Fed-Batch Synthesis of Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate) from Sucrose and 4-Hydroxybutyrate Precursors by Burkholderia sacchari Strain DSM 17165
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Maintenance and Adaptation to Elevated Temperature
2.2. Shaking Flask Cultivation to Assess Production of 4HB-Containing PHA
2.3. Bioreactor Cultivations
2.3.1. PHB Production
2.3.2. P(3HB-co-4HB) Production:
2.4. Cell Dry Mass (CDM) Determination
2.5. Analysis of PHA Content in Biomass and Monomeric PHA Composition
2.6. Preparation of Na-4HB
2.7. Substrate Analysis
2.8. Analysis of Nitrogen Source (NH4+)
2.9. PHA Recovery
2.10. Polymer Characterization
2.10.1. Molecular Mass Distribution
2.10.2. Thermoanalysis
3. Results
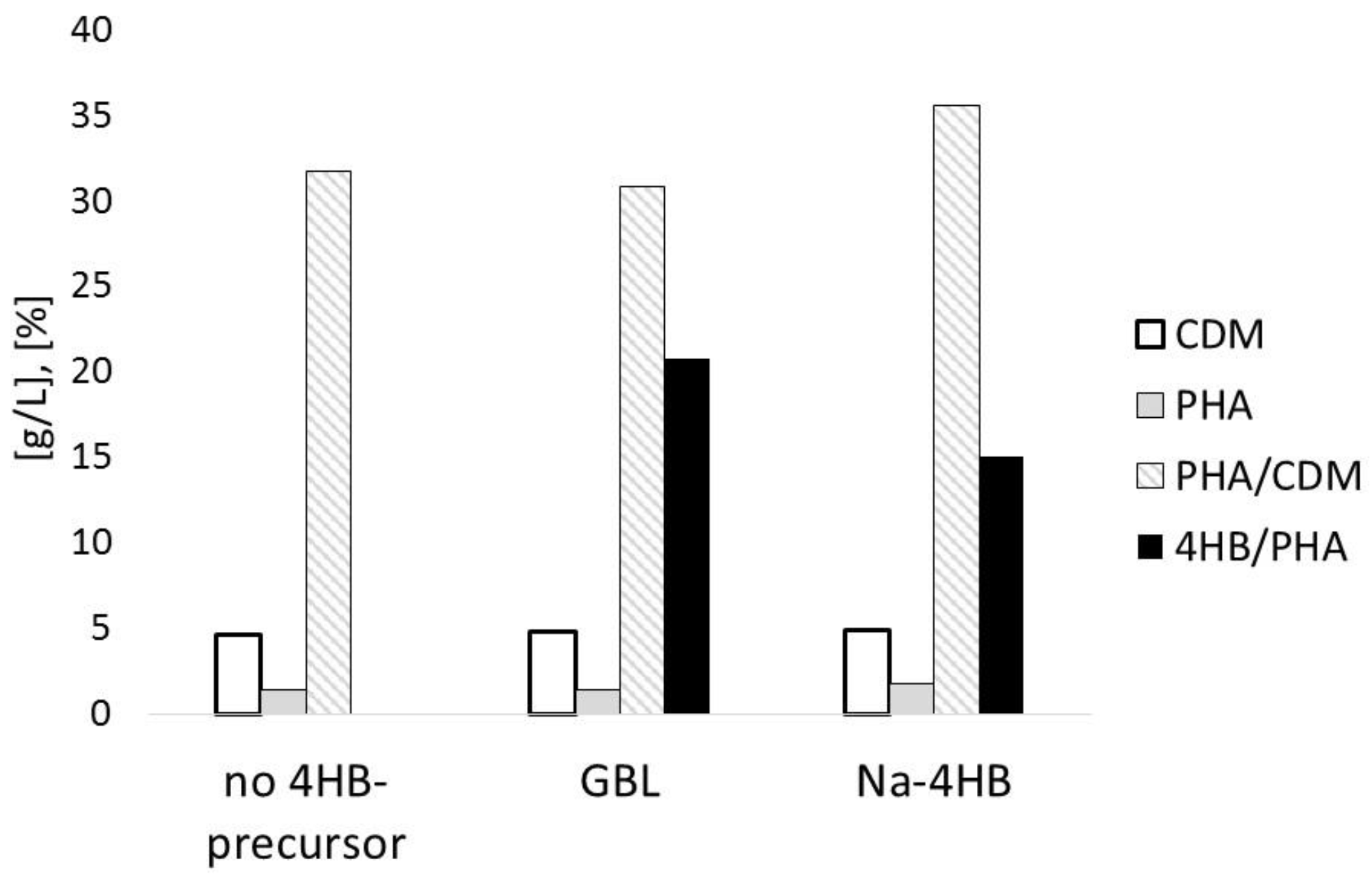
3.1. Impact of 4HB-Precursors GBL and Na-4HB on Poly-(3-hydroxybutyrate-co-4-hydroxybutyrate) (P(3HB-co-4HB)) Biosynthesis by Burkholderia sacchari DSM 17165 on Sucrose
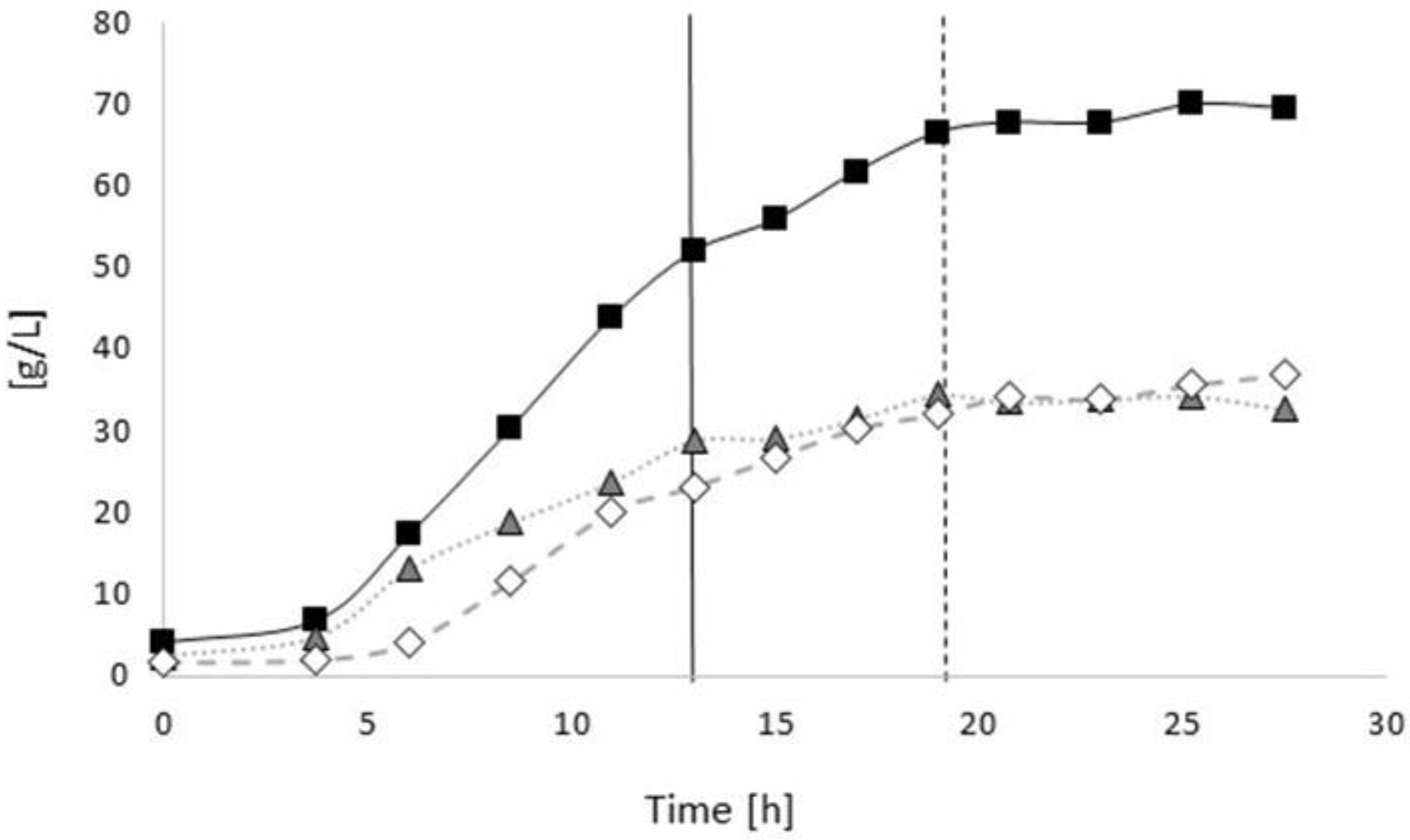
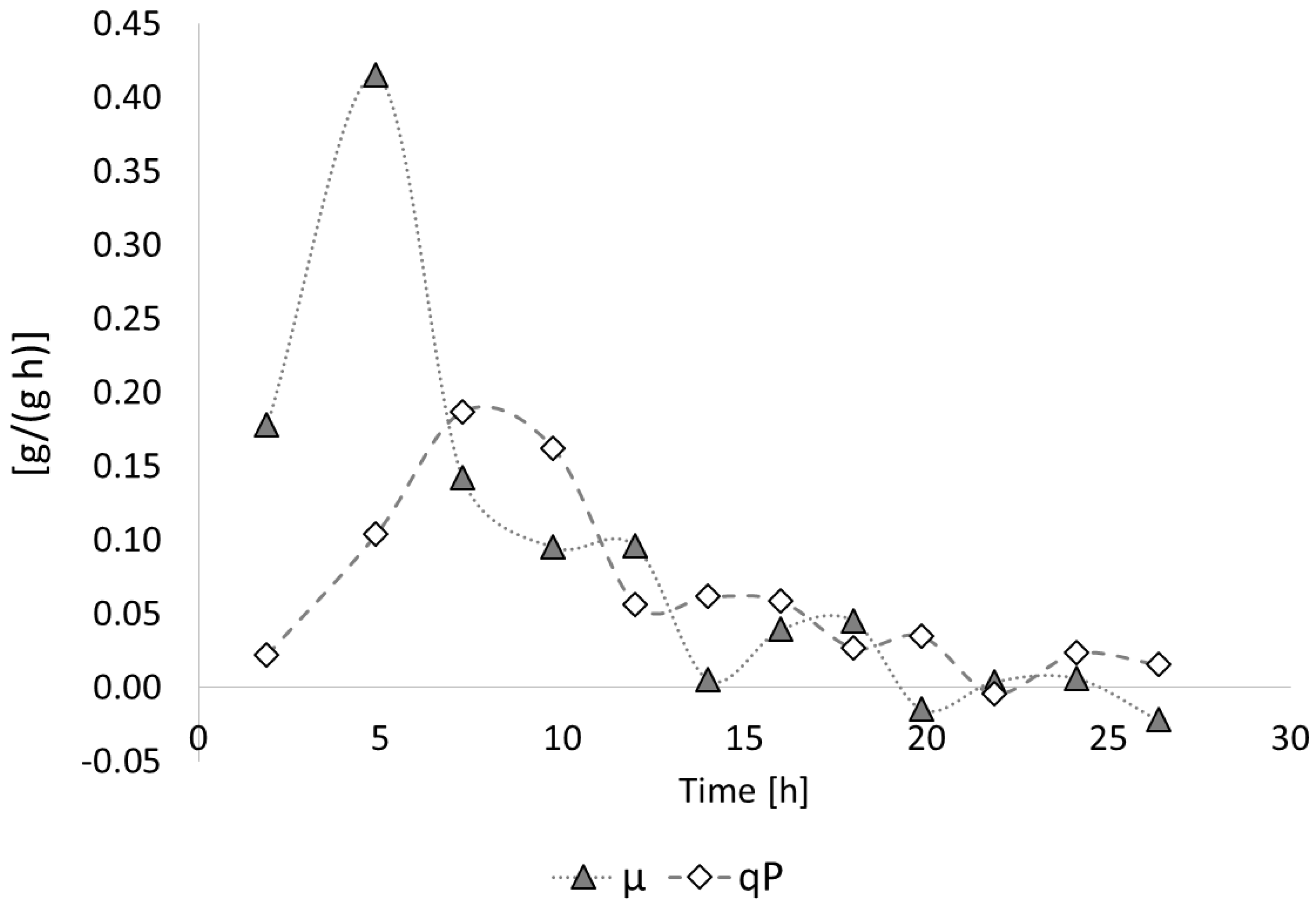
3.2. Poly(3-hydroxybutyrate) (PHB) Production with Burkholderia sacchari on the Bioreactor Scale; Sucrose as the Sole Carbon Source
3.2.1. Bioprocess
3.2.2. Polymer Characterization:
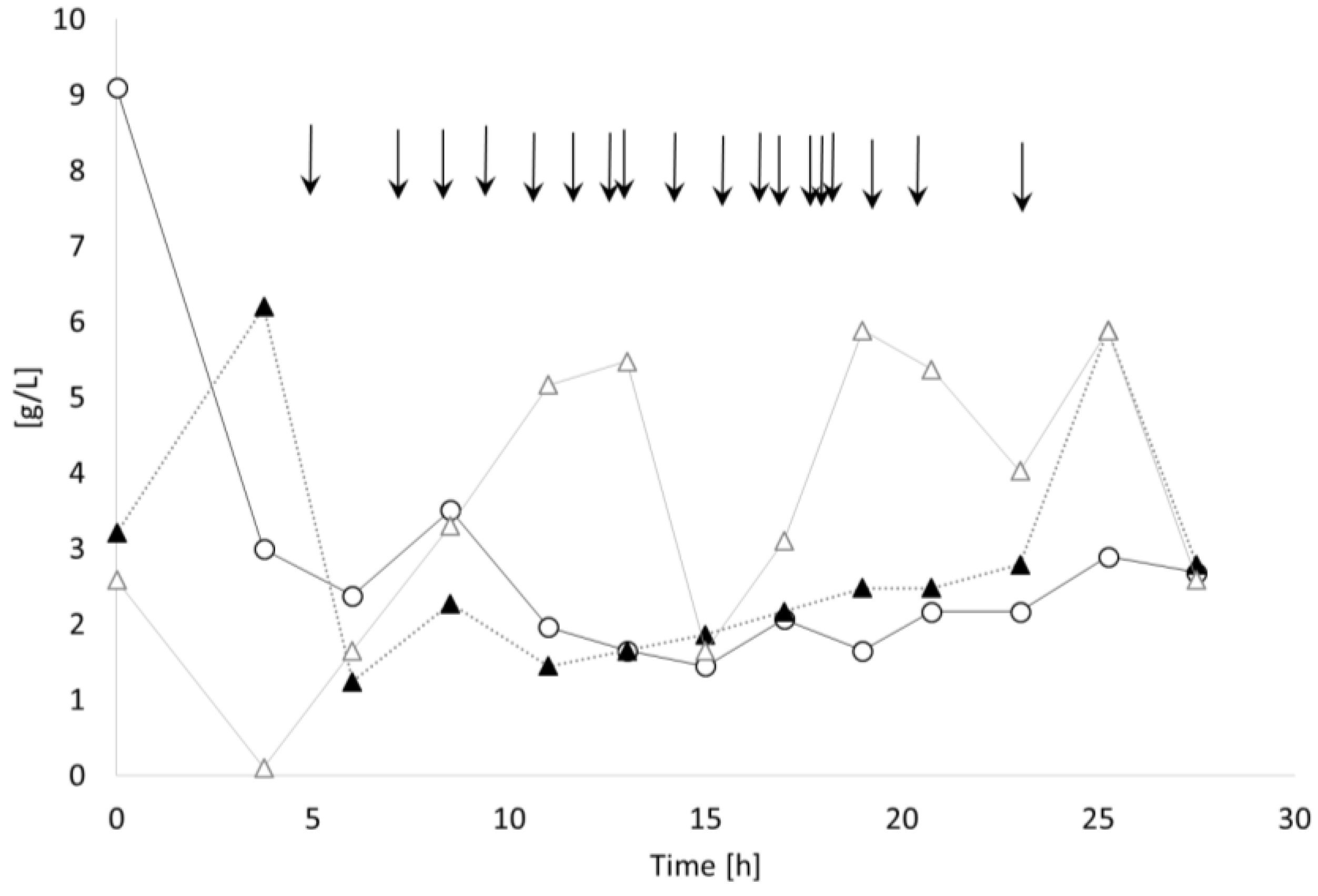
3.3. Controlled Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate) (P(3HB-co-4HB)) Production with Burkholderia sacchari on the Bioreactor Scale: Sucrose plus GBL as Carbon Subsubstrates.
3.3.1. Bioprocess
3.3.2. Polymer Characterization:
4. Discussion
4.1. Bioprocess
4.2. Polymer Characterization:
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jendrossek, D.; Pfeiffer, D. New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ. Microbiol. 2014, 16, 2357–2373. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Hajnal, I. The ‘PHAome’. Trends Biotechnol. 2015, 33, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Y.A.; Chen, C.L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.Y. Start a research on biopolymer polyhydroxyalkanoate (PHA): A review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Mravec, F.; Samek, O.; Marova, I. Evaluation of 3-hydroxybutyrate as an enzyme-protective agent against heating and oxidative damage and its potential role in stress response of poly(3-hydroxybutyrate) accumulating cells. Appl. Microbiol. Biotechnol. 2016, 100, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Ayub, N.D.; Pettinari, M.J.; Ruiz, J.A.; López, N.I. A polyhydroxybutyrate-producing Pseudomonas sp. isolated from Antarctic environments with high stress resistance. Curr. Microbiol. 