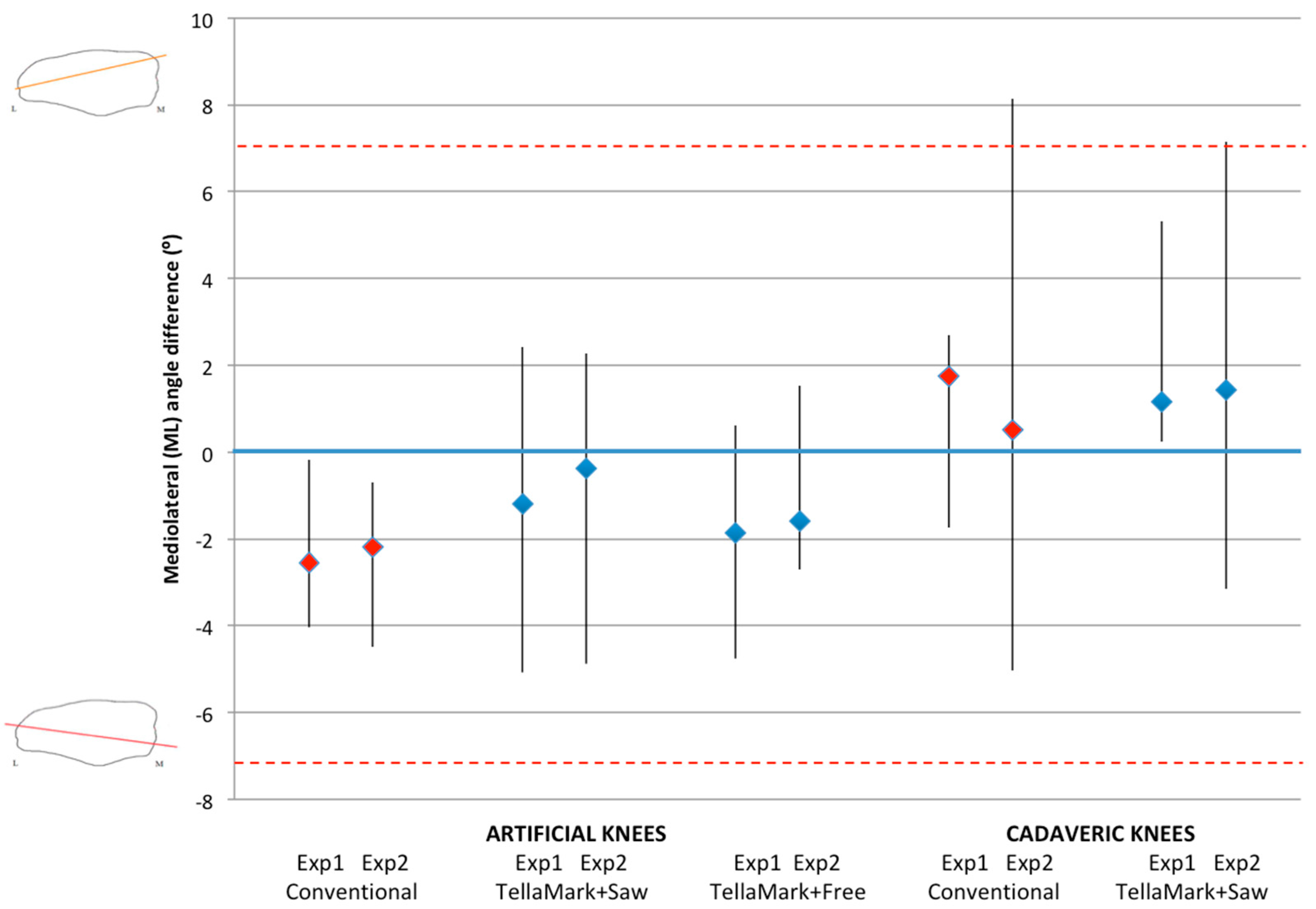
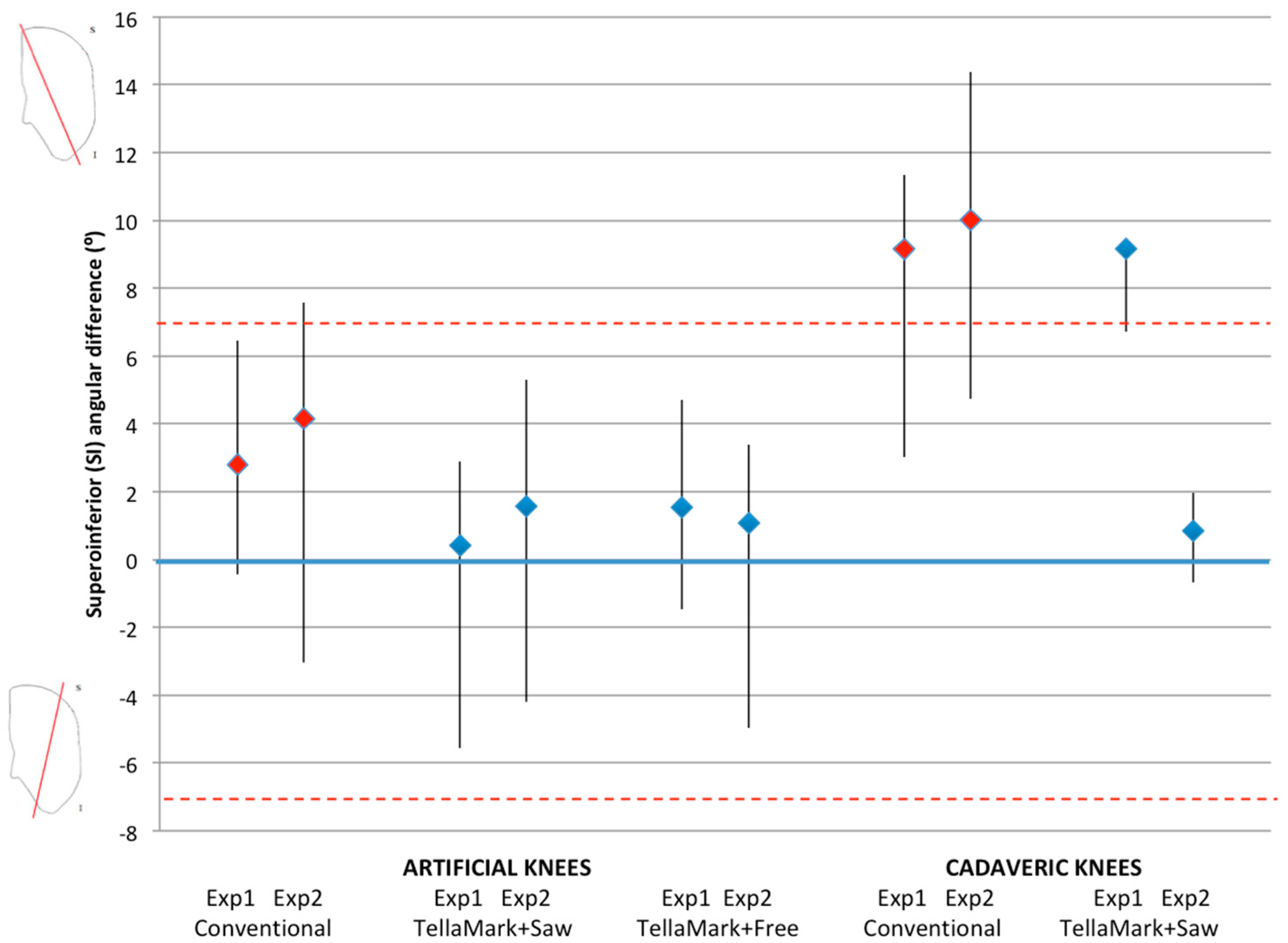
The patellae were CT scanned before and after resection (0.6 mm slice thickness), followed by segmentation of the patellar bone (Amira Version 5.3.1; Visage Imaging, Andover, MA, USA). The resected patellae were aligned to the original surface models using the AlignSurface function in Amira, plus manual fine tuning, and then brought into AutoCAD (Version 2010, AutoDesk, San Rafael, CA, USA). In AutoCAD, an average plane was fitted visually to the resected surface of the patellar model, and then the average resection plane, determined previously from the four surgeons’ input on pseudo X-rays, was applied to the model. This was the particular advantage of making custom molds of the previously-analyzed patellae. The ML and SI angles were measured between the resultant plane and the average surgeon-identified resection plane. The center of the patella was determined from the medial, lateral, superior, and inferior extents of the model, i.e., by drawing a box around the patella. The thickness from the anterior surface to the resected surface was measured at this central point and then compared to the intended remaining thickness specified in the testing process (13 mm for the left, 12 mm for the right).
The angle, thickness and time data were analyzed using ANOVA, followed by Student’s
t-tests when significant, using PASW Statistics 17.0 analysis software (Statistical Package for Social Sciences (SPSS) Inc., Chicago, IL, USA). Shapiro-Wilk tests confirmed the normality of the data. Angles within ±7° were considered symmetric based on previous studies that showed greater anterior knee pain beyond this limit and represented a normal range of results [
8,
10].
