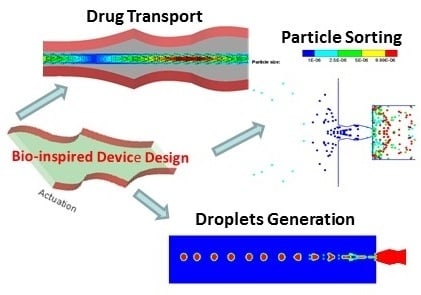
Bio-Inspired Multi-Functional Drug Transport Design Concept and Simulations †
Abstract
:1. Introduction
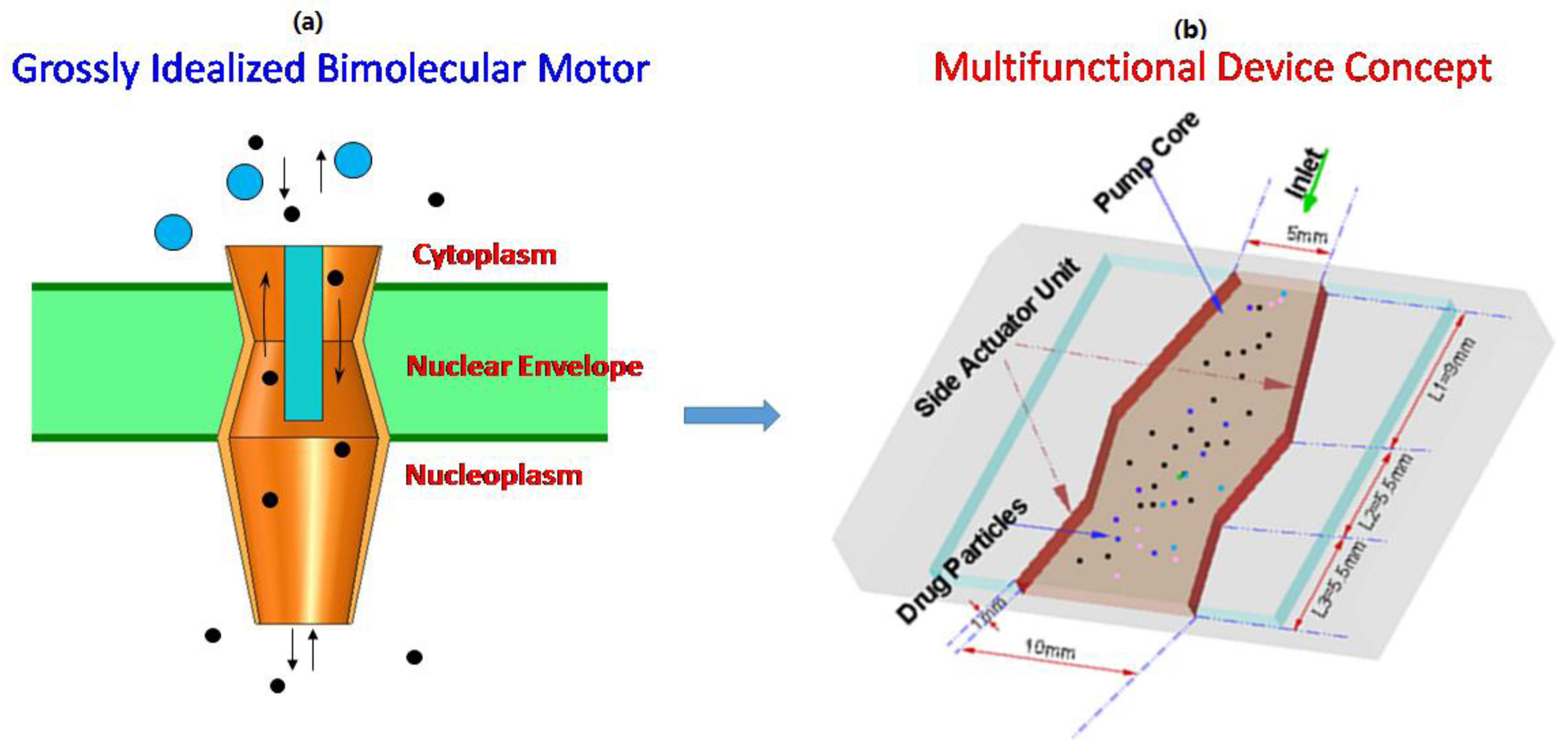
2. Multi-Functional Device Design
3. Design Analysis Methodology
3.1. Design Computational Model
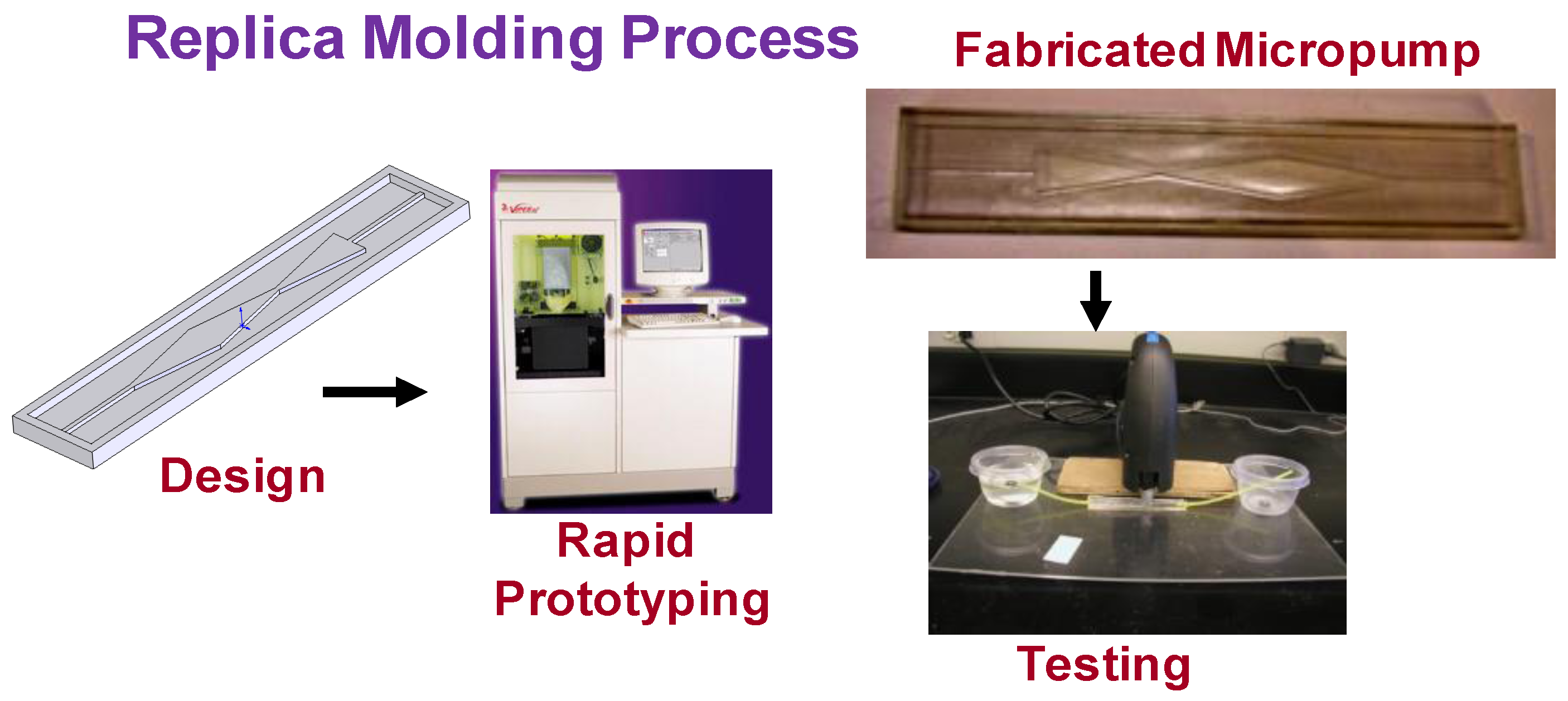
3.2. Prototyping and Testing
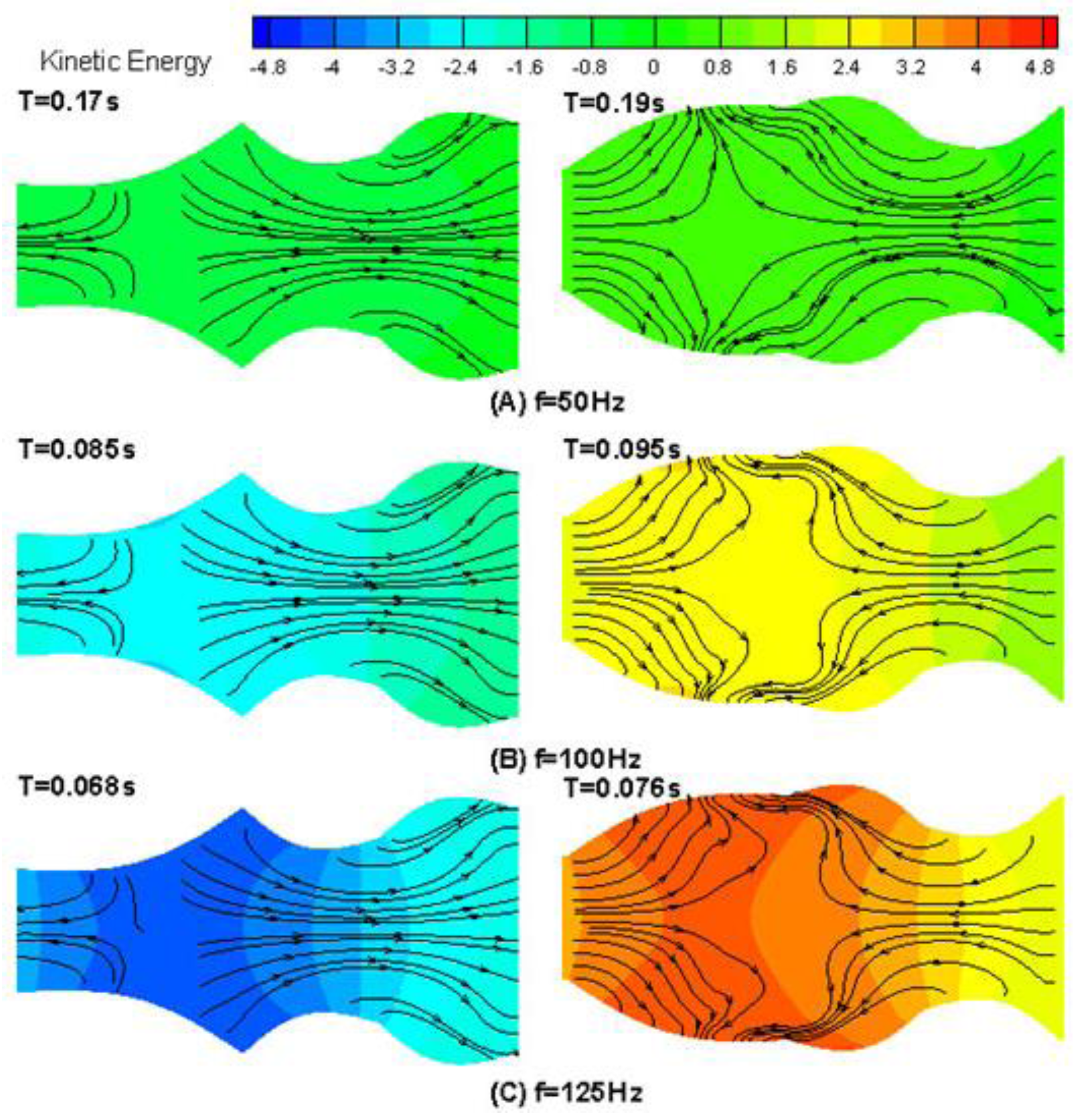
4. Results and Discussion
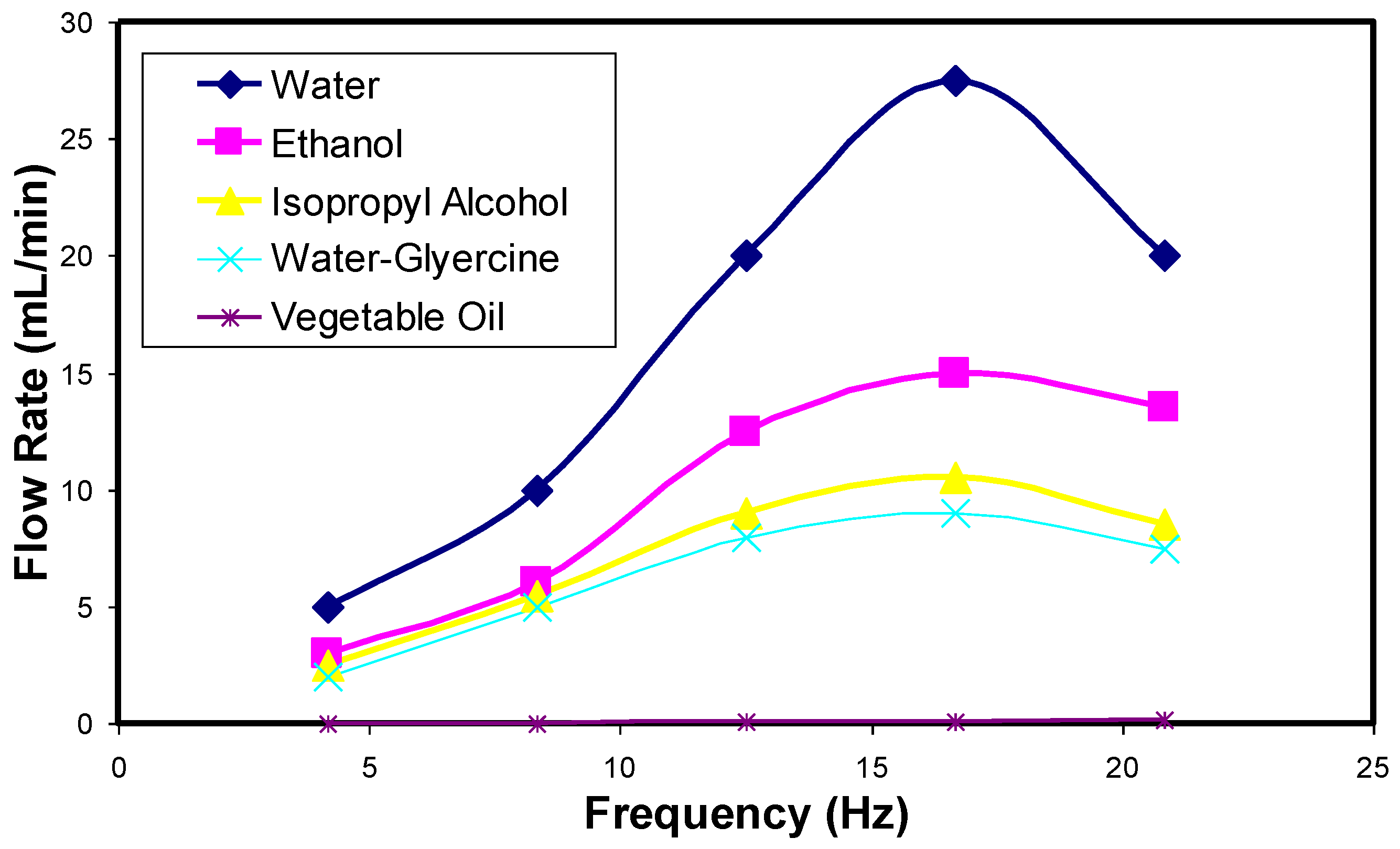
4.1. Drug Transport Design Simulations
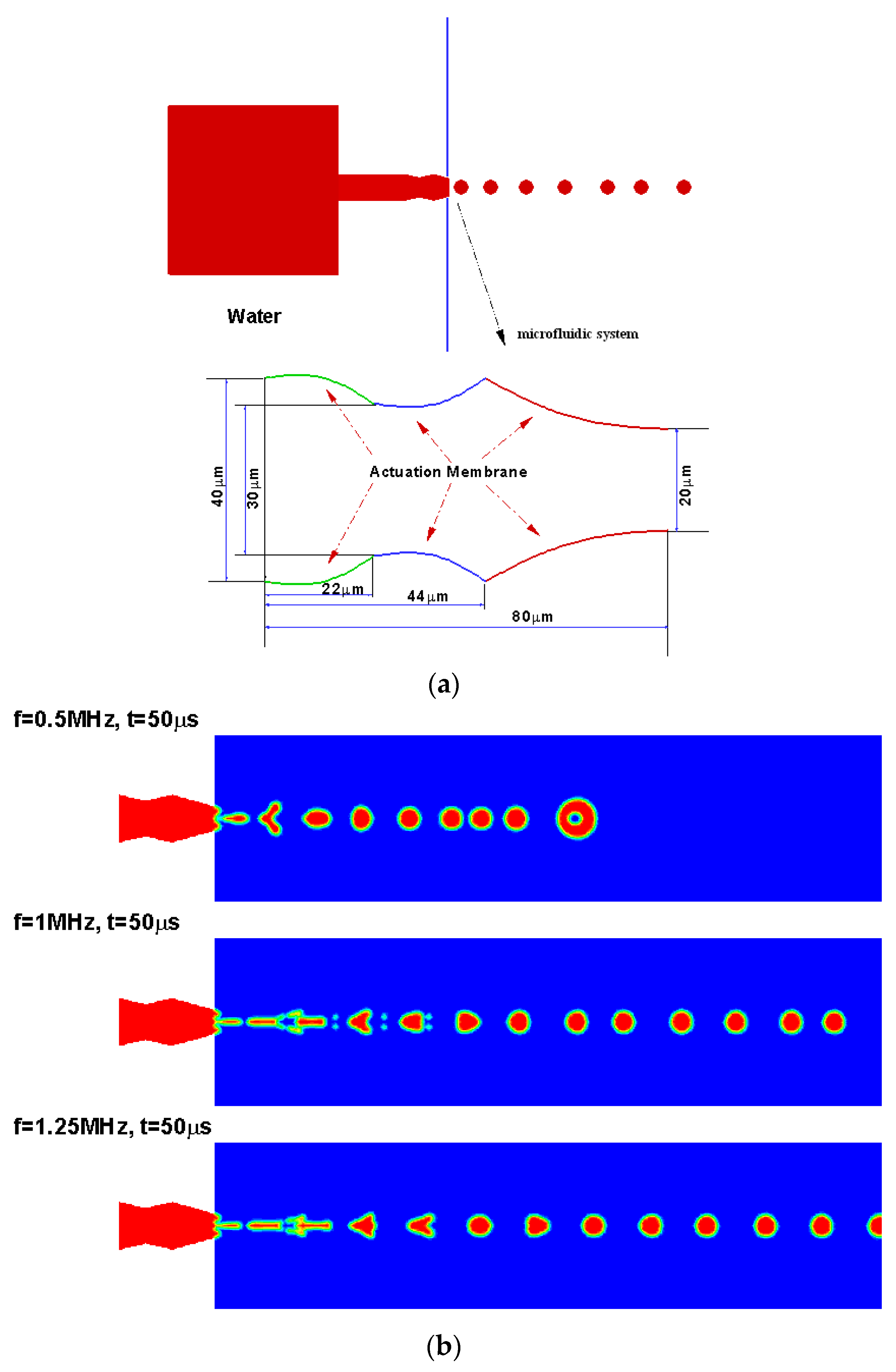
4.2. Droplet Generation Design Simulations
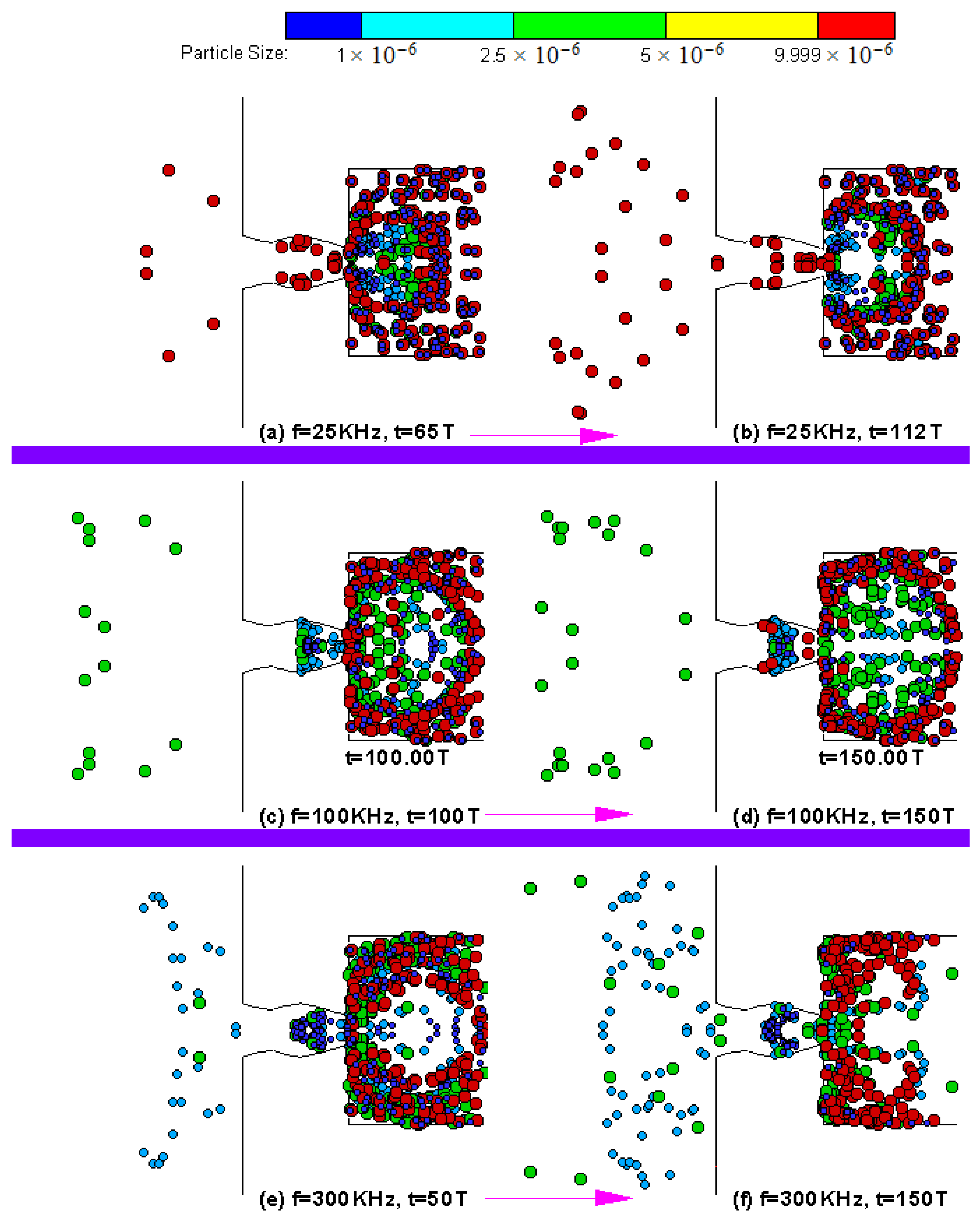
4.3. Particle Sorting Design Simulations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zimon, A.; Di Talia, S.; Chait, B.T.; Rout, M.P.; Magnasco, M.O. Efficiency, selectivity, and robustness of nucleocytoplasmic transport. PLoS Comput. Biol. 2007, 3, 1281–1290. [Google Scholar]