2004, 49, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Marova, I.; Stankova, M.; Mravcova, L.; Svoboda, Z. Effect of ethanol and hydrogen peroxide on poly(3-hydroxybutyrate) biosynthetic pathway in Cupriavidus necator H16. World J. Microbiol. Biotechnol. 2010, 26, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Steinbüchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Zinn, M.; Witholt, B.; Egli, T. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001, 53, 5–21. [Google Scholar] [CrossRef]
- Pérez Amaro, L.; Chen, H.; Barghini, A.; Corti, A.; Chiellini, E. High performance compostable biocomposites based on bacterial polyesters suitable for injection molding and blow extrusion. Chem. Biochem. Eng. Q. 2015, 29, 261–274. [Google Scholar] [CrossRef]
- Kovalcik, A.; Machovsky, M.; Kozakova, Z.; Koller, M. Designing packaging materials with viscoelastic and gas barrier properties by optimized processing of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with lignin. React. Funct. Polym. 2015, 94, 25–34. [Google Scholar] [CrossRef]
- Koller, M. Poly(hydroxyalkanoates) for food packaging: Application and attempts towards implementation. Appl. Food Biotechnol. 2014, 1, 3–15. [Google Scholar]
- Khosravi-Darani, K.; Bucci, D.Z. Application of poly(hydroxyalkanoate) in food packaging: Improvements by nanotechnology. Chem. Biochem. Eng. Q. 2015, 29, 275–285. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Villano, M.; Oliveira, C.; Albuquerque, M.G.; Majone, M.; Reis, M.A.M.; Lopez-Rubio, A.; Lagaron, J.M. Characterization of polyhydroxyalkanoates synthesized from microbial mixed cultures and of their nanobiocomposites with bacterial cellulose nanowhiskers. New Biotechnol. 2014, 31, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Narodoslawsky, M.; Shazad, K.; Kollmann, R.; Schnitzer, H. LCA of PHA production–Identifying the ecological potential of bio-plastic. Chem. Biochem. Eng. Q. 2015, 29, 299–305. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.J.; Del Rio, L.F.; Orsat, V. Producing PHAs in the bioeconomy—Towards a sustainable bioplastic. Sustain. Prod. Consum. 2017, 9, 58–70. [Google Scholar] [CrossRef]
- Koller, M. Poly(hydroxyalkanoate) (PHA) biopolyesters: Production, Performance and processing aspects. Chem. Biochem. Eng. Q. 2015, 29, 261. [Google Scholar]
- Koller, M.; Niebelschütz, H.; Braunegg, G. Strategies for recovery and purification of poly[(R)-3-hydroxyalkanoates] (PHA) biopolyesters from surrounding biomass. Eng. Life Sci. 2013, 13, 549–562. [Google Scholar] [CrossRef]
