Overcoming Opacity: The Role of Intraoperative OCT in Complex Corneal and Anterior Segment Surgery
Abstract
1. Introduction
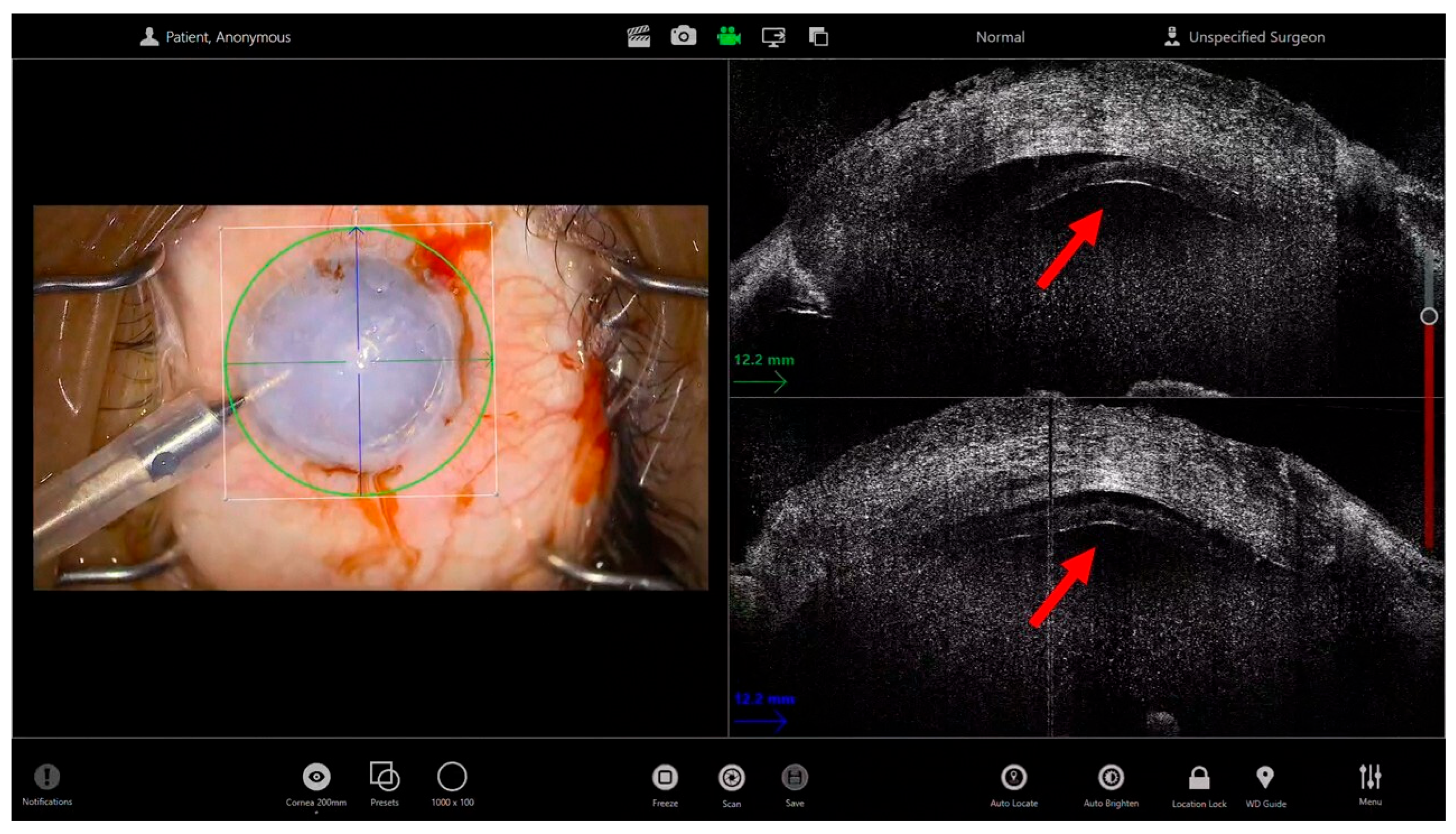
2. Endothelial Keratoplasty in Advanced Corneal Edema
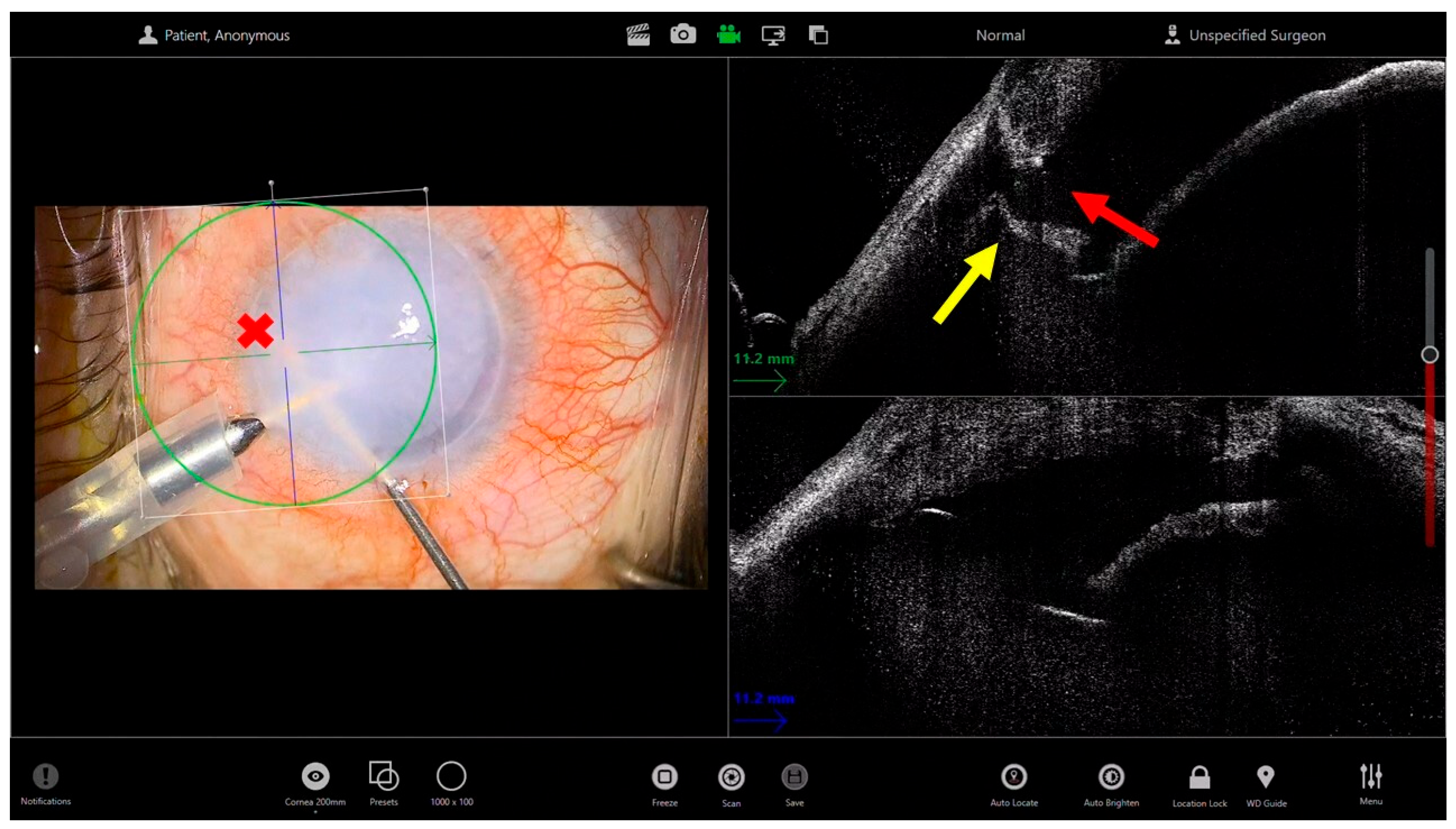
3. Deep Anterior Lamellar Keratoplasty for Leucomatous Corneas
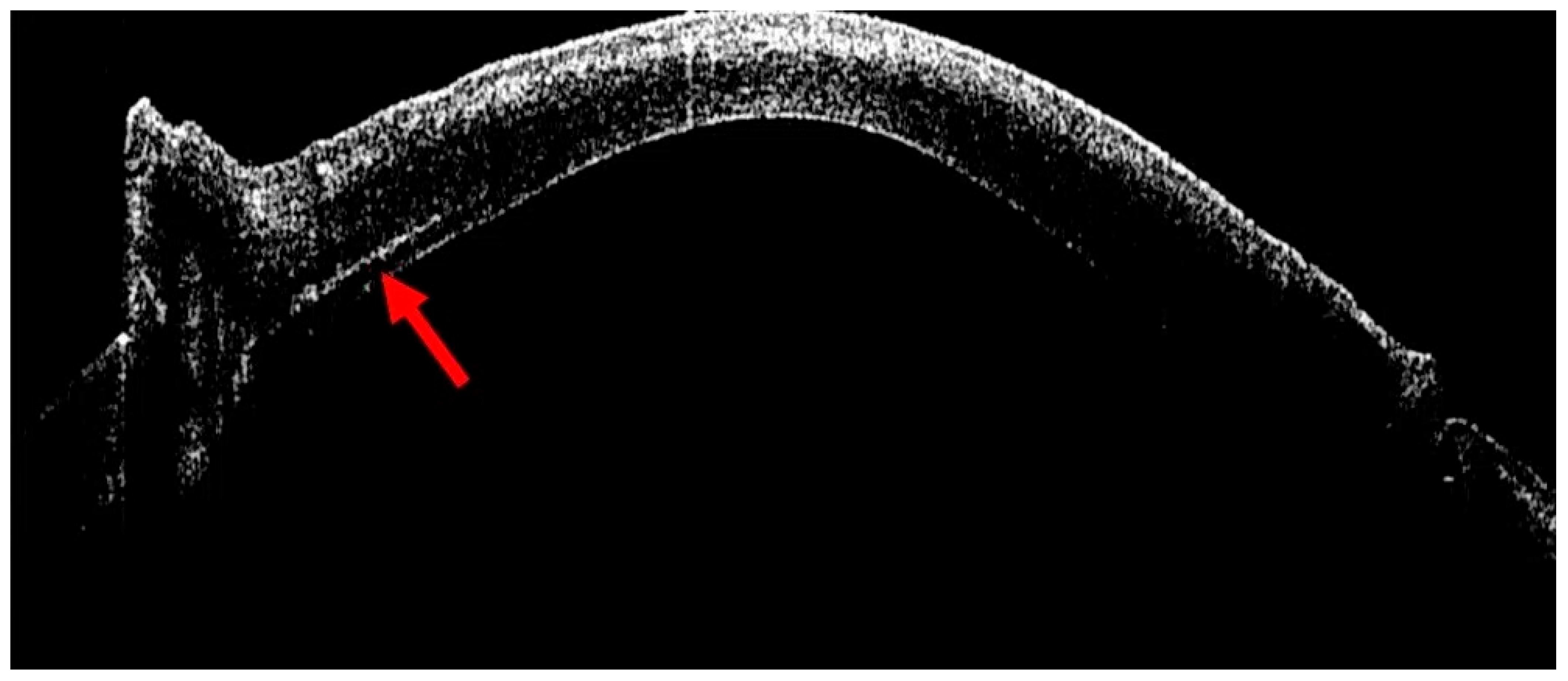
4. Management of Corneal Hydrops
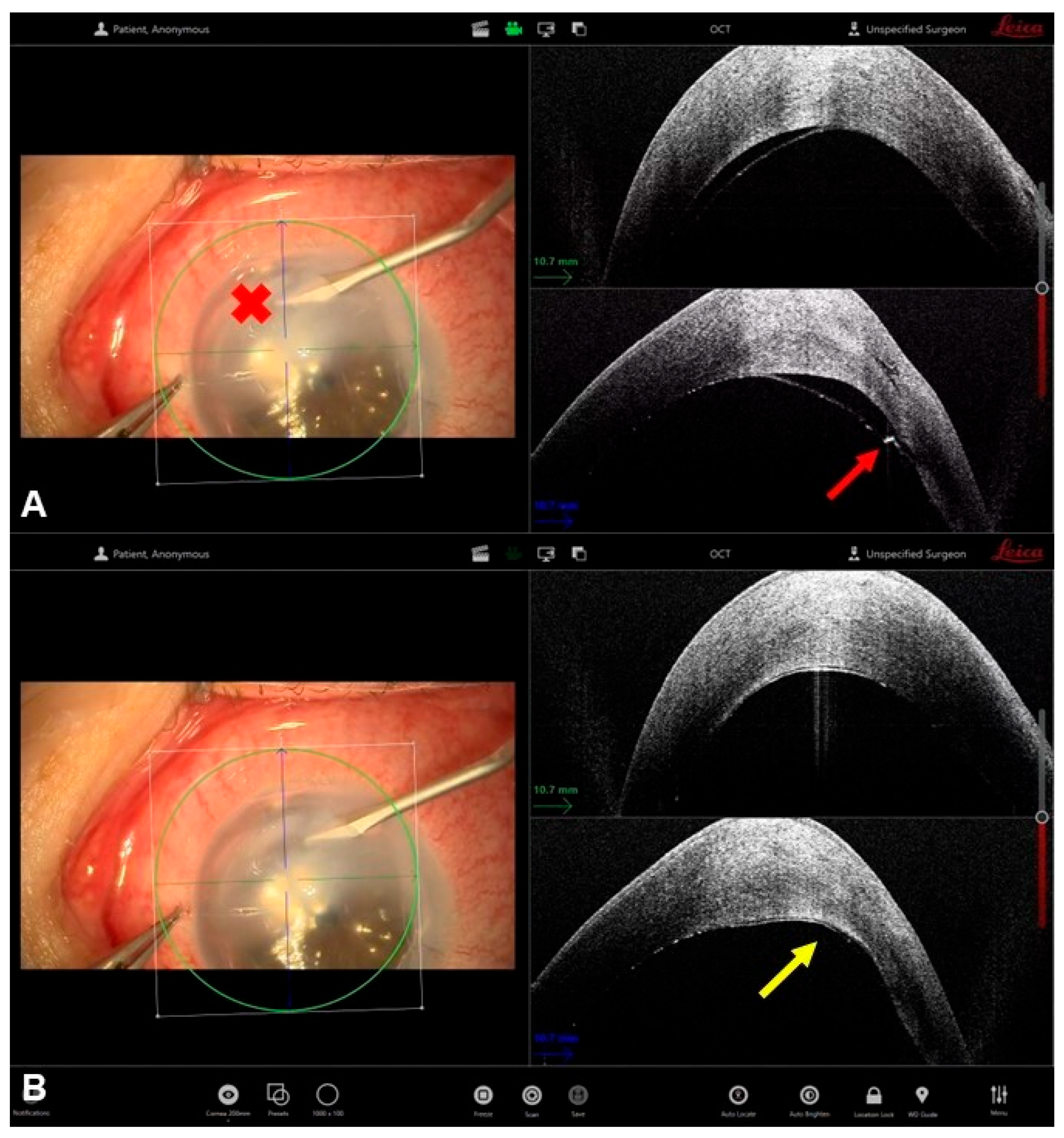
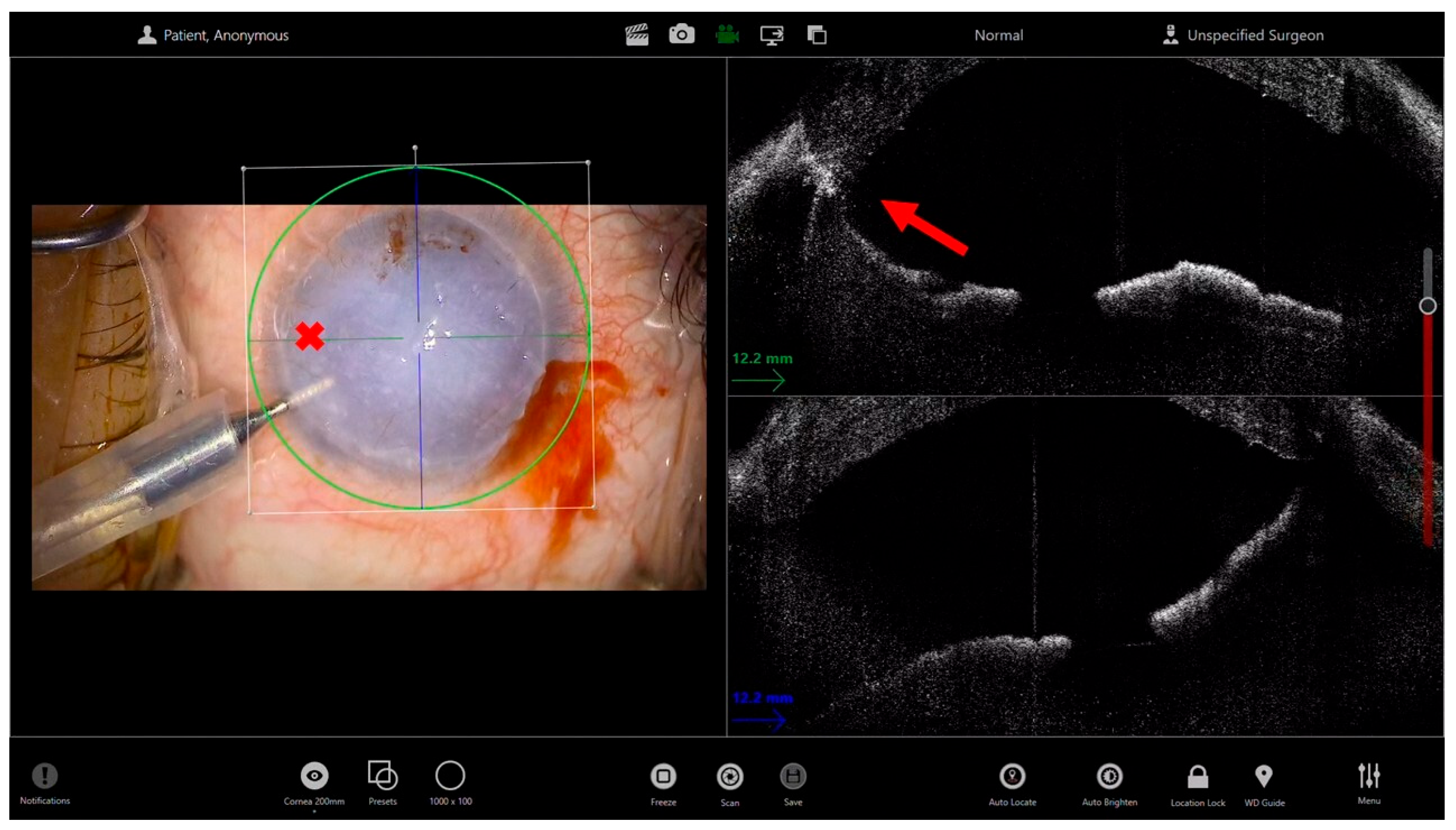
5. Synechiolysis
6. Glaucoma Shunt
7. Trauma
8. Future Directions
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIOCT | Microscopy integrated optical coherence tomography |
| iOCT | Intraoperative OCT |
| PAS | Peripheral anterior synechiae |
| DALK | Deep anterior lamellar keratoplasty |
References
- Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Lin, C.P.; Huang, D.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch. Ophthalmol. 1994, 112, 1584–1589. [Google Scholar] [CrossRef]
- Moramarco, A.; di Geronimo, N.; Airaldi, M.; Gardini, L.; Semeraro, F.; Iannetta, D.; Romano, V.; Fontana, L. Intraoperative OCT for Lamellar Corneal Surgery: A User Guide. J. Clin. Med. 2023, 12, 3048. [Google Scholar] [CrossRef]
