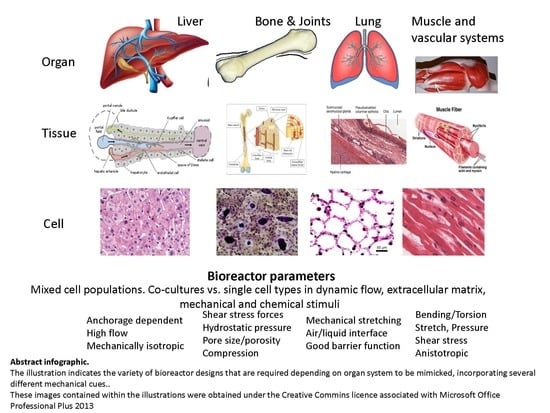
Role of Bioreactor Technology in Tissue Engineering for Clinical Use and Therapeutic Target Design
Abstract
1. Introduction
Requirements for Bioreactor Design
2. Bioreactor Designs
2.1. Perfusion Bioreactors
2.2. Oxygenation
2.3. Sheer Stress
2.4. Mechanical Stimuli
2.5. Mechano-Transduction and Cellular Signalling
2.6. Examples Used in Tissue Engineering and Pathophysiological Studies
2.7. Stretch/Compression
2.8. Cell Seeding
2.9. In Silico Modelling
2.10. Scaffolds Used with Bioreactors
3. Bioreactors Used to Provide Tissue Constructs for Implantation
4. Bioreactors Designed for Disease Modelling
5. Small Bioreactors to Mimic Larger Production Bioreactors as Bioartificial Organs
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Carrier, R.L.; Rupnick, M.; Langer, R.; Schoen, F.J.; Freed, L.E.; Vunjak-Novakovic, G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002, 8, 175–188. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Miller, W.M. Bioreactor development for stem cell expansion and controlled differentiation. Curr. Opin. Chem. Biol. 2007, 11, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, A.; Liu, Y.; Sarker, S.F.; Bonenfant, N.R.; Barrila, J.; Borg, Z.D.; Lee, J.J.; Weiss, D.J.; Nickerson, C.A. Recellularization of decellularized lung scaffolds is enhanced by dynamic suspension culture. PLoS ONE 2015, 10, e0126846. [Google Scholar] [CrossRef] [PubMed]
- Kulig, K.M.; Vacanti, J.P. Hepatic tissue engineering. Transpl. Immunol. 2004, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Nahmias, Y.; Berthiaume, F.; Yarmush, M.L. Integration of technologies for hepatic tissue engineering. Adv. Biochem. Eng. Biotechnol. 2007, 103, 309–329. [Google Scholar] [PubMed]
- Wolff, J. Das Gesetz der Transformation der Knochen; Hirshwald: Berlin, Germany, 1892. [Google Scholar]
- Davis, H. Conservative Surgery; Appleton: New York, NY, USA, 1867. [Google Scholar]
- Gonzalez-Molina, J.; Selden, B.F.C. Extracellular Fluid Viscosity Enhances Cell-Substrate Interaction and Impacts on Cell Size and Morphology; eCM Meeting Abstracts 2016, Collection 5; TCES: London, UK, 2016; p. 74. [Google Scholar]
- McFetridge, P.S.; Abe, K.; Horrocks, M.; Chaudhuri, J.B. Vascular tissue engineering: Bioreactor design considerations for extended culture of primary human vascular smooth muscle cells. ASAIO J. 2007, 53, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shin, H.J.; Cho, I.H.; Kang, Y.M.; Kim, I.A.; Park, K.D.; Shin, J.W. Nanofiber alignment and direction of mechanical strain affect the ecm production of human acl fibroblast. Biomaterials 2005, 26, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Wendt, D.; Marsano, A.; Jakob, M.; Heberer, M.; Martin, I. Oscillating perfusion of cell suspensions through three-dimensional scaffolds enhances cell seeding efficiency and uniformity. Biotechnol. Bioeng. 2003, 84, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Braccini, A.; Wendt, D.; Jaquiery, C.; Jakob, M.; Heberer, M.; Kenins, L.; Wodnar-Filipowicz, A.; Quarto, R.; Martin, I. Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells 2005, 23, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Filipowska, J.; Reilly, G.C.; Osyczka, A.M. A single short session of media perfusion induces osteogenesis in hbmscs cultured in porous scaffolds, dependent on cell differentiation stage. Biotechnol. Bioeng. 2016, 113, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Nieponice, A.; Maul, T.M.; Cumer, J.M.; Soletti, L.; Vorp, D.A. Mechanical stimulation induces morphological and phenotypic changes in bone marrow-derived progenitor cells within a three-dimensional fibrin matrix. J. Biomed. Mater. Res. Part A 2007, 81, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.; Fast, V.G.; Sharifov, O.F.; Iyer, R.K.; Park, H.; Vunjak-Novakovic, G. Optical mapping of impulse propagation in engineered cardiac tissue. Tissue Eng. Part A 2009, 15, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Burk, J.; Plenge, A.; Brehm, W.; Heller, S.; Pfeiffer, B.; Kasper, C. Induction of tenogenic differentiation mediated by extracellular tendon matrix and short-term cyclic stretching. Stem Cells Int. 2016, 2016, 7342379. [Google Scholar] [CrossRef] [PubMed]