- Madkour, M.H.; Heinrich, D.; Alghamdi, M.A.; Shabbaj, I.I.; Steinbüchel, A. PHA recovery from biomass. Biomacromolecules 2013, 14, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Murugan, P.; Han, L.; Gan, C.Y.; Maurer, F.H.; Sudesh, K. A new biological recovery approach for PHA using mealworm, Tenebrio molitor. J. Biotechnol. 2016, 239, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Rosengart, A.; Cesário, M.T.; de Almeida, M.C.M.; Raposo, R.S.; Espert, A.; de Apodaca, E.D.; da Fonseca, M.M.R. Efficient P(3HB) extraction from Burkholderia sacchari cells using non-chlorinated solvents. Biochem. Eng. J. 2015, 103, 39–46. [Google Scholar] [CrossRef]
- Kaur, G.; Roy, I. Strategies for large-scale production of polyhydroxyalkanoates. Chem. Biochem. Eng. Q. 2015, 29, 157–172. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A. Continuous production mode as a viable process-engineering tool for efficient poly(hydroxyalkanoate) (PHA) bio-production. Chem. Biochem. Eng. Q. 2014, 28, 65–77. [Google Scholar]
- Sindhu, R.; Pandey, A.; Binod, P. Solid-state fermentation for the production of poly(hydroxyalkanoates). Chem. Biochem. Eng. Q. 2015, 29, 173–181. [Google Scholar] [CrossRef]
- Haas, C.; El-Najjar, T.; Virgolini, N.; Smerilli, M.; Neureiter, M. High cell-density production of poly(3-hydroxybutyrate) in a membrane bioreactor. New Biotechnol. 2017, 37, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Koller, M.; Braunegg, M.; Horvat, P. Mathematical modelling as a tool for optimized PHA production. Chem. Biochem. Eng. Q. 2015, 29, 183–220. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Chiellini, E.; Fernandes, E.G.; Horvat, P.; Kutschera, C.; Hesse, P.; Braunegg, G. Polyhydroxyalkanoate production from whey by Pseudomonas hydrogenovora. Bioresour. Technol. 2008, 99, 4854–4863. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Benesova, P.; Marsalek, L.; Marova, I. Use of lignocellulosic materials for PHA production. Chem. Biochem. Eng. Q. 2015, 29, 135–144. [Google Scholar] [CrossRef]
- Cesário, M.T.; Raposo, R.S.; de Almeida, M.C.M.; Van Keulen, F.; Ferreira, B.S.; Telo, J.P.; da Fonseca, M.M.R. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Burkholderia sacchari using wheat straw hydrolysates and gamma-butyrolactone. Int. J. Biol. Macromol. 2014, 71, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.; Steinwandter, V.; Diaz De Apodaca, E.; Maestro Madurga, B.; Smerilli, M.; Dietrich, T.; Neureiter, M. Production of PHB from chicory roots—Comparison of three Cupriavidus necator strains. Chem. Biochem. Eng. Q. 2015, 29, 99–112. [Google Scholar] [CrossRef]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Schiller, M.; Kwiecień, M.; Adamus, G.; Kowalczuk, M.; Strohmeier, K.; Schober, S.; et al. Biodegradable latexes from animal-derived waste: Biosynthesis and characterization of mcl-PHA accumulated by Ps. citronellolis. React. Funct. Polym. 