- Ray, R.; Barañano, D.E.; Fortun, J.A.; Schwent, B.J.; Cribbs, B.E.; Bergstrom, C.S.; Hubbard, G.B.; Srivastava, S.K. Intraoperative microscope-mounted spectral domain optical coherence tomography for evaluation of retinal anatomy during macular surgery. Ophthalmology 2011, 118, 2212–2217. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Dupps, W.J.; Kaiser, P.K.; Goshe, J.; Singh, R.P.; Petkovsek, D.; Srivastava, S.K. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-year results. Am. J. Ophthalmol. 2014, 158, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Yee, P.; Sevgi, D.D.; Abraham, J.; Srivastava, S.K.; Le, T.; Uchida, A.; Figueiredo, N.; Rachitskaya, A.V.; Sharma, S.; Reese, J.; et al. iOCT-assisted macular hole surgery: Outcomes and utility from the DISCOVER study. Br. J. Ophthalmol. 2021, 105, 403–409. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Berrocal, A.M.; Schefler, A.C.; Uhlhorn, S.R.; Ruggeri, M.; Hess, D. Intraoperative OCT of a full-thickness macular hole before and after internal limiting membrane peeling. Ophthalmic Surg. Lasers Imaging Off. J. Int. Soc. Imaging Eye 2010, 41, 7–11. [Google Scholar] [CrossRef]
