In Vivo GFP Knockdown by Cationic Nanogel-siRNA Polyplexes
Abstract
:1. Introduction
2. Experimental Section
2.1. Nanogel NSP Synthesis
2.2. Cell Culture
2.3. siRNA Protection Assay
2.4. In vitro GAPDH Knockdown
2.5. In vivo GFP Knockdown
2.6. Statistical Analyses
3. Results and Discussion
3.1. Results



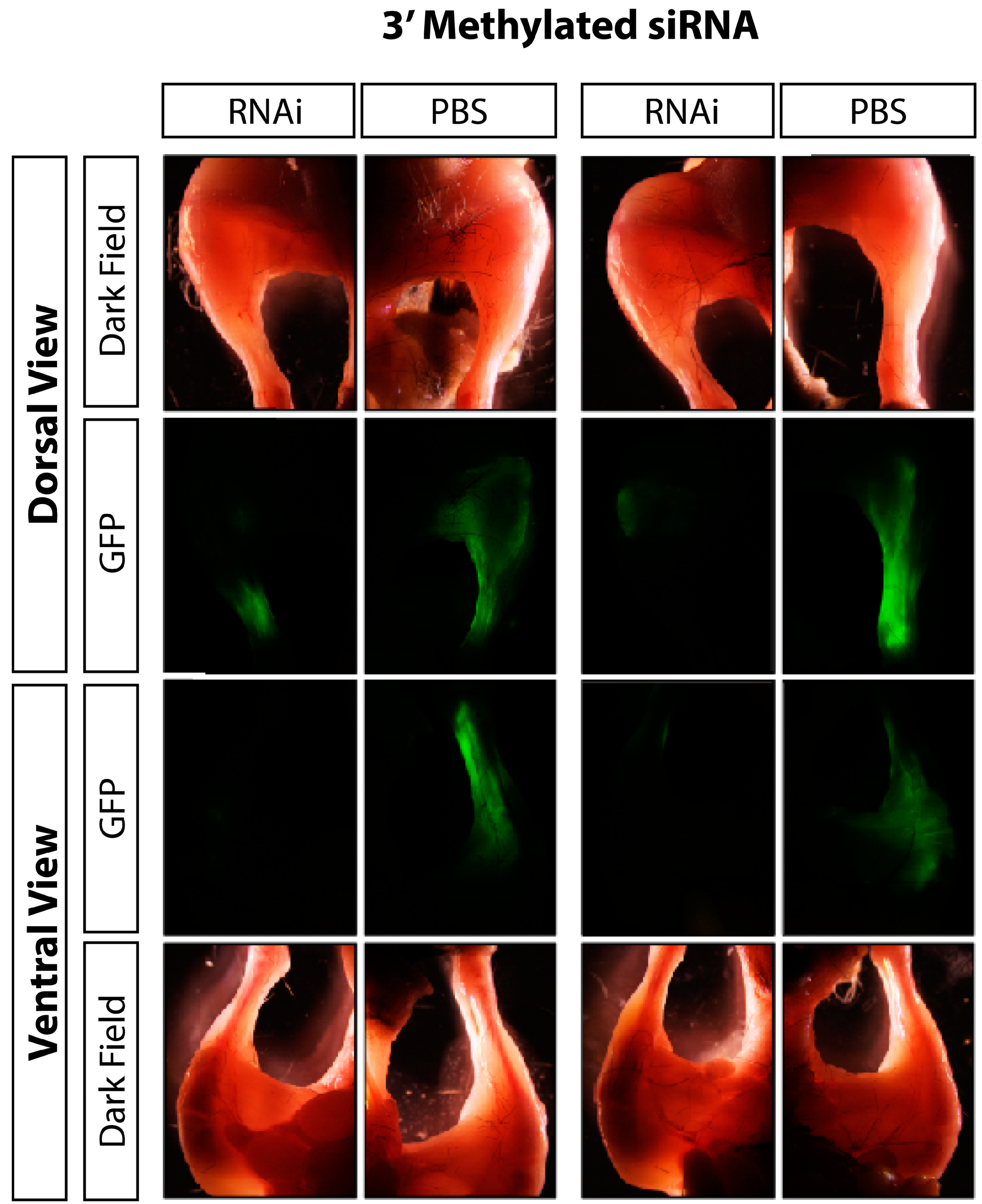
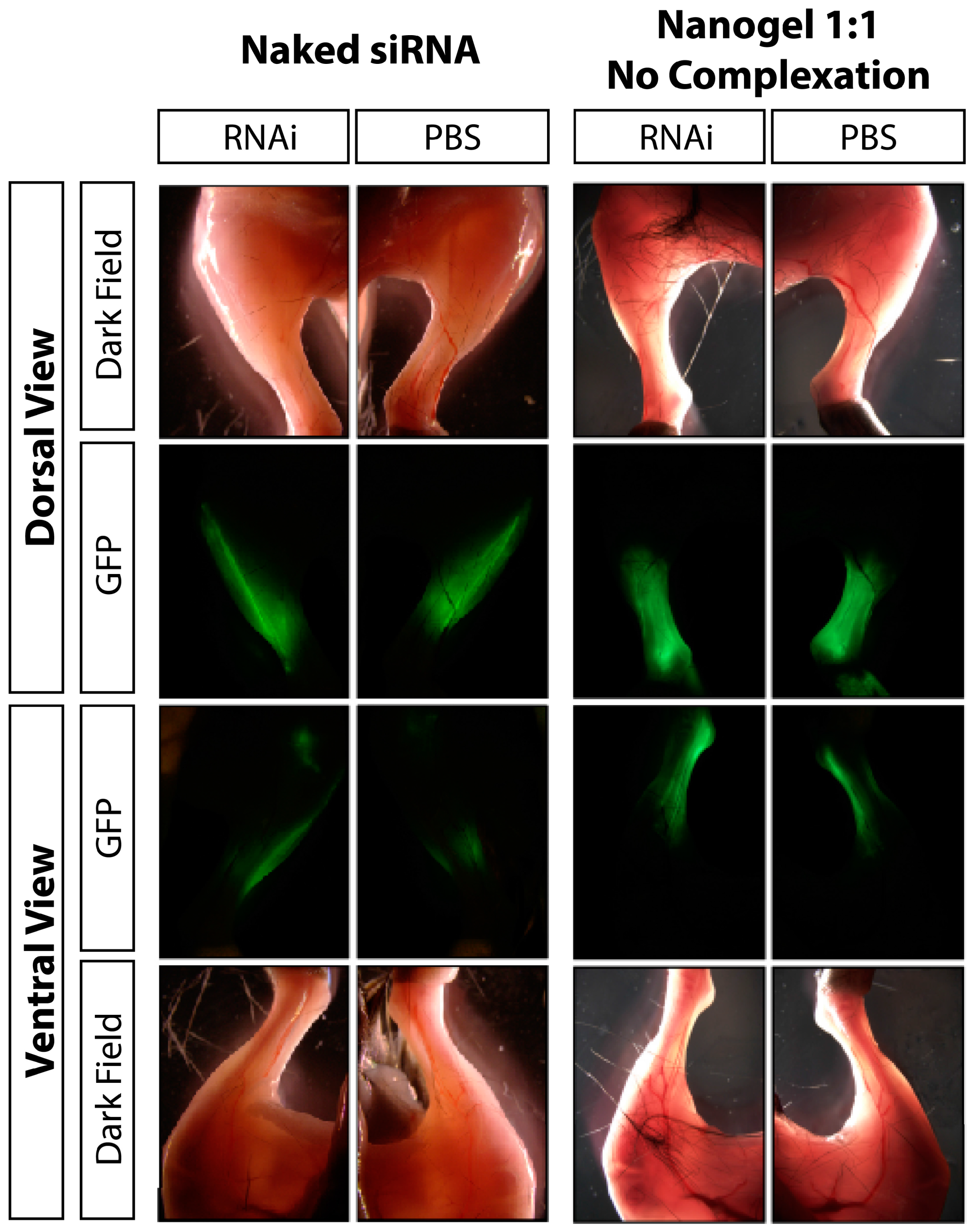
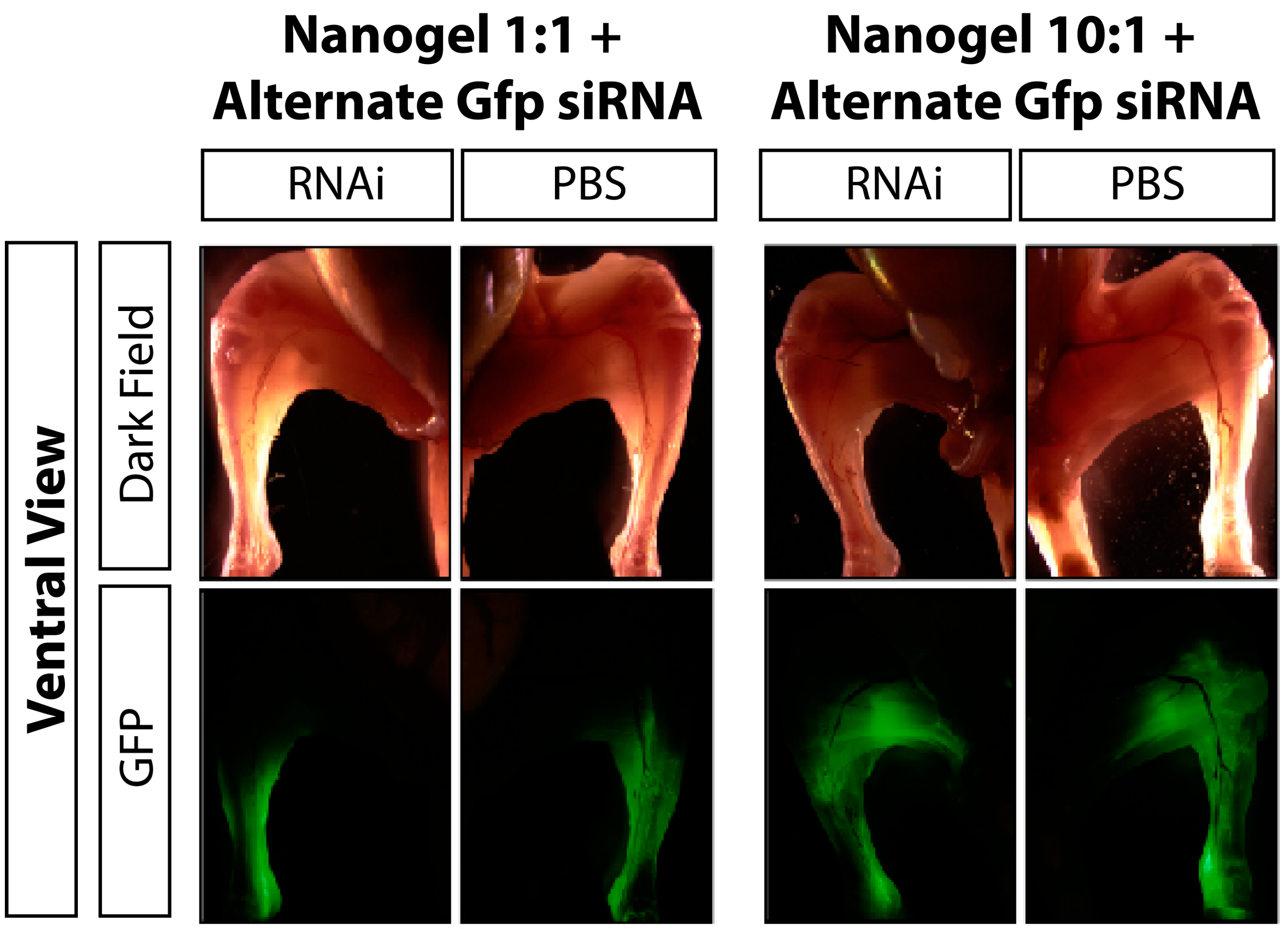
3.2. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dykxhoorn, D.M.; Palliser, D.; Lieberman, J. The silent treatment: siRNAs as small molecule drugs. Gene Ther. 2006, 13, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W. RNAi: The nuts and bolts of the risc machine. Cell 2005, 122, 17–20. [Google Scholar] [CrossRef] [PubMed]
- De Fougerolles, A.; Vornlocher, H.P.; Maraganore, J.; Lieberman, J. Interfering with disease: A progress report on siRNA-based therapeutics. Nat. Rev. Drug Discov. 2007, 6, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K.; Symons, R.C.; Shah, S.M.; Quinlan, E.J.; Tabandeh, H.; Do, D.V.; Reisen, G.; Lockridge, J.A.; Short, B.; Guerciolini, R.; et al. RNAi-based treatment for neovascular age-related macular degeneration by siRNA-027. Amer. J. Ophthalmol. 2010, 150, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ban, H.S.; Kim, S.S.; Wu, H.; Pearson, T.; Greiner, D.L.; Laouar, A.; Yao, J.; Haridas, V.; Habiro, K.; et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell 2008, 134, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Takigami, I.; Ohno, T.; Kitade, Y.; Hara, A.; Nagano, A.; Kawai, G.; Saitou, M.; Matsuhashi, A.; Yamada, K.; Shimizu, K. Synthetic siRNA targeting the breakpoint of EWS/FLI-1 inhibits growth of ewing sarcoma xenografts in a mouse model. Int. J. Cancer 2011, 128, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.S.; Sharp, P.A. Pyruvate kinase M2-specific siRNA induces apoptosis and tumor regression. J. Exp. Med. 2012, 209, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Strumberg, D.; Schultheis, B.; Traugott, U.; Vank, C.; Santel, A.; Keil, O.; Giese, K.; Kaufmann, J.; Drevs, J. Phase I clinical development of Atu027, a siRNA formulation targeting PKN3 in patients with advanced solid tumors. Int. J. Clin. Pharm. Therapeutics 2012, 50, 76–78. [Google Scholar] [CrossRef]
- Hamamichi, S.; Rivas, R.N.; Knight, A.L.; Cao, S.; Caldwell, K.A.; Caldwell, G.A. Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson’s disease model. Proc. Natl. Acad. Sci. USA 2008, 105, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Koyama, A.; Koyama, S.; Yoshina, S.; Ren, C.H.; Kato, T.; Mitani, S.; Iwatsubo, T. A systematic RNAi screen reveals involvement of endocytic pathway in neuronal dysfunction in alpha-synuclein transgenic C. elegans. Hum. Mol. Genet. 2008, 17, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Behlke, M.A. Progress towards in vivo use of sirnas. Mol. Ther. 2006, 13, 644–670. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E. Polymers for siRNA delivery: Inspired by viruses to be targeted, dynamic, and precise. Acc. Chem. Res. 2012, 45, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Wientjes, M.G.; Au, J.L. Delivery of siRNA therapeutics: Barriers and carriers. AAPS 2010, 12, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Devroe, E.; Silver, P.A. Retrovirus-delivered siRNA. BMC Biotechnology 2002, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Mao, Q.; Paulson, H.L.; Davidson, B.L. siRNA-mediated gene silencing in vitro and in vivo. Nat. Biotechnol. 2002, 20, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Brummelkamp, T.R.; Bernards, R.; Agami, R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2002, 2, 243–247. [Google Scholar] [CrossRef]