2013, 73, 1391–1398. [Google Scholar] [CrossRef]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Malli, K.; Strohmeier, K.; Schober, S; Mittelbach, M.; Koller, M. Novel description of mcl-PHA biosynthesis by Pseudomonas chlororaphis from animal-derived waste. J. Biotechnol. 2013, 165, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Titz, M.; Kettl, K.H.; Shahzad, K.; Koller, M.; Schnitzer, H.; Narodoslawsky, M. Process optimization for efficient biomediated PHA production from animal-based waste streams. Clean Technol. Environ. Policy 2012, 14, 495–503. [Google Scholar] [CrossRef]
- Obruca, S.; Marova, I.; Snajdar, O.; Mravcova, L.; Svoboda, Z. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator from waste rapeseed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol. Lett. 2010, 32, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Snajdar, O.; Svoboda, Z.; Marova, I. Application of random mutagenesis to enhance the production of polyhydroxyalkanoates by Cupriavidus necator H16 on waste frying oil. World J. Microbiol. Biotechnol. 2013, 29, 2417–2428. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; O’Connor, K.; Babu, R.; Woods, T.; Kenny, S. Plant oils and products of their hydrolysis as substrates for polyhydroxyalkanoate synthesis. Chem. Biochem. Eng. Q. 2015, 29, 123–133. [Google Scholar] [CrossRef]
- Hermann-Krauss, C.; Koller, M.; Muhr, A.; Fasl, H.; Stelzer, F.; Braunegg, G. Archaeal production of polyhydroxyalkanoate (PHA) co-and terpolyesters from biodiesel industry-derived by-products. Archaea 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, J.M.; Raposo, R.S.; de Almeida, M.C.M.; Cesário, M.T.; Sevrin, C.; Grandfils, C.; Da Fonseca, M.M.R. Effect of cultivation parameters on the production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and poly(3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour. Technol. 2012, 111, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Contreras, A.; Koller, M.; Miranda-de Sousa Dias, M.; Calafell-Monfort, M.; Braunegg, G.; Marqués-Calvo, M.S. Influence of glycerol on poly(3-hydroxybutyrate) production by Cupriavidus necator and Burkholderia sacchari. Biochem. Eng. J. 2015, 94, 50–57. [Google Scholar] [CrossRef]