- Abraham, J.R.; Srivastava, S.K.; Le, T.K.; Sharma, S.; Rachitskaya, A.; Reese, J.L.; Ehlers, J.P. Intraoperative OCT-Assisted Retinal Detachment Repair in the DISCOVER Study: Impact and Outcomes. Ophthalmol. Retina 2020, 4, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Falkner-Radler, C.I.; Glittenberg, C.; Gabriel, M.; Binder, S. Intrasurgical microscope-integrated spectral domain optical coherence tomography-assisted membrane peeling. Retina 2015, 35, 2100–2106. [Google Scholar] [CrossRef]
- Abraham, J.R.; Srivastava, S.K.; Reese, J.L.; Ehlers, J.P. Intraoperative OCT Features and Postoperative Ellipsoid Mapping in Primary Macula-Involving Retinal Detachments from the PIONEER Study. Ophthalmol. Retina 2019, 3, 252–257. [Google Scholar] [CrossRef]
- Heindl, L.M.; Siebelmann, S.; Dietlein, T.; Hüttmann, G.; Lankenau, E.; Cursiefen, C.; Steven, P. Future prospects: Assessment of intraoperative optical coherence tomography in ab interno glaucoma surgery. Curr. Eye Res. 2015, 40, 1288–1291. [Google Scholar] [CrossRef]
- Toro, M.D.; Milan, S.; Tognetto, D.; Rejdak, R.; Costagliola, C.; Zweifel, S.A.; Posarelli, C.; Figus, M.; Rejdak, M.; Avitabile, T.; et al. Intraoperative Anterior Segment Optical Coherence Tomography in the Management of Cataract Surgery: State of the Art. J. Clin. Med. 2022, 11, 3867. [Google Scholar] [CrossRef]
