Non-Transfusional Hemocomponents: From Biology to the Clinic—A Literature Review
Abstract
1. Introduction
2. Definition and History
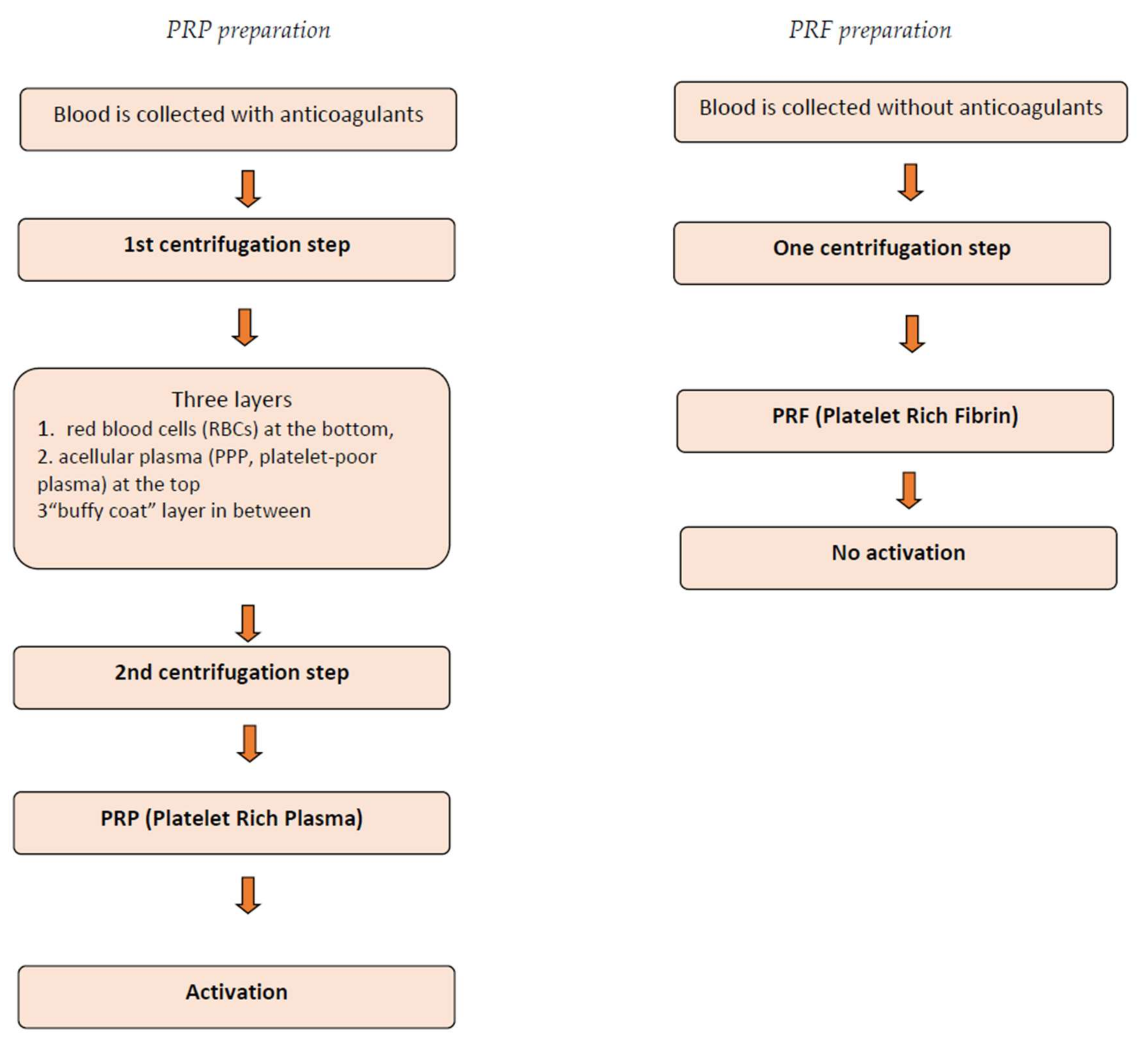
3. Classification and Techniques
4. Biological Behaviour
5. Clinical Applications of Platelet Concentrates
5.1. Reconstructive and Implant Surgery
5.2. Prevention of Hemorrhagic Complications after Dental Extraction
5.3. Periodontology
5.4. Orthopedic and Sports Medicine
5.5. Plastic Surgery and Dermatology
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bielecki, T.; Dohan Ehrenfest, D.M. Platelet-rich plasma (PRP) and Platelet-Rich Fibrin (PRF): Surgical adjuvants, preparations for in situ regenerative medicine and tools for tissue engineering. Curr. Pharm. Biotechnol. 2012, 13, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Goubran, H.A.; Chen, T.M.; Ou, K.L.; El-Ekiaby, M.; Radosevic, M. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013, 27, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Sammartino, G.; Dohan Ehrenfest, D.M.; Carile, F.; Tia, M.; Bucci, P. Prevention of hemorrhagic complications after dental extractions into open heart surgery patients under anticoagulant therapy: The use of leukocyte- and platelet-rich fibrin. J. Oral Implantol. 2011, 37, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Hersant, B.; Bosc, R.; Meningaud, J.P. The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: A review and a proposal for a new standard care. Wound Repair Regen. 2015, 23, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: Periodontal and dentoalveolar surgery. Curr. Pharm. Biotechnol. 2012, 13, 1207–1230. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.Q.; Pinto, N.R.; Bielecki, T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.S.; Kumar, A.S.; Kirit, R.; Konathan, R.; Sivamani, R.K. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J. Cosmet. Dermatol. 2015, 14, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Sammartino, G.; Shibli, J.A.; Wang, H.L.; Zou, D.R.; Bernard, J.P. Guidelines for the publication of articles related to platelet concentrates (Platelet-Rich Plasma—PRP, or Platelet-Rich Fibrin—PRF): The international classification of the POSEIDO. Poseido J. 2013, 1, 17–27. [Google Scholar]
- Borzini, P.; Balbo, V.; Mazzucco, L. Platelet concentrates for topical use: Bedside device and blood transfusion technology. Quality and versatility. Curr. Pharm. Biotechnol. 2012, 13, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, L.; Balbo, V.; Cattana, E.; Guaschino, R.; Borzini, P. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet®, RegenPRP-Kit®, Plateltex® and one manual procedure. Vox Sang. 2009, 97, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Weibrich, G.; Hansen, T.; Kleis, W.; Buch, R.; Hitzler, W.E. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 2004, 34, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.R.; Harbury, C.; Egbert, P.R.; Rubenstein, E. Use of a platelet-fibrinogen-thrombin mixture as a corneal adhesive: Experiments with sutureless lamellar keratoplasty in the rabbit. Investig. Ophthalmol. 1975, 14, 872–875. [Google Scholar]
- Rosenthal, A.R.; Egbert, P.R.; Harbury, C.; Hopkins, J.L.; Rubenstein, E. Use of platelet-fibrinogen-thrombin mixture to seal experimental penetrating corneal wounds. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 1978, 207, 111–115. [Google Scholar] [CrossRef]
- Pearl, R.M.; Wustrack, K.O.; Harbury, C.; Rubenstein, E.; Kaplan, E.N. Microvascular anastomosis using a blood product sealant-adhesive. Surg. Gynecol. Obstet. 1977, 144, 227–231. [Google Scholar] [PubMed]