- Koller, M.; Marsalek, L. Potential of diverse prokaryotic organisms for glycerol-based polyhydroxyalkanoate production. Appl. Food Biotechnol. 2015, 2, 3–15. [Google Scholar]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Benesova, P.; Petrik, S.; Oborna, J.; Prikryl, R.; Marova, I. Production of polyhydroxyalkanoates using hydrolysate of spent coffee grounds. Process Biochem. 2014, 49, 1409–1414. [Google Scholar] [CrossRef]
- Carvalho, G.; Oehmen, A.; Albuquerque, M.G.; Reis, M.A. The relationship between mixed microbial culture composition and PHA production performance from fermented molasses. New Biotechnol. 2014, 31, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Khosravi-Darani, K.; Mokhtari, Z.B.; Amai, T.; Tanaka, K. Microbial production of poly(hydroxybutyrate) from C1 carbon sources. Appl. Microbiol. Biotechnol. 2013, 97, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Drosg, B.; Fritz, I.; Gattermayr, F.; Silvestrini, L. Photo-autotrophic production of poly(hydroxyalkanoates) in cyanobacteria. Chem. Biochem. Eng. Q. 2015, 29, 145–156. [Google Scholar] [CrossRef]
- Koller, M.; Marsalek, L. Cyanobacterial Polyhydroxyalkanoate Production: Status Quo and Quo Vadis? Curr. Biotechnol. 2015, 4, 464–480. [Google Scholar] [CrossRef]
- Tanaka, K.; Miyawaki, K.; Yamaguchi, A.; Khosravi-Darani, K.; Matsusaki, H. Cell growth and P(3HB) accumulation from CO2 of a carbon monoxide-tolerant hydrogen-oxidizing bacterium, Ideonella sp. O-1. Appl. Microbiol. Biotechnol. 2011, 92, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Nonato, R.; Mantelatto, P.; Rossell, C. Integrated production of biodegradable plastic, sugar and ethanol. Appl. Microbiol. Biotechnol. 2001, 57, 1–5. [Google Scholar] [PubMed]
- Brämer, C.O.; Vandamme, P.; da Silva, L.F.; Gomez, J.G.; Steinbüchel, A. Polyhydroxyalkanoate-accumulating bacterium isolated from soil of a sugar-cane plantation in Brazil. Int. J. Syst. Evol. Microbiol. 2001, 51, 1709–1713. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, V.M.; Silva, L.F.; Gomez, J.G.C.; Fonseca, G.G. Growth of Burkholderia sacchari LFM 101 cultivated in glucose, sucrose and glycerol at different temperatures. Sci. Agric. 2016, 73, 429–433. [Google Scholar] [CrossRef]
- Alexandrino, P.M.R.; Mendonça, T.T.; Bautista, L.P.G.; Cherix, J.; Lozano-Sakalauskas, G.C.; Fujita, A.; Ramos Filho, E.; Long, P.; Padilla, G.; Taciro, M.K.; et al. Draft genome sequence of the polyhydroxyalkanoate-producing bacterium Burkholderia sacchari LMG 19450 isolated from Brazilian sugarcane plantation soil. Genome Announc. 2015, 3, e00313-15. [Google Scholar] [CrossRef] [PubMed]