- Eguchi, H.; Hotta, F.; Kusaka, S.; Shimomura, Y. Intraoperative Optical Coherence Tomography Imaging in Corneal Surgery: A Literature Review and Proposal of Novel Applications. J. Ophthalmol. 2020, 2020, 1497089. [Google Scholar] [CrossRef]
- Fu, L.M.; Hollick, E.J.M. Comparison of Long-Term Outcomes of DSEK and DMEK in Fuchs Endothelial Dystrophy. Cornea 2024, 43, 184–189. [Google Scholar] [CrossRef]
- Weisenthal, R.W.; Yin, H.Y.; Jarstad, A.R.; Wang, D.; Verdier, D.D. Long-term Outcomes in Fellow Eyes Comparing DSAEK and DMEK for Treatment of Fuchs Corneal Dystrophy. Am. J. Ophthalmol. 2022, 233, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.W.; de Benito-Llopis, L. Comparison of repeat penetrating keratoplasty, DSAEK and DMEK for the management of endothelial failure of previous PK. Eye Lond. Engl. 2023, 37, 3596–3601. [Google Scholar] [CrossRef]
- Hos, D.; Matthaei, M.; Bock, F.; Maruyama, K.; Notara, M.; Clahsen, T.; Hou, Y.; Le, V.N.H.; Salabarria, A.-C.; Horstmann, J.; et al. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog. Retin. Eye Res. 2019, 73, 100768. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, N.D.B.; Shieh, C.; Carrasco-Zevallos, O.M.B.; Keller, B.B.; Izatt, J.A.; Toth, C.A.; Kuo, A.N. Real-Time Microscope-Integrated OCT to Improve Visualization in DSAEK for Advanced Bullous Keratopathy. Cornea 2015, 34, 1606–1610. [Google Scholar] [CrossRef]
- Wylegala, E.; Nowinska, A.K.; Wroblewska-Czajka, E.; Janiszewska, D. Donor disc attachment assessment with intraoperative spectral optical coherence tomography during descemet stripping automated endothelial keratoplasty. Indian J. Ophthalmol. 2013, 61, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sahay, P.; Maharana, P.K.; Kumar, P.; Ahsan, S.; Titiyal, J.S. Microscope Integrated Intraoperative Optical Coherence Tomography-Guided DMEK in Corneas with Poor Visualization. Clin. Ophthalmol. 2020, 14, 643–651. [Google Scholar] [CrossRef]
- Moramarco, A.; Gardini, L.; Di Mola, I.; di Geronimo, N.; Iannetta, D.; Romano, V.; Hannush, S.B.; Fontana, L. Big-bubble DALK: A technique in evolution. Ocul. Surf. 2024, 34, 418–429. [Google Scholar] [CrossRef]
- Gadhvi, K.A.; Romano, V.; Fernández-Vega Cueto, L.; Aiello, F.; Day, A.C.; Allan, B.D. Deep Anterior Lamellar Keratoplasty for Keratoconus: Multisurgeon Results. Am. J. Ophthalmol. 2019, 201, 54–62. [Google Scholar] [CrossRef]