- Silverberg, G.D.; Harbury, C.B.; Rubenstein, E. A physiological sealant for cerebrospinal fluid leaks. J. Neurosurg. 1977, 46, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Knighton, D.R.; Ciresi, K.F.; Fiegel, V.D.; Austin, L.L.; Butler, E.L. Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF). Ann. Surg. 1986, 204, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Mishra, A.; Borzini, P.; Inchingolo, F.; Sammartino, G.; Rasmusson, L.; Everts, P.A. In search of a consensus terminology in the field of platelet concentrates for surgical use: Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin gel polymerization and leukocytes. Curr. Pharm. Biotechnol. 2012, 13, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.L. Platelet secretory mechanisms. Semin. Thromb. Hemost. 2004, 30, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Demidova-Rice, T.N.; Wolf, L.; Deckenback, J.; Hamblin, M.R.; Herman, I.M. Human platelet-rich plasma- and extracellular matrix-derived peptides promote impaired cutaneous wound healing in vivo. PLoS ONE 2012, 7, e32146. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.B.; Blashki, D.; Buchanan, R.M.; Yazdi, I.K.; Ferrari, M.; Simmons, P.J.; Tasciotti, E. Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials 2012, 33, 5308–5316. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Thakur, A. Platelet concentrates: Past, present and future. J. Maxillofac. Oral Surg. 2011, 10, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Keen, D. A review of research examining the regulatory role of lymphocytes in normal wound healing. J. Wound Care 2008, 17, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; de Peppo, G.M.; Doglioli, P.; Sammartino, G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): A gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 2009, 27, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Passaretti, F.; Tia, M.; D’Esposito, V.; De Pascale, M.; Del Corso, M.; Sepulveres, R.; Liguoro, D.; Valentino, R.; Beguinot, F.; Formisano, P.; et al. Growth-promoting action and growth factor release by different platelet derivatives. Platelets 2014, 25, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Cabaro, S.; D’Esposito, V.; Gasparro, R.; Borriello, F.; Granata, F.; Mosca, G.; Passaretti, F.; Sammartino, J.C.; Beguinot, F.; Sammartino, G.; et al. White cell and platelet content affects the release of bioactive factors in different blood-derived scaffolds. Platelets 2017, 21, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hosny, N.; Goubran, F.; BadrEldin Hasan, B.; Kamel, N. Assessment of vascular endothelial growth factor in fresh versus frozen platelet rich plasma. J. Blood Transfus. 2015, 2015, 706903. [Google Scholar] [CrossRef] [PubMed]
- Roffi, A.; Filardo, G.; Assirelli, E.; Cavallo, C.; Cenacchi, A.; Facchini, A.; Grigolo, B.; Kon, E.; Mariani, E.; Pratelli, L.; et al. Does platelet-rich plasma freeze-thawing influence growth factor release and their effects on chondrocytes and synoviocytes? Biomed. Res. Int. 2014, 2014, 692913. [Google Scholar] [CrossRef] [PubMed]
- Shiga, Y.; Orita, S.; Kubota, G.; Kamoda, H.; Yamashita, M.; Matsuura, Y.; Yamauchi, K.; Eguchi, Y.; Suzuki, M.; Inage, K.; et al. Freeze-dried platelet-rich plasma accelerates bone union with adequate rigidity in posterolateral lumbar fusion surgery model in rats. Sci. Rep. 2016, 6, 36715. [Google Scholar] [CrossRef] [PubMed]
- Simonpieri, A.; Del Corso, M.; Vervelle, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr. Pharm. Biotechnol. 2012, 13, 1231–1256. [Google Scholar] [CrossRef] [PubMed]
- Whitman, D.H.; Berry, R.L.; Green, D.M. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 1997, 55, 1294–1299. [Google Scholar] [CrossRef]
