A Proof-of-Concept Study: Simple and Effective Detection of P and T Waves in Arrhythmic ECG Signals
Abstract
:1. Introduction
2. Data
- The MIT-BIH database contains 30-min recordings for each patient, which is considerably longer than the recordings in many other databases, such as the Common Standards for Electrocardiography (CSE) database that contains 10-s recordings [20].
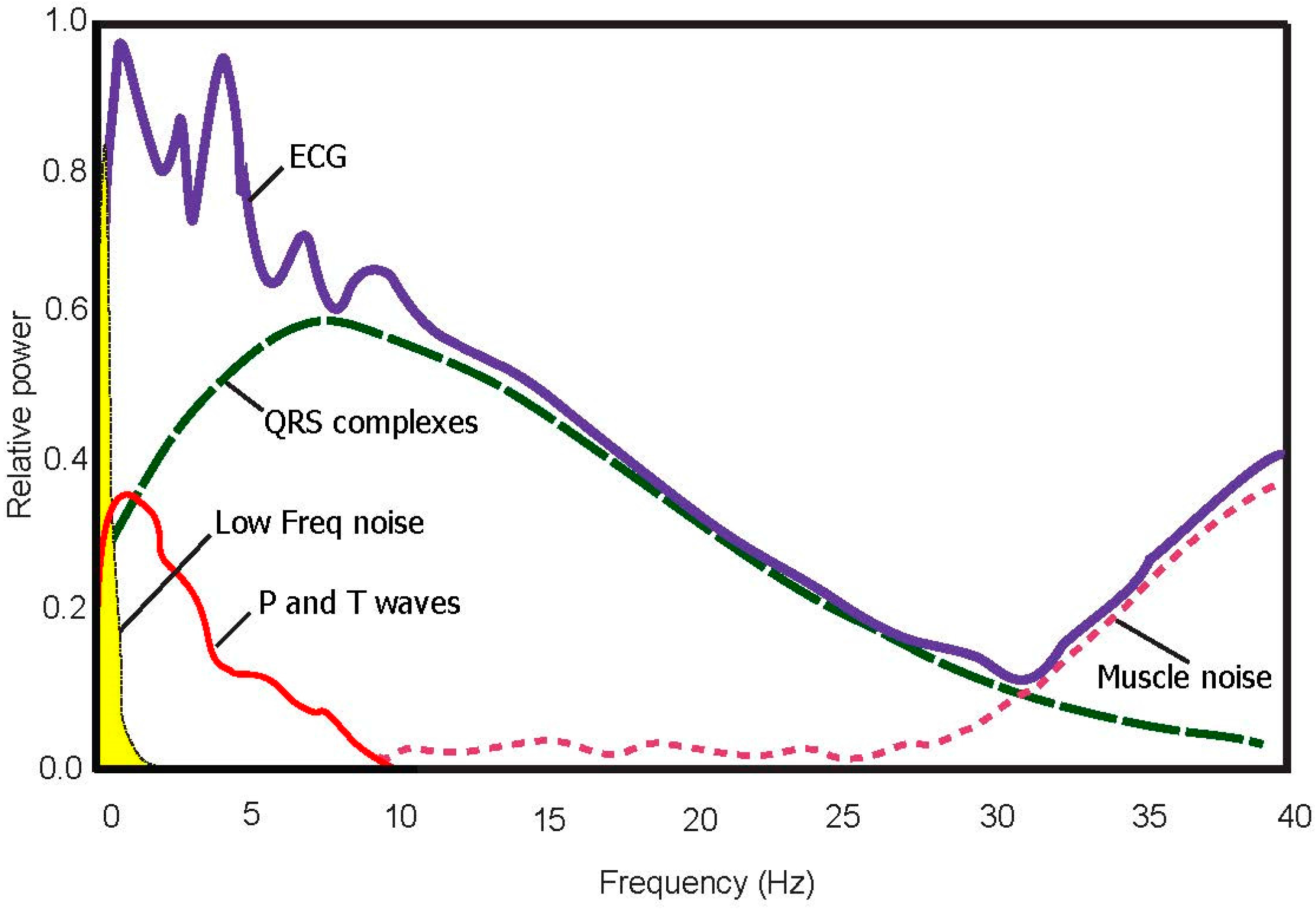
- Arrhythmic ECG signals provided by the MIT-BIH Arrhythmia Database are impacted by multiple factors that affect signal quality. For example, we noted premature atrial complexes, non-stationary effects, premature ventricular complexes, low signal-to-noise ratio, left bundle blocks, and right bundle blocks. These challenges provide an opportunity to test the robustness of the P and T wave detection algorithm. These issues are expected to present significant difficulties for any ECG signal analysis algorithm [21].