- Gadhvi, K.A.; Romano, V.; Cueto, L.F.-V.; Aiello, F.; Day, A.C.; Gore, D.M.; Allan, B.D. Femtosecond Laser-Assisted Deep Anterior Lamellar Keratoplasty for Keratoconus: Multi-surgeon Results. Am. J. Ophthalmol. 2020, 220, 191–202. [Google Scholar] [CrossRef]
- Han, D.C.Y.; Mehta, J.S.; Por, Y.M.; Htoon, H.M.; Tan, D.T.H. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am. J. Ophthalmol. 2009, 148, 744–751.e1. [Google Scholar] [CrossRef]
- Lucisano, A.; Yu, G.L.A.C.; Giannaccare, G.; D’Angelo, S.; Busin, M.; Scorcia, V. Outcomes of Conventional 8.0-mm Versus Large 9.0-mm Diameter Deep Anterior Lamellar Keratoplasty for Keratoconus. Cornea 2023, 42, 815–820. [Google Scholar] [CrossRef]
- Anwar, M.; Teichmann, K.D. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J. Cataract Refract. Surg. 2002, 28, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Goweida, M.B.B. Intraoperative review of different bubble types formed during pneumodissection (big-bubble) deep anterior lamellar keratoplasty. Cornea 2015, 34, 621–624. [Google Scholar] [CrossRef]