- Broggini, N.; Hofstetter, W.; Hunziker, E.; Bosshardt, D.D.; Bornstein, M.M.; Seto, I.; Weibrich, G.; Buser, D. The influence of PRP on early bone formation in membrane protected defects. A histological and histomorphometric study in the rabbit calvaria. Clin. Implant Dent. Relat. Res. 2011, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Tamimi, F.M.; Tresguerres, I.F.; Alkhraisat, M.H.; Khraisat, A.; Lopez-Cabarcos, E.; Blanco, L. Effect of solely applied platelet-rich plasma on osseous regeneration compared to Bio-Oss: A morphometric and densitometric study on rabbit calvaria. Clin. Implant Dent. Relat. Res. 2008, 10, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Simonpieri, A.; Del Corso, M.; Sammartino, G.; Dohan Ehrenfest, D.M. The relevance of Choukroun’s platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. Part I: A new grafting protocol. Implant Dent. 2009, 18, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Simonpieri, A.; Del Corso, M.; Sammartino, G.; Dohan Ehrenfest, D.M. The relevance of Choukroun’s platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. Part II: Implant surgery, prosthodontics, and survival. Implant Dent. 2009, 18, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, A.P.; Touati, B. Soft tissue recession around implants: Is it still unavoidable?—Part II. Pract. Proced. Aesthet. Dent. 2007, 19, 81–87. [Google Scholar]
- Scully, C.; Wolff, A. Oral surgery in patients on anticoagulant therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 94, 57–64. [Google Scholar] [CrossRef]
- Della Valle, A.; Sammartino, G.; Marenzi, G.; Tia, M.; Espedito di Lauro, A.; Ferrari, F.; Lo Muzio, L. Prevention of postoperative bleeding in anticoagulated patients undergoing oral surgery: Use of platelet-rich plasma gel. J. Oral Maxillofac. Surg. 2003, 61, 1275–1278. [Google Scholar] [CrossRef]
- Sammartino, G.; Tia, M.; Marenzi, G.; di Lauro, A.E.; D’Agostino, E.; Claudio, P.P. Use of autologous platelet-rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J. Oral Maxillofac. Surg. 2005, 63, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Thorat, M.; Pradeep, A.R.; Pallavi, B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: A controlled clinical trial. J. Clin. Periodontol. 2011, 38, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, S.; Aleksic, Z.; Klokkevold, P.; Lekovic, V.; Dimitrijevic, B.; Kenney, E.B.; Camargo, P. Use of platelet-rich fibrin membrane following treatment of gingival recession: A randomized clinical trial. Int. J. Periodontics Restor. Dent. 2012, 32, e41–e50. [Google Scholar]
- Mishra, A.; Harmon, K.; Woodall, J.; Vieira, A. Sports medicine applications of platelet rich plasma. Curr. Pharm. Biotechnol. 2012, 13, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, Y.; Morihara, T.; Sakamoto, H.; Matsuda, K.; Oshima, Y.; Yoshida, A.; Nagae, M.; Arai, Y.; Kawata, M.; Kubo, T. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J. Cell. Physiol. 2008, 215, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Anitua, E.; Orive, G.; Mujika, I.; Andia, I. Platelet-rich therapies in the treatment of orthopedic sport injuries. Sports Med. 2009, 39, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Delcogliano, M.; Presti, M.L.; Russo, A.; Bondi, A.; Di Martino, A.; Cenacchi, A.; Fornasari, P.M.; Marcacci, M. Platelet-rich plasma: New clinical application: A pilot study for treatment of jumper’s knee. Injury 2009, 40, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Sommeling, C.E.; Heyneman, A.; Hoeksema, H.; Verbelen, J.; Stillaert, F.B.; Monstrey, S. The use of platelet-rich plasma in plastic