- Kohn, D.B.; Sadelain, M.; Glorioso, J.C. Occurrence of leukaemia following gene therapy of X-linked SCID. Nature Rev. Cancer 2003, 3, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Huang, L. Nonviral vectors in the new millennium: Delivery barriers in gene transfer. Hum. Gene Ther. 2001, 12, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Midoux, P.; Breuzard, G.; Gomez, J.P.; Pichon, C. Polymer-based gene delivery: A current review on the uptake and intracellular trafficking of polyplexes. Curr. Gene Ther. 2008, 8, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Tsarevsky, N.V. Macromolecular engineering by atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 6513–6533. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.J.J.; Yang, W.T.T. Polymer vectors via controlled/living radical polymerization for gene delivery. Prog. Polym. Sci. 2011, 36, 1099–1131. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Harashima, H. The polyethyleneglycol dilemma: Advantage and disadvantage of pegylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol Pharm Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Averick, S.E.; Paredes, E.; Irastorza, A.; Shrivats, A.R.; Srinivasan, A.; Siegwart, D.J.; Magenau, A.J.; Cho, H.Y.; Hsu, E.; Averick, A.A.; et al. Preparation of cationic nanogels for nucleic acid delivery. Biomacromolecules 2012, 13, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mahato, R.I.; Sung, Y.K.; Kim, S.W. Development of biomaterials for gene therapy. Mol. Ther. 2000, 2, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.W.; Liu, S.; Shrivats, A.R.; Watt, A.; McBride, S.; Averick, S.E.; Cho, H.Y.; Matyjaszewski, K.; Hollinger, J.O. Cationic nanostructured polymers for siRNA delivery in murine calvarial pre-osteoblasts. J. Biomed. Nanotechnol. 2014, 10, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Shrivats, A.R.; Hsu, E.; Averick, S.; Klimak, M.; Watt, A.C.; DeMaio, M.; Matyjaszewski, K.; Hollinger, J.O. Cationic nanogel-mediated Runx2 and osterix siRNA delivery decreases mineralization in MC3T3 cells. Clin. Orthop. Related Res. 2014, 473, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hodges, E.; Redelius, J.; Hoog, C. A novel approach for evaluating the efficiency of siRNAs on protein levels in cultured cells. Nucl. Acid. Res. 2004, 32, e17. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Mok, H.; Jeong, J.H.; Kim, S.W.; Park, T.G. Comparative evaluation of target-specific GFP gene silencing efficiencies for antisense ODN, synthetic siRNA, and siRNA plasmid complexed with PEI-PEG-FOL conjugate. Bioconjug. Chem. 2006, 17, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Tiscornia, G.; Singer, O.; Ikawa, M.; Verma, I.M. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc. Nat. Acad. Sci. USA 2003, 100, 1844–1848. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, B.R.; Cronican, J.J.; Thompson, D.B.; Liu, D.R. Mammalian cell penetration, siRNA transfection, and DNA transfection by supercharged proteins. Proc. Nat. Acad. Sci. USA 2009, 106, 6111–6116. [Google Scholar] [CrossRef] [PubMed]
- Waite, C.L.; Sparks, S.M.; Uhrich, K.E.; Roth, C.M. Acetylation of PAMAM dendrimers for cellular delivery of siRNA. BMC Biotechnology 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Yezhelyev, M.V.; Qi, L.; O'Regan, R.M.; Nie, S.; Gao, X. Proton-sponge coated quantum dots for siRNA delivery and intracellular imaging. J. Am. Chem. Soc. 2008, 130, 9006–9012. [Google Scholar] [CrossRef] [PubMed]
- Behlke, M.A. Chemical modification of siRNAs for in vivo use. Oligonucleotides 2008, 18, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Shrivats, A.R.; Hollinger, J.O. The delivery and evaluation of RNAi therapeutics for heterotopic ossification pathologies. In Biomimetics and Stem Cells: Methods and Protocols; Turksen, K., Vunjak-Novakovic, G., Eds.; SpringerLink: New York, NY, USA, 2014. [Google Scholar]
- Ewert, K.K.; Zidovska, A.; Ahmad, A.; Bouxsein, N.F.; Evans, H.M.; McAllister, C.S.; Samuel, C.E.; Safinya, C.R. Cationic liposome-nucleic acid complexes for gene delivery and silencing: Pathways and mechanisms for plasmid DNA and siRNA. Top. Curr. Chem. 2010, 296, 191–226. [Google Scholar] [PubMed]