- Groeber, F.; Engelhardt, L.; Lange, J.; Kurdyn, S.; Schmid, F.F.; Rucker, C.; Mielke, S.; Walles, H.; Hansmann, J. A first vascularized skin equivalent as an alternative to animal experimentation. Altex 2016, 33, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Egger, D.; Spitz, S.; Fischer, M.; Handschuh, S.; Glosmann, M.; Friemert, B.; Egerbacher, M.; Kasper, C. Application of a parallelizable perfusion bioreactor for physiologic 3D cell culture. Cells Tissues Organs 2017, 203, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, S.; Cowin, S.C.; Zeng, Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994, 27, 339–360. [Google Scholar] [CrossRef]
- Hung, C.T.; Mauck, R.L.; Wang, C.C.; Lima, E.G.; Ateshian, G.A. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann. Biomed. Eng. 2004, 32, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Seidel, J.O.; Pei, M.; Gray, M.L.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorheology 2004, 41, 445–458. [Google Scholar] [PubMed]
- Liu, C.; Abedian, R.; Meister, R.; Haasper, C.; Hurschler, C.; Krettek, C.; von Lewinski, G.; Jagodzinski, M. Influence of perfusion and compression on the proliferation and differentiation of bone mesenchymal stromal cells seeded on polyurethane scaffolds. Biomaterials 2012, 33, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Van Wachem, P.B.; Stronck, J.W.; Koers-Zuideveld, R.; Dijk, F.; Wildevuur, C.R. Vacuum cell seeding: A new method for the fast application of an evenly distributed cell layer on porous vascular grafts. Biomaterials 1990, 11, 602–606. [Google Scholar] [CrossRef]
- Ito, A.; Ino, K.; Hayashida, M.; Kobayashi, T.; Matsunuma, H.; Kagami, H.; Ueda, M.; Honda, H. Novel methodology for fabrication of tissue-engineered tubular constructs using magnetite nanoparticles and magnetic force. Tissue Eng. 2005, 11, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Friend, J.R.; Yeo, L.Y. A scaffold cell seeding method driven by surface acoustic waves. Biomaterials 2007, 28, 4098–4104. [Google Scholar] [CrossRef] [PubMed]
- Tresoldi, C.; Bianchi, E.; Pellegata, A.F.; Dubini, G.; Mantero, S. Estimation of the physiological mechanical conditioning in vascular tissue engineering by a predictive fluid-structure interaction approach. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 1077–1088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arenas-Herrera, J.E.; Ko, I.K.; Atala, A.; Yoo, J.J. Decellularization for whole organ bioengineering. Biomed. Mater. (Bristol Engl.) 2013, 8, 014106. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.H.; Park, K.M.; Kang, K.S.; Woo, H.M. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Alkhawaji, A.; Ding, Y.; Mei, J. Decellularized scaffolds in regenerative medicine. Oncotarget 2016, 7, 58671–58683. [Google Scholar] [CrossRef] [PubMed]
- Atias, S.; Mizrahi, S.S.; Shaco-Levy, R.; Yussim, A. Preservation of pancreatic tissue morphology, viability and energy metabolism during extended cold storage in two-layer oxygenated university of wisconsin/perfluorocarbon solution. Isr. Med. Assoc. J. 2008, 10, 273–276. [Google Scholar] [PubMed]
- Pacifici, A.; Laino, L.; Gargari, M.; Guzzo, F.; Velandia Luz, A.; Polimeni, A.; Pacifici, L. Decellularized hydrogels in bone tissue engineering: A topical review. Int. J. Med. Sci. 2018, 15, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Zhang, Z.; Lou, K.; Zhang, Q.; Wang, S.; Ni, J.; Liu, W.; Fan, S.; Lin, X. Current advances in the development of natural meniscus scaffolds: Innovative approaches to decellularization and recellularization. Cell Tissue Res. 2017, 370, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Milan, A.; Urbani, L.; De Coppi, P.; Lowdell, M.W. Decellularized material as scaffolds for tissue engineering studies in long gap esophageal atresia. Expert Opin. Biol. Ther. 2017, 17, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nicolas, C.T.; Chen, H.S.; Ross, J.J.; De Lorenzo, S.B.; Nyberg, S.L. Recent advances in decellularization and recellularization for tissue-engineered liver grafts. Cells Tissues Organs 2017, 203, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; Bottagisio, M.; Moretti, M. Decellularized and engineered tendons as biological substitutes: A critical review. Stem Cells Int. 2016, 2016, 7276150. [Google Scholar] [CrossRef] [PubMed]