2.3. Cadaveric Testing
Eight pairs of fresh-frozen cadaveric knee specimens (six female, two male; mean age 82, range 67 to 90 years) were used for testing, following ethics approval. They were CT scanned prior to testing and then prepared with a midline incision followed by a standard parapatellar capsulotomy: medial in 14 cases, lateral in two, providing the opportunity to test both approaches. The soft tissues were released to allow for eversion of the patella, and cleared around the circumference to allow for the application of the saw guide, as done clinically. The specimens varied from no arthritis to severe arthritis, with the majority having moderate arthritis (grades 2–3). The arthritic state did not affect the experiment since the device relies only on the anterior surface, not the articulating surface, one of the advantages of the device.
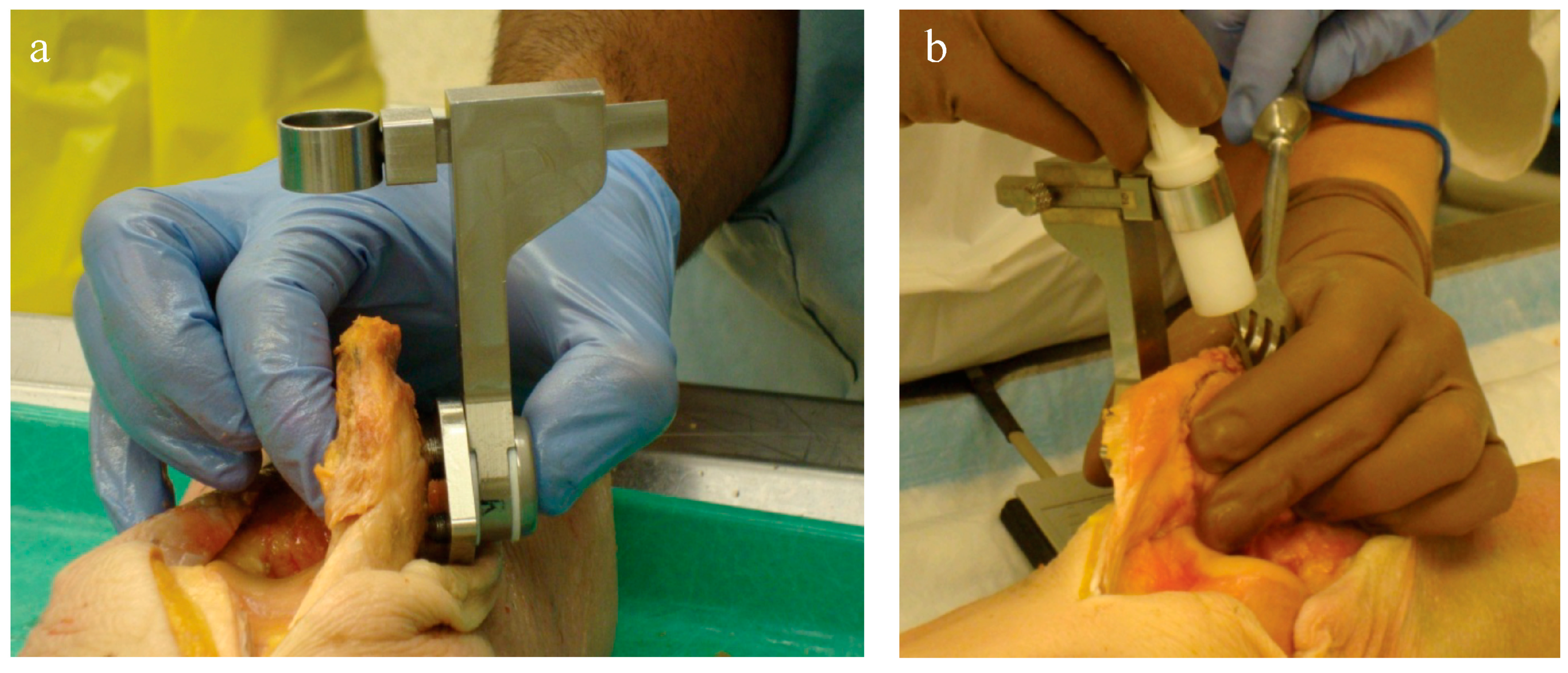
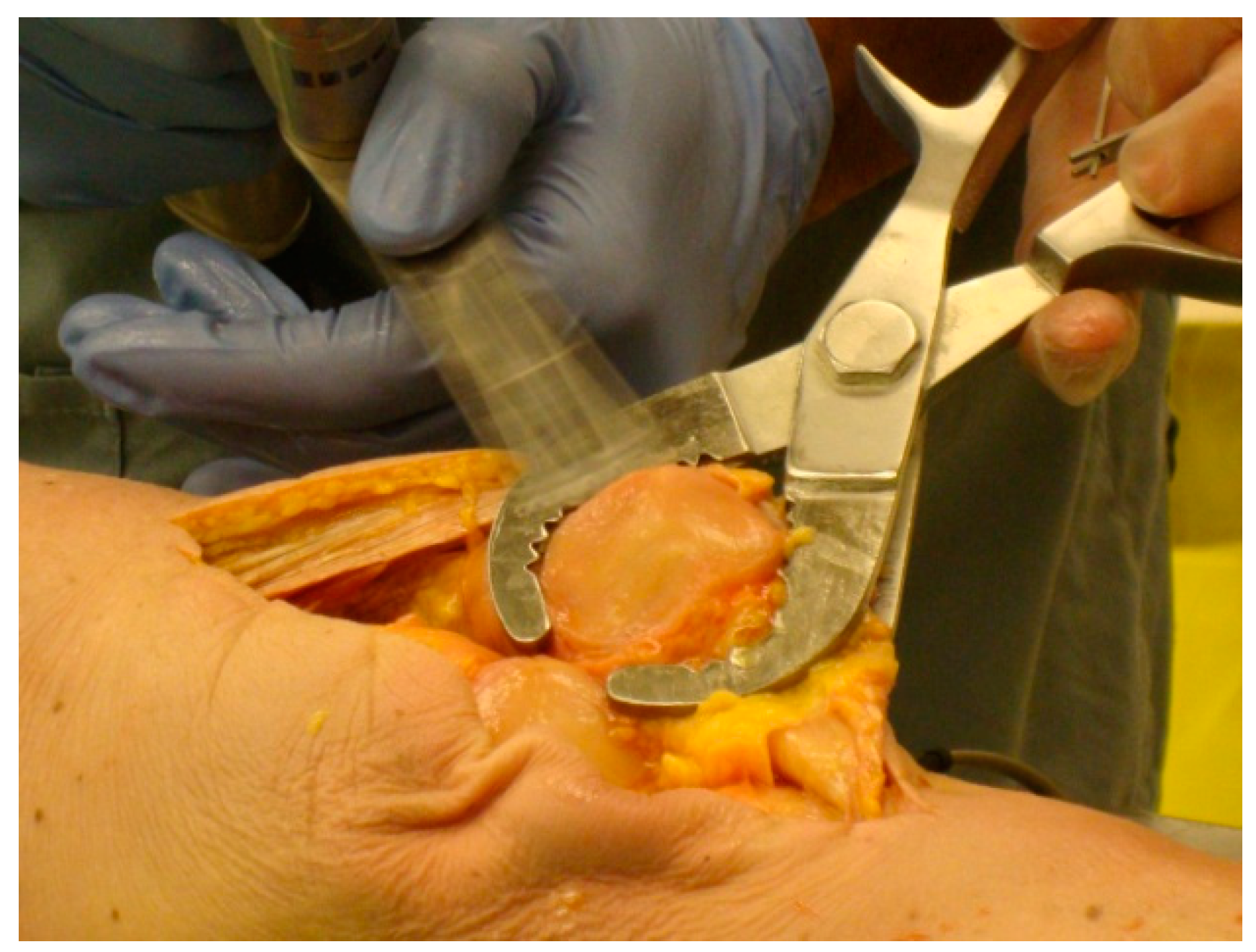
For each specimen pair, a TellaMark with saw-guide resection was performed on one side (
Figure 5) and a conventional saw-guide resection was performed on the other (
Figure 6), in a randomized order. The same two senior residents who performed the artificial bone testing performed the cadaveric testing. The cautery tool produced a clear, precise line, about 1 mm in thickness. As with the artificial bones, in the TellaMark case, the first cut was taken as the final cut; small corrections to the resection were allowed, such as removing a ridge, but the resection plane itself was not allowed to be recut or otherwise modified. In the conventional case, the experimenter could correct the cut until satisfied; cuts after the initial saw-guide cut were usually done freehand, with the patella being secured with towel clips (
Figure 4). Experimenter 1 set the TellaMark device in such a way that the line would be cut off with the saw; Experimenter 2 set it in such a way that the saw cut just above the line, leaving the line visible afterward. This latter technique had the advantage of confirming that the cut made corresponded to the cut recommended by the device, and is now the recommended technique. The desired thickness was determined from caliper measurements, with the prosthesis thickness being subtracted from the total thickness.
Once the resections were complete, CT images were acquired and used to calculate the desired resection plane as well as the achieved resection plane for each patella, by importing the segmented surfaces into AutoCAD. From this the ML and SI angles as well as the remaining bone thickness were measured, using the same method as for the artificial bone models. The three-peg model of the device was applied to the surface to determine the expected TellaMark resection angle. ANOVA tests of the ML angle, SI angle, bone remnant thickness, and time results were performed with p < 0.05 being considered significant. Normality was confirmed.