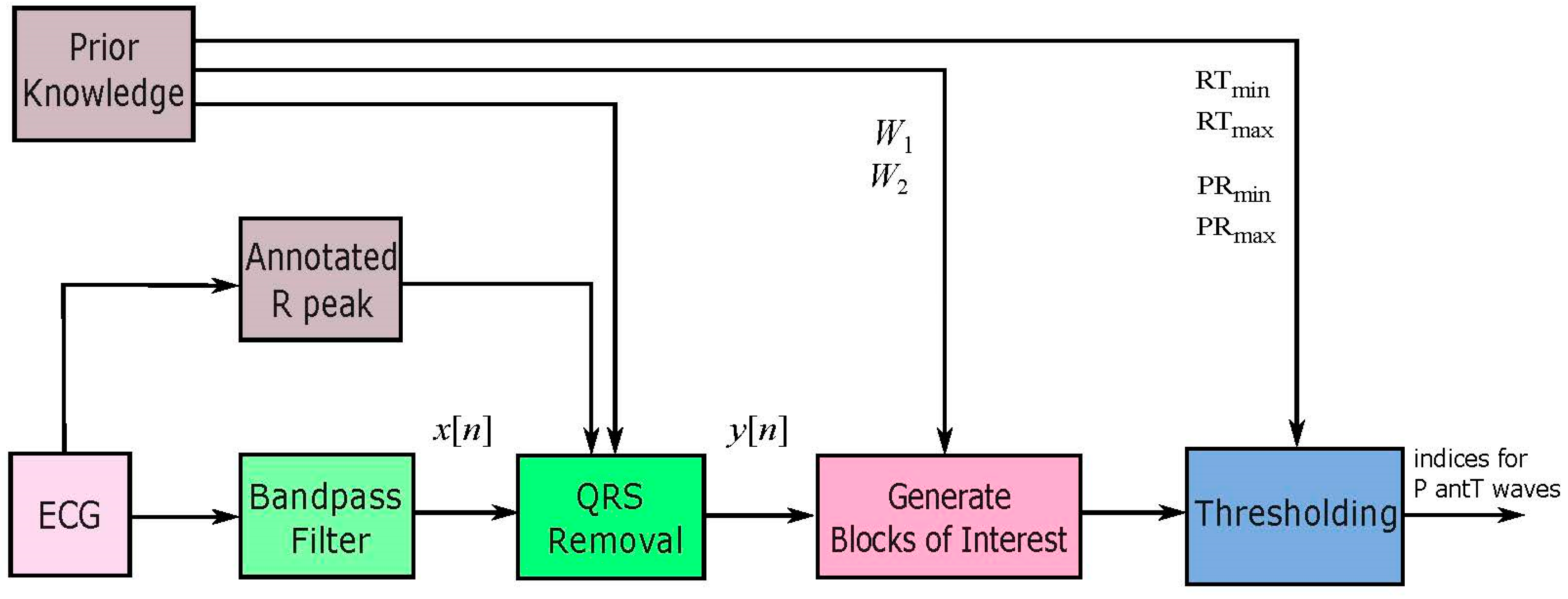
3. Methodology
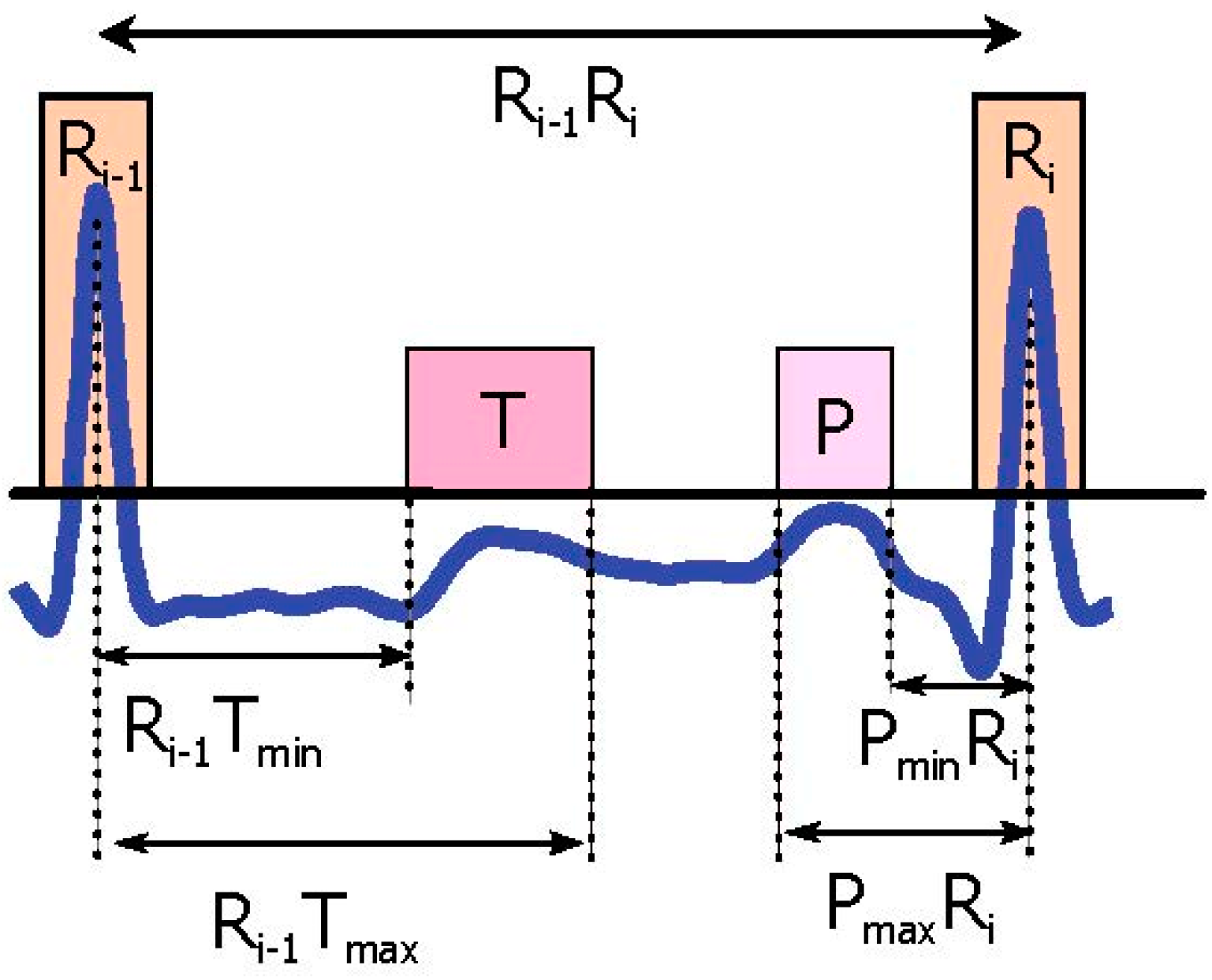
3.1. Prior Information Analysis