- Raposo, R.S.; de Almeida, M.C.M.; de Oliveira, M.D.C.M.; da Fonseca, M.M.; Cesário, M.T. A Burkholderia sacchari cell factory: Production of poly-3-hydroxybutyrate, xylitol and xylonic acid from xylose-rich sugar mixtures. New Biotechnol. 2017, 34, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Cesário, M.T.; Raposo, R.S.; de Almeida, M.C.M.; van Keulen, F.; Ferreira, B.S.; da Fonseca, M.M.R. Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol. 2014, 31, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.G.; Gomez, J.G.C.; Silva, L.F. Cloning and overexpression of the xylose isomerase gene from Burkholderia sacchari and production of polyhydroxybutyrate from xylose. Can. J. Microbiol. 2009, 55, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.C.; da Silva, L.F.; Taciro, M.K.; Pradella, J.G. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) P(3HB-co-3HV) with a broad range of 3HV content at high yields by Burkholderia sacchari IPT 189. World J. Microbiol. Biotechnol. 2008, 24, 427–431. [Google Scholar] [CrossRef]
- Mendonça, T.T.; Gomez, J.G.C.; Buffoni, E.; Sánchez Rodriguez, R.J.; Schripsema, J.; Lopes, M.S.G.; Silva, L.F. Exploring the potential of Burkholderia sacchari to produce polyhydroxyalkanoates. J. Appl. Microbiol. 2014, 116, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz Pradella, J.G.; Taciro, M.K.; Mateus, A.Y.P. High-cell-density poly(3-hydroxybutyrate) production from sucrose using Burkholderia sacchari culture in airlift bioreactor. Bioresour. Technol. 2010, 101, 8355–8360. [Google Scholar] [CrossRef] [PubMed]
- Brämer, C.O.; Silva, L.F.; Gomez, J.G.C.; Priefert, H.; Steinbüchel, A. Identification of the 2-methylcitrate pathway involved in the catabolism of propionate in the polyhydroxyalkanoate-producing strain Burkholderia sacchari IPT101T and analysis of a mutant accumulating a copolyester with higher 3-hydroxyvalerate content. Appl. Environ. Microbiol. 2002, 68, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.; Gomez, J.G.C.; Oliveira, M.S.; Torres, B.B. Propionic acid metabolism and poly-3-hydroxybutyrate-co-3-hydroxyvalerate (P3HB-co-3HV) production by Burkholderia sp. J. Biotechnol. 2000, 76, 165–174. [Google Scholar] [CrossRef]