- Blobel, G.; Wozniak, R.W. Structural Biology: Proteomics for the Pore. Nature 2000, 403, 835–836. [Google Scholar] [CrossRef] [PubMed]
- Pante, N.; Aebi, U. Towards understanding the 3-D structure of the Nuclear Pore Complex at the molecular level. Curr. Opin. Struct. Biol. 1996, 4, 187–196. [Google Scholar] [CrossRef]
- Fahrenkrog, B.; Aebi, U. The nuclear pore complex: Nucleocytoplasmic transport and beyond. Nat. Rev. Mol. Cell Biol. 2003, 4, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Chait, B.T.; Sali, A.; Rout, M.P. The molecular architecture of the nuclear pore complex. Nature 2007, 450, 695–701. [Google Scholar]
- Rout, M.P.; Aitchison, J.D.; Suprapto, A.; Hjertaas, K.; Zhao, Y.; Chait, B.T. The yeast nuclear pore complex: Composition, architecture, and transport mechanism. J. Cell Biol. 2000, 148, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Suntharalingam, M.; Wente, S.R. Peering through the pore: Nuclear pore complex structure, assembly and function. Dev. Cell 2003, 4, 775–789. [Google Scholar] [CrossRef]
- Akey, C.W. Interactions and structure of the Nuclear Pore Complex revealed by cryo-electron microscopy. J. Cell Biol. 1989, 109, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Foerster, F.; Ecke, M.; Plitzko, J.M.; Melchior, F.; Gerisch, G.; Baumeister, W.; Medalia, O. Nuclear Pore Complex structure and dynamics revealed by cryoelectron tomography. Science 2004, 306, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Pante, N. Nuclear pore complex structure: Unplugged and dynamic pores. Dev. Cell 2004, 7, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Mantell, S.; Polla, D. Design and simulation of an implantable medical drug delivery system using microelectromechanical systems technology. Sens. Actuators A 2001, 94, 117–125. [Google Scholar] [CrossRef]