3.2. Bandpass Filter
3.3. QRS Removal
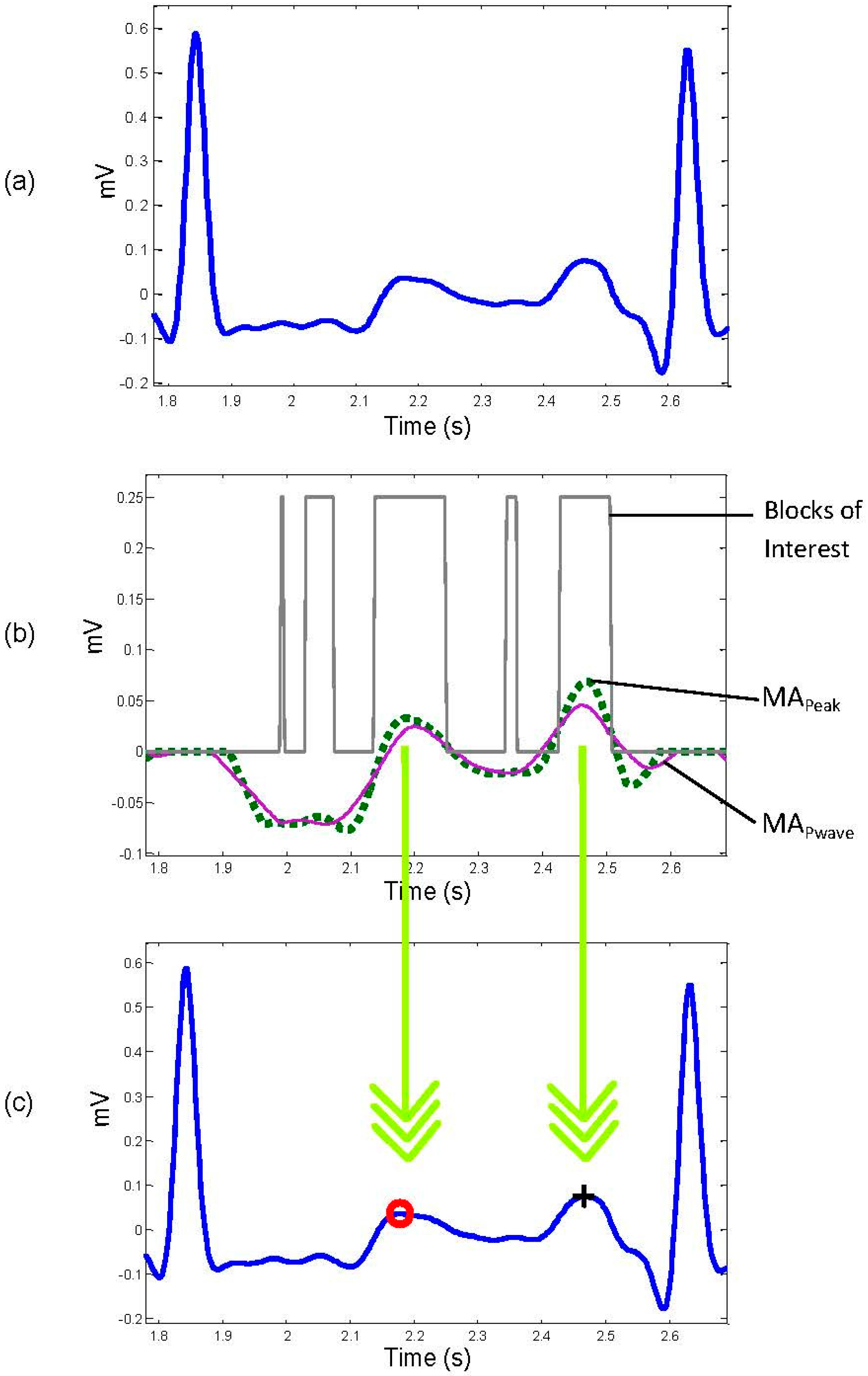
3.4. Select Potential Blocks
3.5. Thresholding
- No blocks detected: No detection of P or T wave in the processed RR interval.
- One block detected: Most likely the P and T waves are merged within one block.