- Mendonça, T.T.; Tavares, R.R.; Cespedes, L.G.; Sánchez-Rodriguez, R.J.; Schripsema, J.; Taciro, M.K.; Gomez, J.G.C.; Silva, L.F. Combining molecular and bioprocess techniques to produce poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) with controlled monomer composition by Burkholderia sacchari. Int. J. Biol. Macromol. 2017, 98, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.; Taciro, M.K.; Raicher, G.; Piccoli, R.A.M.; Mendonça, T.T.; Lopes, M.S.G.; Gomez, J.G.C. Perspectives on the production of polyhydroxyalkanoates in biorefineries associated with the production of sugar and ethanol. Int. J. Biol. Macromol. 2014, 71, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Küng, W. Wachstum und Poly-d-(-)-3-Hydroxybuttersäure-Akkumulation bei Alcaligenes latus. Diploma Thesis, Graz University of Technology, Graz, Austria, 1982. [Google Scholar]
- Braunegg, G.; Sonnleitner, B.; Lafferty, R. A rapid gaschromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur. J. Appl. Microbiol. 1978, 6, 29–37. [Google Scholar] [CrossRef]
- Kunioka, M.; Kawaguchi, Y.; Doi, Y. Production of biodegradable copolyesters of 3-hydroxybutyrate and 4-hydroxybutyrate by Alcaligenes eutrophus. Appl. Microbiol. Biotechnol. 1989, 30, 569–573. [Google Scholar] [CrossRef]
- Choi, M.H.; Yoon, S.C.; Lenz, R.W. Production of poly(3-hydroxybutyric acid-co-4-hydroxybutyric acid) and poly(4-hydroxybutyric acid) without subsequent degradation by Hydrogenophaga pseudoflava. Appl. Environ. Microbiol. 1999, 65, 1570–1577. [Google Scholar] [PubMed]
- Valentin, H.E.; Zwingmann, G.; Schönebaum, A.; Steinbüchel, A. Metabolic pathway for biosynthesis of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from 4-hydroxybutyrate by Alcaligenes eutrophus. Eur. J. Biochem. 1995, 227, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kang, M.S.; Jung, Y.M. Regulating the molar fraction of 4-hydroxybutyrate in poly(3-hydroxybutyrate-4-hydroxybutyrate) biosynthesis by Ralstonia eutropha using propionate as a stimulator. J. Biosci. Bioeng. 2000, 89, 380–383. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Braunegg, G.; Hermann, C.; Horvat, P.; Kroutil, M.; Martinz, J.; Neto, J.; Pereira, L.; Varila, P. Production of polyhydroxyalkanoates from agricultural waste and surplus materials. Biomacromolecules 2005, 6, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.A.; Anderson, A.J.; Shah, D.T.; Asrar, J. Chain termination in polyhydroxyalkanoate synthesis: Involvement of exogenous hydroxy-compounds as chain transfer agents. Int. J. Biol. Macromol. 1999, 25, 43–53. [Google Scholar] [CrossRef]
- Atlić, A.; Koller, M.; Scherzer, D.; Kutschera, C.; Grillo-Fernandes, E.; Horvat, P.; Chiellini, E.; Braunegg, G. Continuous production of poly([R]-3-hydroxybutyrate) by Cupriavidus necator in a multistage bioreactor cascade. Appl. Microbiol. Biotechnol. 2011, 91, 295–304. [Google Scholar] [CrossRef] [PubMed]
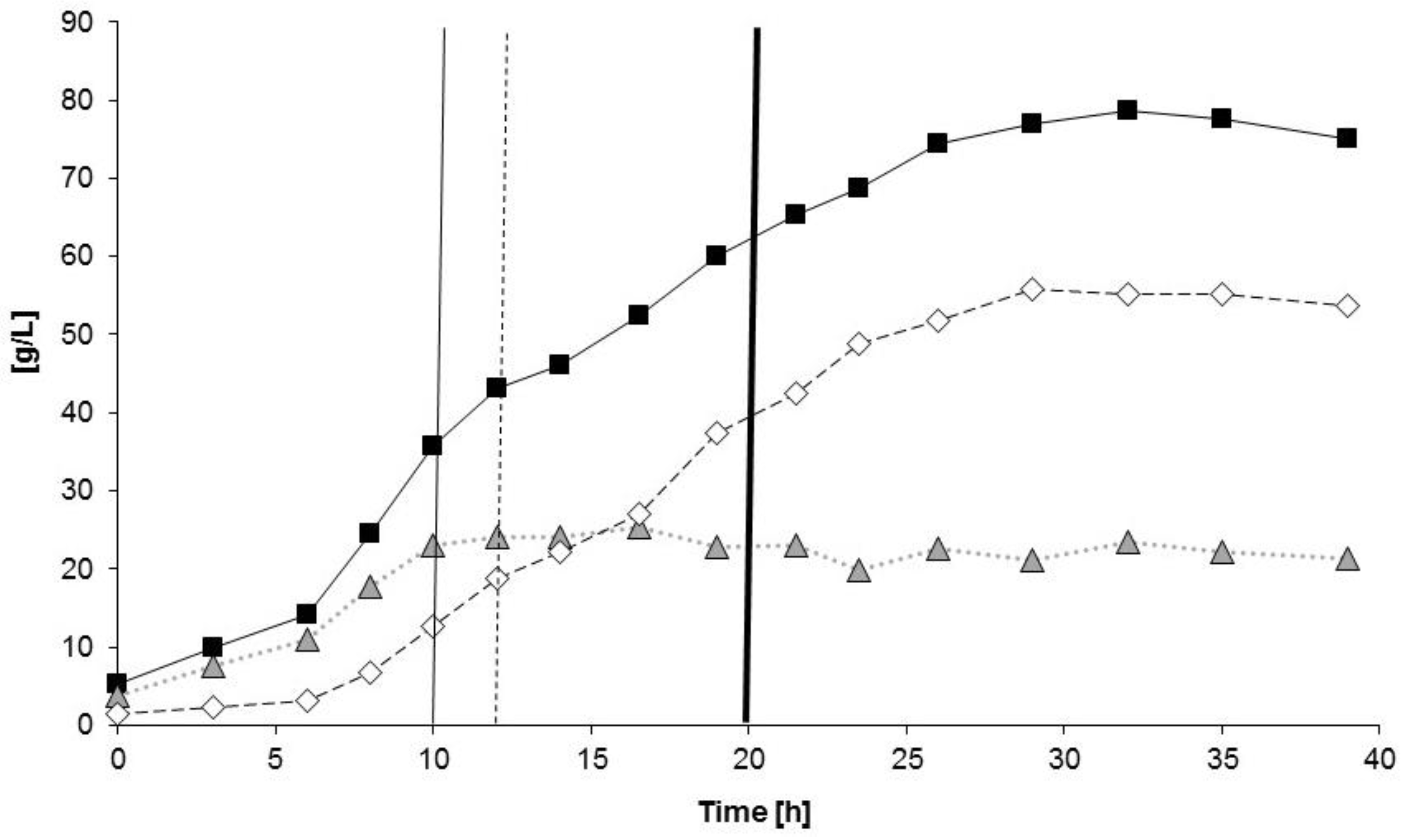