- Junwu, K.; Zhigang, Y.; Taijiang, P.; Guangming, C.; Boda, W. Design and test of a high-performance piezoelectric micropump for drug delivery. Sens. Actuators A 2005, 121, 156–161. [Google Scholar] [CrossRef]
- Teymoori, M.M.; Abbaspour-Sani, E. Design and simulation of a novel electrostatic peristaltic micromachined pump for drug delivery applications. Sens. Actuators A 2005, 117, 222–229. [Google Scholar] [CrossRef]
- Jang, L.; Kan, W. Peristaltic piezoelectric micropump system for biomedical applications. Biomed. Microdevices 2007, 9, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Q. Valveless piezoelectric micropump for fuel delivery in direct methanol fuel cell (DMFC) devices. J. Power Sources 2005, 140, 72–80. [Google Scholar] [CrossRef]
- Kohler, J.; Bejhed, J.; Kratz, H.; Bruhn, F.; Lindberg, U.; Hjort, K.; Stenmark, L. A hybrid cold gas microthruster system for spacecraft. Sens. Actuators A 2002, 97–98, 587–598. [Google Scholar] [CrossRef]
- Rossi, C.; Rouhani, M.D.; Esteve, D. Prediction of the performance of a Si-micromachined microthruster by computing the subsonic gas flow inside the thruster. Sens. Actuators A 2000, 87, 96–104. [Google Scholar] [CrossRef]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Koombua, K.; Pidaparti, R.M.; Longest, P.W.; Atkinson, G.M. Design Evaluation for Performance Characteristics of a Novel Valveless Micropump. J. Nanoscale Microscale Thermophys. Eng. 2010, 14, 34–50. [Google Scholar] [CrossRef]
- Su, G.; Longest, P.W.; Pidaparti, R.M. A Novel Micropump Droplet Generator for Aerosol Drug Delivery: Design Simulations. Biomicrofluidics 2010, 4, 044108. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Pidaparti, R.M. Drug Particle Delivery Investigation through a Valveless Micropump. ASME J. Microelectromech. Syst. 2010, 19, 1390–1399. [Google Scholar] [CrossRef]
- Su, G.; Pidaparti, R.M. Separation of Particles for Drug Delivery using a Microfluidic Device with Actuation. ASME J. Nanotechnol. Eng. Med. 2011, 2, 021006. [Google Scholar] [CrossRef]
- FLUENT; Version 13; ANSYS, Inc.: Pittsburgh, PA, USA, 2010.
- Morsi, S.A.; Alexander, A.J. An Investigation of Particle Trajectories in Two-Phase Flow Systems. J. Fluid Mech. 1972, 55, 193–208. [Google Scholar] [CrossRef]
- Brackbill, J.U.; Kothe, D.B.; Zemach, C.A. Continuum Method for Modelling Surface Tension. J. Comput. Phys. 1992, 100, 335–354. [Google Scholar] [CrossRef]
- Charles, P.C.; Ramana, M.P. Design, Fabrication and Testing of a Nozzle/Diffuser PDMS Micropump with Top Actuation. Micro Nanosyst. 2011, 3, 8–13. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pidaparti, R.M.; Cartin, C.; Su, G. Bio-Inspired Multi-Functional Drug Transport Design Concept and Simulations. Bioengineering 2017, 4, 37. https://doi.org/10.3390/bioengineering4020037
Pidaparti RM, Cartin C, Su G. Bio-Inspired Multi-Functional Drug Transport Design Concept and Simulations. Bioengineering. 2017; 4(2):37. https://doi.org/10.3390/bioengineering4020037
Chicago/Turabian StylePidaparti, Ramana M., Charles Cartin, and Guoguang Su. 2017. "Bio-Inspired Multi-Functional Drug Transport Design Concept and Simulations" Bioengineering 4, no. 2: 37. https://doi.org/10.3390/bioengineering4020037
APA StylePidaparti, R. M., Cartin, C., & Su, G. (2017). Bio-Inspired Multi-Functional Drug Transport Design Concept and Simulations. Bioengineering, 4(2), 37. https://doi.org/10.3390/bioengineering4020037