- More than one block detected: Most likely the signal is noisy and therefore multiple blocks are generated. This step has two sub steps:
- ❖
- ❖
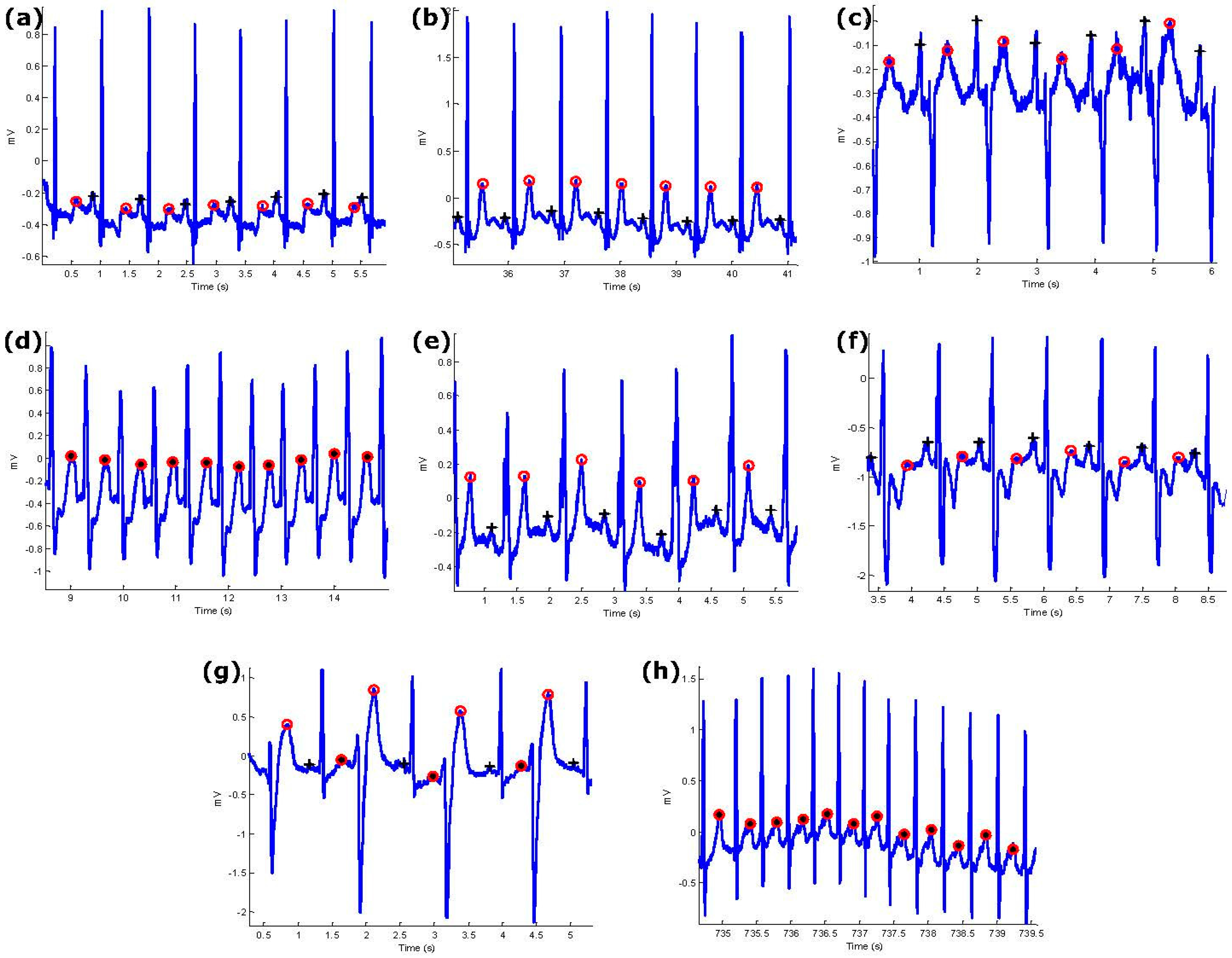
4. Results
5. Discussion
6. Conclusions
Author Contributions
Conflicts of Interest
References
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; Geneva, Switzerland, 2014. [Google Scholar]
- Pearson, T.A.; Palaniappan, L.P.; Artinian, N.T.; Carnethon, M.R.; Criqui, M.H.; Daniels, S.R.; Fonarow, G.C.; Fortmann, S.P.; Franklin, B.A.; Galloway, J.M.; et al. American Heart Association Guide for Improving Cardiovascular Health at the Community Level, 2013 Update: A Scientific Statement for Public Health Practitioners, Healthcare Providers, and Health Policy Makers. Circulation 2013, 127, 1730–1753. [Google Scholar] [CrossRef] [PubMed]
- Kligfield, P.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Hancock, E.W.; van Herpen, G.; Kors, J.A.; Macfarlane, P.; Mirvis, D.M.; et al. Recommendations for the Standardization and Interpretation of the Electrocardiogram: Part I: The Electrocardiogram and Its Technology: A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. Circulation 2007, 115, 1306–1324. [Google Scholar] [PubMed]
- Elgendi, M.; Eskofier, B.; Dokos, S.; Abbott, D. Revisiting QRS Detection Methodologies for Portable, Wearable, Battery-Operated, and Wireless ECG Systems. PLoS ONE 2014, 9, e84018. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Zipes, D.; Libby, P.; Bonow, R. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 10th ed.; Saunders: Philadelphia, PA, USA, 2004. [Google Scholar]
- Murthy, I.S.N.; Prasad, G.S.S.D. Analysis of ECG from pole-zero models. IEEE Trans. Biomed. Eng. 1992, 39, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Mailhesm, C.; Tourneret, J.-Y. P- and T-wave delineation in ECG signals using a Bayesian approach and a partially collapsed Gibbs sampler. IEEE Trans. Biomed. Eng. 2010, 57, 2840–2849. [Google Scholar] [PubMed]
- Karimipour, A.; Homaeinezhad, M.R. Real-time electrocardiogram P-QRS-T detection–delineation algorithm based on quality-supported analysis of characteristic templates. Comput. Bio. Med. 2014, 52, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, H.; Gupta, R.; Mitra, M. Real time P and T wave detection from ECG using FPGA. Proced. Technol. 2012, 4, 840–844. [Google Scholar] [CrossRef]