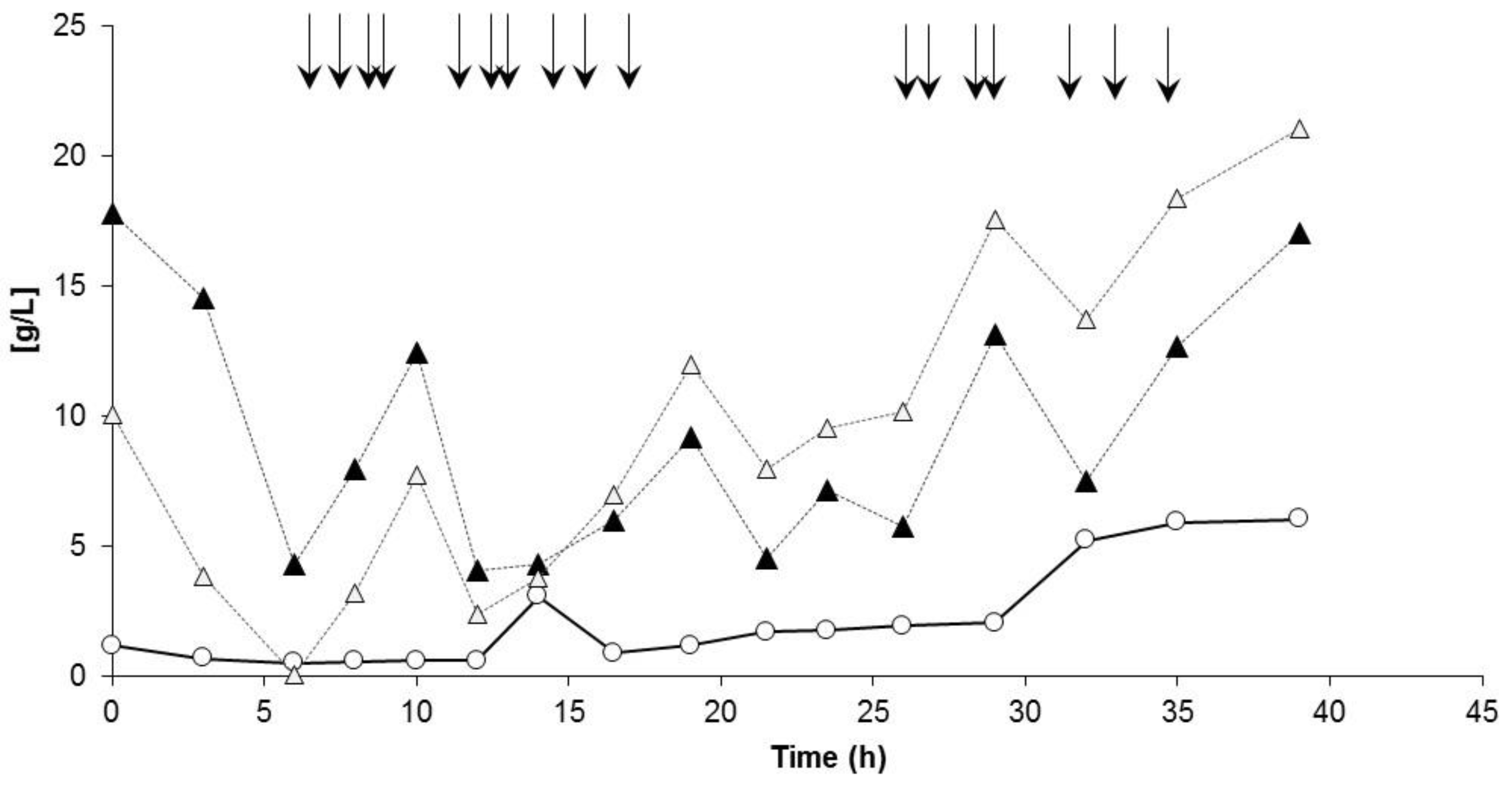
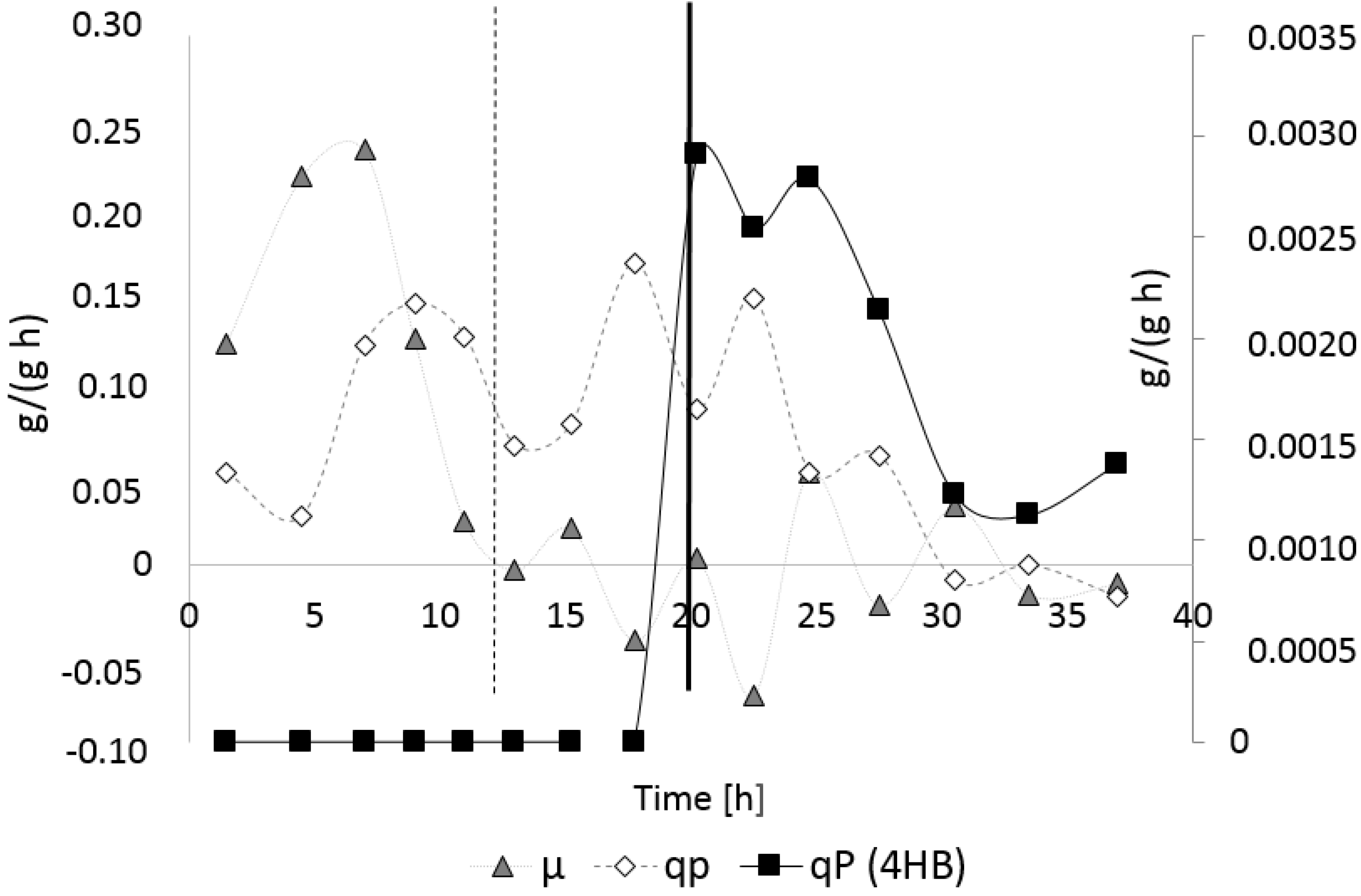

| Kinetic Parameter | PHB Production Process (1st Bioreactor Cultivation) | P(3HB-co-4HB) Production Process (2nd Bioreactor Cultivation) |
|---|---|---|
| µmax. (1/h) | 0.41 (t = 3.75–6 h) | 0.23 (t = 6–8 h) |
| max. CDM (g/L) | 70.0 (t = 25.25 h) | 78.6 (t = 32 h) |
| max. PHA concentration (g/L) | 36.8 (t = 27.5 h) | 55.8 (t = 29 h) |
| max. fraction of PHA in CDM (% w/w) | 53.0 (t = 27.5 h) | 72.6 (t = 29 h) |
| max. fraction of 4HB in PHA (% mol/mol) | - | 1.6 (t = 39 h) |
| Volumetric productivity for PHA (g/L·h) | 1.29 (t = 0–27.5 h) | 1.87 (t = 0–39 h) |
| YieldCDM/sucorse (g/g) | 0.18 | 0.38 |
| Yield 4HB/GBL (g/g) | - | 0.05 |
| max. specific productivity qP (g/(g·h)) | 0.19 (t = 7.25 h) | 0.17 (t = 17.75 h) |
| Material Characterization | ||
| Weight average molecular mass Mw (kDa) | 627 ± 13 | 315 ± 24 |
| Polydispersity Pi (Mw/Mn) | 2.66 ± 0.13 | 2.51 ± 0.15 |
| Glass transition temperature Tg (°C) | 1.0 ± 0.6 | 1.8 ± 0.2 |
| Melting point Tm (°C) | 177.6 ± 0.6 | 160.9 ± 0.8 |
| Degree of crystallinity Xc (%) | 70.9 ± 0.9 | 24.0 ± 3.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda De Sousa Dias, M.; Koller, M.; Puppi, D.; Morelli, A.; Chiellini, F.; Braunegg, G. Fed-Batch Synthesis of Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate) from Sucrose and 4-Hydroxybutyrate Precursors by Burkholderia sacchari Strain DSM 17165. Bioengineering 2017, 4, 36. https://doi.org/10.3390/bioengineering4020036
Miranda De Sousa Dias M, Koller M, Puppi D, Morelli A, Chiellini F, Braunegg G. Fed-Batch Synthesis of Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate) from Sucrose and 4-Hydroxybutyrate Precursors by Burkholderia sacchari Strain DSM 17165. Bioengineering. 2017; 4(2):36. https://doi.org/10.3390/bioengineering4020036
Chicago/Turabian StyleMiranda De Sousa Dias, Miguel, Martin Koller, Dario Puppi, Andrea Morelli, Federica Chiellini, and Gerhart Braunegg. 2017. "Fed-Batch Synthesis of Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate) from Sucrose and 4-Hydroxybutyrate Precursors by Burkholderia sacchari Strain DSM 17165" Bioengineering 4, no. 2: 36. https://doi.org/10.3390/bioengineering4020036
APA StyleMiranda De Sousa Dias, M., Koller, M., Puppi, D., Morelli, A., Chiellini, F., & Braunegg, G. (2017). Fed-Batch Synthesis of Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate) from Sucrose and 4-Hydroxybutyrate Precursors by Burkholderia sacchari Strain DSM 17165. Bioengineering, 4(2), 36. https://doi.org/10.3390/bioengineering4020036