- Dokka, S.; Toledo, D.; Shi, X.; Castranova, V.; Rojanasakul, Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharmaceut. Res. 2000, 17, 521–525. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, F.W.; Bendifallah, N.; Zachar, V.; Shiraishi, T.; Fink, T.; Ebbesen, P.; Nielsen, P.E.; Koppelhus, U. Evaluation of transfection protocols for unmodified and modified peptide nucleic acid (PNA) oligomers. Oligonucleotides 2006, 16, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Alemany, R.; Wang, J.; Koch, P.E.; Ordonez, N.G.; Roth, J.A. Safety evaluation of Ad5CMV-p53 in vitro and in vivo. Hum. Gene Ther. 1995, 6, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Abriss, B.; van de Leur, E.; Weiskirchen, S.; Gressner, A.M.; Weiskirchen, R. Comparative analysis of adenoviral transgene delivery via tail or portal vein into rat liver. Arch. Virol. 2004, 149, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrivats, A.R.; Mishina, Y.; Averick, S.; Matyjaszewski, K.; Hollinger, J.O. In Vivo GFP Knockdown by Cationic Nanogel-siRNA Polyplexes. Bioengineering 2015, 2, 160-175. https://doi.org/10.3390/bioengineering2030160
Shrivats AR, Mishina Y, Averick S, Matyjaszewski K, Hollinger JO. In Vivo GFP Knockdown by Cationic Nanogel-siRNA Polyplexes. Bioengineering. 2015; 2(3):160-175. https://doi.org/10.3390/bioengineering2030160
Chicago/Turabian StyleShrivats, Arun R., Yuji Mishina, Saadyah Averick, Krzysztof Matyjaszewski, and Jeffrey O. Hollinger. 2015. "In Vivo GFP Knockdown by Cationic Nanogel-siRNA Polyplexes" Bioengineering 2, no. 3: 160-175. https://doi.org/10.3390/bioengineering2030160
APA StyleShrivats, A. R., Mishina, Y., Averick, S., Matyjaszewski, K., & Hollinger, J. O. (2015). In Vivo GFP Knockdown by Cationic Nanogel-siRNA Polyplexes. Bioengineering, 2(3), 160-175. https://doi.org/10.3390/bioengineering2030160