- Li, C.; Zheng, C.; Tai, C. Detection of ECG characteristic points using wavelet transforms. IEEE Trans. Biomed. Eng. 1995, 42, 21–28. [Google Scholar] [PubMed]
- De Azevedo Botter, E.; Nascimento, C.L., Jr.; Yoneyama, T. A neural network with asymmetric basis functions for feature extraction of ECG P waves. IEEE Trans. Neural Netw. 2001, 12, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Strumillo, P. Nested median filtering for detecting T-wave offset in ECGs. Electron. Lett. 2002, 38, 682–683. [Google Scholar] [CrossRef]
- Martinez, J.P.; Almeida, R.; Olmos, S.; Rocha, A.P.; Laguna, P. A wavelet-based ECG delineator: Evaluation on standard databases. IEEE Trans. Biomed. Eng. 2004, 51, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Sovilj, S.; Jeras, M.; Magjarevic, R. Real time P-wave detector based on wavelet analysis. In Proceedings of the 12th IEEE Mediterranean Electrotechnical Conference, Dubrovnik, Hrvatska, 12–15 May 2004; pp. 403–407.
- Chouhan, V.; Mehta, S. Threshold-based Detection of P and T-wave in ECG using New Feature Signal. Int. J. Comput Sci. Netw. Secur. 2008, 8, 2. [Google Scholar]
- Lay-Ekuakille, A.; Vergallo, P.; Trabacca, A.; De Rinaldis, M.; Angelillo, F.; Conversano, F.; Casciaro, S. Low-frequency detection in ECG signals and joint EEG-Ergospirometric measurements for precautionary diagnosis. Measurement 2013, 46, 97–107. [Google Scholar] [CrossRef]
- Clifford, G.D.; Azuaje, F.; McSharry, P. Advanced Methods And Tools for ECG Data Analysis; Artech House Publishers: Norwood, MA, USA, 2006. [Google Scholar]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Moody, G.B.; Mark, R.G. The impact of the MIT-BIH Arrhythmia Database. IEEE Eng. Med. Biol. Mag. 2001, 20, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Willems, J.L.; Arnaud, P.; Van Bemmel, J.H.; Degani, R.; Macfarlane, P.W.; Zywietz, C. Common standards for quantitative electrocardiography: goals and main results. CSE Working Party. Methods Inf. Med. 1990, 29, 263–271. [Google Scholar] [PubMed]
- Moody, G.B.; Mark, R.G. The MIT-BIH arrhythmia database on CD-ROM and software for use with it. In Proceedings of the Computers in Cardiology, Chicago, IL, USA, 23–26 September 1990; pp. 185–188.