surgery: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, L.; Medici, D.; Serra, M.; Panizza, R.; Rivara, G.; Orecchia, S.; Libener, R.; Cattana, E.; Levis, A.; Betta, P.G.; et al. The use of autologous platelet gel to treat difficult-to-heal wounds: A pilot study. Transfusion 2004, 44, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Saad Setta, H.; Elshahat, A.; Elsherbiny, K.; Massoud, K.; Safe, I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: A comparative study. Int. Wound J. 2011, 8, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Grimalt, R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol. Surg. 2016, 42, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Cervelli, V. The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial. Stem. Cells Transl. Med. 2015, 4, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
| References | Study Type | Results |
|---|---|---|
| Hard and soft tissue regeneration | ||
| Marx R.E. et al., 1998 [3] | Clinical study | PRP enhanced bone graft |
| Weibrich G. et al., 2004 [12] | Clinical study | PRP was not beneficial in accelerating osseointegration |
| Broggini N. et al., 2011 [34] | Histological study | PRP did not lead to greater bone remodeling |
| Torres J. et al., 2008 [35] | Morphometric study | PRP was not beneficial in osseous regeneration |
| Simonpieri et al, 2009 [36] | Clinical study | PRF was helpful for periosteum healing and maturation |
| Sammartino et al., 2005 [41] | Clinical study | PRP was effective in accelerating bone regeneration |
| Thorat M. et al., 2011 [42] | Clinical study | PRF improved intra-bony defect fill |
| Sommeling et al., 2013 [48] | Systematic review | PRP enhanced bone graft regeneration |
| Hemostasis | ||
| Della Valle A. et al., 2003 [40] | Clinical study | PRP reduced postoperative hemorrhage |
| Sammartino G. et al., 2011 [4] | Clinical study | PRF reduced postoperative hemorrhage |
| Wound healing | ||
| Picard F. et al., 2015 [5] | Literature Review | PRP may be beneficial in diabetic chronic wounds |
| Knighton D.R. et al., 1986 [17] | Clinical study | PDWHF promoted the healing of chronic cutaneous ulcers |
| Jankovic S. et al., 2012 [43] | Clinical study | PRF enhanced wound healing in gingival recession |
| Sommeling et al., 2013 [48] | Systematic review | PRP improved the wound healing rate |
| Mazzucco et al., 2004 [49] | Clinical study | Platelet gel improved chronic unhealing wounds |
| Saad Setta et al., 2011 [50] | Clinical study | PRP enhanced healing of chronic diabetic foot ulcers |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasparro, R.; Qorri, E.; Valletta, A.; Masucci, M.; Sammartino, P.; Amato, A.; Marenzi, G. Non-Transfusional Hemocomponents: From Biology to the Clinic—A Literature Review. Bioengineering 2018, 5, 27. https://doi.org/10.3390/bioengineering5020027
Gasparro R, Qorri E, Valletta A, Masucci M, Sammartino P, Amato A, Marenzi G. Non-Transfusional Hemocomponents: From Biology to the Clinic—A Literature Review. Bioengineering. 2018; 5(2):27. https://doi.org/10.3390/bioengineering5020027
Chicago/Turabian StyleGasparro, Roberta, Erda Qorri, Alessandra Valletta, Michele Masucci, Pasquale Sammartino, Alessandra Amato, and Gaetano Marenzi. 2018. "Non-Transfusional Hemocomponents: From Biology to the Clinic—A Literature Review" Bioengineering 5, no. 2: 27. https://doi.org/10.3390/bioengineering5020027
APA StyleGasparro, R., Qorri, E., Valletta, A., Masucci, M., Sammartino, P., Amato, A., & Marenzi, G. (2018). Non-Transfusional Hemocomponents: From Biology to the Clinic—A Literature Review. Bioengineering, 5(2), 27. https://doi.org/10.3390/bioengineering5020027