- Nachlas, A.L.Y.; Li, S.; Davis, M.E. Developing a clinically relevant tissue engineered heart valve—A review of current approaches. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Swinehart, I.T.; Badylak, S.F. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2016, 245, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Boccafoschi, F.; Botta, M.; Fusaro, L.; Copes, F.; Ramella, M.; Cannas, M. Decellularized biological matrices: An interesting approach for cardiovascular tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; He, Z.; Li, L.; Liu, G.; Li, Q.; Yang, D.; Zhang, Y.; Li, N. Development and in vivo validation of tissue-engineered, small-diameter vascular grafts from decellularized aortae of fetal pigs and canine vascular endothelial cells. J. Cardiothoracic. Surg. 2017, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Le, A.V.; Hatachi, G.; Beloiartsev, A.; Rocco, K.; Sivarapatna, A.; Mendez, J.J.; Baevova, P.; Dyal, R.N.; Leiby, K.L.; et al. Bioengineered lungs generated from human ipscs-derived epithelial cells on native extracellular matrix. J. Tissue Eng. Regen. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Tondreau, M.Y.; Laterreur, V.; Gauvin, R.; Vallieres, K.; Bourget, J.M.; Lacroix, D.; Tremblay, C.; Germain, L.; Ruel, J.; Auger, F.A. Mechanical properties of endothelialized fibroblast-derived vascular scaffolds stimulated in a bioreactor. Acta Biomater. 2015, 18, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, G.; Rossoni, G.; Sala, A.; Polvani, G.L.; Berti, F.; Dainese, L.; Porqueddu, M.; Biglioli, P. Endothelial-dependent dynamic and antithrombotic properties of porcine aortic and pulmonary valves. Ann. Thorac. Surg. 1998, 65, 986–992. [Google Scholar] [CrossRef]
- Lichtenberg, A.; Cebotari, S.; Tudorache, I.; Sturz, G.; Winterhalter, M.; Hilfiker, A.; Haverich, A. Flow-dependent re-endothelialization of tissue-engineered heart valves. J. Heart Valve Dis. 2006, 15, 287–293. [Google Scholar] [PubMed]
- Hussein, K.H.; Park, K.M.; Kang, K.S.; Woo, H.M. Heparin-gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers. Acta Biomater. 2016, 38, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Nietzer, S.; Baur, F.; Sieber, S.; Hansmann, J.; Schwarz, T.; Stoffer, C.; Hafner, H.; Gasser, M.; Waaga-Gasser, A.M.; Walles, H.; et al. Mimicking metastases including tumor stroma: A new technique to generate a three-dimensional colorectal cancer model based on a biological decellularized intestinal scaffold. Tissue Eng. Part C Methods 2016, 22, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Aron, J.; Agarwal, B.; Davenport, A. Extracorporeal support for patients with acute and acute on chronic liver failure. Expert. Rev. Med. Devices 2016, 13, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Selden, C.; Bundy, J.; Erro, E.; Puschmann, E.; Miller, M.; Kahn, D.; Hodgson, H.; Fuller, B.; Gonzalez-Molina, J.; Le Lay, A.; et al. A clinical-scale bioartificial liver, developed for gmp, improved clinical parameters of liver function in porcine liver failure. Sci. Rep. 2017, 7, 14518. [Google Scholar] [CrossRef] [PubMed]
- Erro, E.; Bundy, J.; Massie, I.; Chalmers, S.A.; Gautier, A.; Gerontas, S.; Hoare, M.; Sharratt, P.; Choudhury, S.; Lubowiecki, M.; et al. Bioengineering the liver: Scale-up and cool chain delivery of the liver cell biomass for clinical targeting in a bioartificial liver support system. Biores Open Access 2013, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Selden, C.; Spearman, C.W.; Kahn, D.; Miller, M.; Figaji, A.; Erro, E.; Bundy, J.; Massie, I.; Chalmers, S.A.; Arendse, H.; et al. Evaluation of encapsulated liver cell spheroids in a fluidised-bed bioartificial liver for treatment of ischaemic acute liver failure in pigs in a translational setting. PLoS ONE 2013, 8, e82312. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Bundy, J.E.E.; Chalmers, S.; Mukhopadhyay, T.; Fuller, B.; Selden, C. Development of a Small Scale Fluidised Bed Bioreactor for Encapsulated Cell Systems to Be Tested Simultaneously, Thereby Speeding up the r&d Process; eCM Meeting Abstracts 2016, Collection 5; TCES: London, UK, 2016; p. 73. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selden, C.; Fuller, B. Role of Bioreactor Technology in Tissue Engineering for Clinical Use and Therapeutic Target Design. Bioengineering 2018, 5, 32. https://doi.org/10.3390/bioengineering5020032
Selden C, Fuller B. Role of Bioreactor Technology in Tissue Engineering for Clinical Use and Therapeutic Target Design. Bioengineering. 2018; 5(2):32. https://doi.org/10.3390/bioengineering5020032
Chicago/Turabian StyleSelden, Clare, and Barry Fuller. 2018. "Role of Bioreactor Technology in Tissue Engineering for Clinical Use and Therapeutic Target Design" Bioengineering 5, no. 2: 32. https://doi.org/10.3390/bioengineering5020032
APA StyleSelden, C., & Fuller, B. (2018). Role of Bioreactor Technology in Tissue Engineering for Clinical Use and Therapeutic Target Design. Bioengineering, 5(2), 32. https://doi.org/10.3390/bioengineering5020032