- Elgendi, M.; Eskofier, B.; Abbott, D. Fast T Wave Detection Calibrated by Clinical Knowledge with Annotation of P and T Waves. Sensors 2015, 15, 17693. [Google Scholar] [CrossRef] [PubMed]
- Elgendi, M. Fast QRS Detection with an Optimized Knowledge-Based Method: Evaluation on 11 Standard ECG Databases. PLoS ONE 2013, 8, 73557. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, A.; Shafer, R. Discrete-Time Signal Processing, 3nd ed.; Prentice Hall: Bergen County, NJ, USA, 1989; pp. 411–425. [Google Scholar]
- Sahambi, J.S.; Tandon, S.N.; Bhatt, R.K.P. Using wavelet transforms for ECG characterization. An on-line digital signal processing system. IEEE Eng. Med. Biol. Mag. 1997, 16, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Arafat, A.; Hasan, K. Automatic detection of ECG wave boundaries using empirical mode decomposition. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing, Taipei, Taiwan, 19–24 April 2009; pp. 461–464.
- Diery, A. Novel Applications of the Wavelet Transform for Analysis of P Waves in Clinical ECG Recordings. Ph.D. Thesis, Griffith University, Brisbane, Australia, November 2008. [Google Scholar]
- Ktata, S.; Ouni, K.; Ellouze, N. ECG signal maxima detection using wavelet transform. In Proceedings of the IEEE International Symposium on Industrial Electronics, Montreal, QC, Canada, 9–13 July 2006; pp. 700–703.
- Mahmoodabadi, S.Z.; Alirezaie, J.; Babyn, P. Bio-signal characteristics detection utilizing frequency ordered wavelet packets. In Proceedings of the IEEE International Symposium on Signal Processing and Information Technology, Cairo, Egypt, 15–18 December 2007; pp. 748–753.
- Krimi, S.; Ouni, K.; Ellouze, N. An approach combining wavelet transform and hidden markov models for ECG segmentation. In Proceedings of the 3rd International Conference on Information and Communication Technologies: From Theory to Applications, Damascus, Syria, 7–11 April 2008; pp. 1–6.
- Sun, Y.; Chan, K.L.; Krishnan, S.M. Characteristic wave detection in ECG signal using morphological transform. BMC Cardiovasc. Disor. 2005, 5. [Google Scholar] [CrossRef] [PubMed]
- Goutas, A.; Ferdi, Y.; Herbeuval, J.; Boudraa, M.; Boucheham, B. Digital fractional order differentiation-based algorithm for P and T-waves detection and delineation. ITBM RBM 2005, 26, 127–132. [Google Scholar] [CrossRef]
- Elgendi, M. Eventogram: A Visual Representation of Main Events in Biomedical Signals. Bioengineering 2016, 3, 22. [Google Scholar] [CrossRef]
- Elgendi, M. TERMA Framework for Biomedical Signal Analysis: An Economic-Inspired Approach. Biosensors 2016. submitted for publication. [Google Scholar]
- Taylor, J. Introduction to Error Analysis, the Study of Uncertainties in Physical Measurements; University Science Books: Mill Valley, CA, USA, 1996; p. 327. [Google Scholar]
- Elgendi, M.; Jonkman, M.; De Boer, F. Recognition of T waves in ECG signals. In Proceedings of the IEEE 35th Annual Northeast Bioengineering Conference, Boston, MA, USA, 3–5 April 2009; pp. 1–2.
- Elgendi, M.; Jonkman, M.; De Boer, F. P wave demarcation in electrocardiogram. In Proceedings of the IEEE 35th Annual Northeast Bioengineering Conference, Boston, MA, USA, 3–5 April 2009; pp. 1–2.

| Feature | Normal Value | Normal Limit | Normal duration (fs = 360 Hz) |
|---|---|---|---|
| P width | 110 ms | ± 20 ms | 33–47 samples |
| PQ/PR interval | 160 ms | ± 40 ms | 43–72 samples |
| QRS width | 100 ms | ± 20 ms | 29–43 samples |
| QTc interval | 400 ms | ± 40 ms | 130–158 samples |
| P Wave Detection Performance | T Wave Detection Performance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Record | No. of Beats | TP | FP | FN | SE (%) | +P (%) | TP | FP | FN | SE (%) | +P (%) |
| 100 | 2274 | 2274 | 0 | 0 | 100.00 | 100.00 | 2274 | 0 | 0 | 100.00 | 100.00 |
| 101 | 1866 | 1866 | 0 | 0 | 100.00 | 100.00 | 1863 | 3 | 0 | 100.00 | 99.84 |
| 102 | 2187 | 2021 | 87 | 79 | 96.37 | 96.02 | 2187 | 0 | 0 | 100.00 | 100.00 |
| 103 | 2084 | 2076 | 4 | 4 | 99.81 | 99.81 | 2084 | 0 | 0 | 100.00 | 100.00 |
| 104 | 2229 | 2071 | 82 | 76 | 96.58 | 96.32 | 2228 | 1 | 0 | 100.00 | 99.96 |
| 105 | 2602 | 2557 | 33 | 12 | 99.53 | 98.72 | 2579 | 15 | 8 | 99.69 | 99.42 |
| 106 | 2026 | 2013 | 12 | 1 | 99.95 | 99.41 | 2013 | 13 | 0 | 100.00 | 99.36 |
| 107 | 2136 | 2136 | 0 | 0 | 100.00 | 100.00 | 2136 | 0 | 0 | 100.00 | 100.00 |
| 108 | 1765 | 1363 | 244 | 158 | 90.56 | 86.13 | 1710 | 36 | 19 | 98.91 | 97.95 |
| 109 | 2533 | 2342 | 135 | 56 | 97.72 | 94.67 | 2532 | 1 | 0 | 100.00 | 99.96 |
| 21702 | 20719 | 597 | 386 | 98.05 | 97.11 | 21,606 | 69 | 27 | 99.86 | 99.65 | |
| Comparison of P Wave Detection Algorithms | Comparison of T Wave Detection Algorithms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Algorithm | Method | Data Used | Se (%) | +P (%) | Algorithm | Method | Data Used | Se (%) | +P (%) |
| Proposed algorithm | Blocks of Interest | 10 records | 98.05 | 97.11 | Proposed algorithm | Blocks of Interest | 10 records | 99.86 | 99.65 |
| Arafat et al. [26] | EMD | 10,000 beats | N/R | N/R | Arafat et al. [26] | EMD | 10,000 beats | N/R | N/R |
| Diery [27] | Wavelet | 39 records (10 s each) | N/R | N/R | Ktata et al. [28] | Wavelet | Selected segments | N/R | N/R |
| Mahmoodabadi et al. [29] | Wavelet | Selected segments | 51.69 | 53.64 | Krimi et al. [30] | Wavelet | Selected beats | 94.65 | N/R |
| Ktata et al. [28] | Wavelet | Selected segments | N/R | N/R | Sun et al. [31] | Multiscale morphological derivative | Selected segments | TON = 99.8 TOFF = 99.6 | N/R |
| Sun et al. [31] | Multiscale derivatives | Selected segments | PON = 97.2 POFF = 94.8 | N/R | Goutas et al. [32] | Fractional differentiation | Selected segments | N/R | N/R |
| Goutas et al. [32] | Fractional differentiation differentiation | Selectedsegments | N/R | N/R | Sun et al. [31] | Multi-scale morphological derivative | Selected segments | N/R | N/R |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgendi, M.; Meo, M.; Abbott, D. A Proof-of-Concept Study: Simple and Effective Detection of P and T Waves in Arrhythmic ECG Signals. Bioengineering 2016, 3, 26. https://doi.org/10.3390/bioengineering3040026
Elgendi M, Meo M, Abbott D. A Proof-of-Concept Study: Simple and Effective Detection of P and T Waves in Arrhythmic ECG Signals. Bioengineering. 2016; 3(4):26. https://doi.org/10.3390/bioengineering3040026
Chicago/Turabian StyleElgendi, Mohamed, Marianna Meo, and Derek Abbott. 2016. "A Proof-of-Concept Study: Simple and Effective Detection of P and T Waves in Arrhythmic ECG Signals" Bioengineering 3, no. 4: 26. https://doi.org/10.3390/bioengineering3040026
APA StyleElgendi, M., Meo, M., & Abbott, D. (2016). A Proof-of-Concept Study: Simple and Effective Detection of P and T Waves in Arrhythmic ECG Signals. Bioengineering, 3(4), 26. https://doi.org/10.3390/bioengineering3040026