- Parker, J.S.; Birbal, R.S.; van Dijk, K.; Oellerich, S.; Dapena, I.; Melles, G.R.J. Are Descemet Membrane Ruptures the Root Cause of Corneal Hydrops in Keratoconic Eyes? Am. J. Ophthalmol. 2019, 205, 147–152. [Google Scholar] [CrossRef]
- Sharma, N.; Maharana, P.K.; Jhanji, V.; Vajpayee, R.B. Management of acute corneal hydrops in ectatic corneal disorders. Curr. Opin. Ophthalmol. 2012, 23, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Moramarco, A.; Elifani, M.; Zimbru, M.S.; Rosolia, A.; Mete, M.; Fontana, L. Air-Assisted Dome Drainage in Acute Corneal Hydrops: A 3D-OCT-Guided Approach. Bioengineering 2025, 12, 867. [Google Scholar] [CrossRef] [PubMed]
- Siebelmann, S.; Händel, A.; Matthaei, M.; Bachmann, B.; Cursiefen, C. Microscope-Integrated Optical Coherence Tomography-Guided Drainage of Acute Corneal Hydrops in Keratoconus Combined With Suturing and Gas-Aided Reattachment of Descemet Membrane. Cornea 2019, 38, 1058–1061. [Google Scholar] [CrossRef]
- Kaur, M.; Balaji, A.; Titiyal, J.S.; Bansal, M.; Raj, R.; Namdev, V. Intraoperative optical coherence tomography-guided compression sutures in acute corneal hydrops-Surgical technique and review of literature. Indian J. Ophthalmol. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, F.; Alsharif, H.; Almutlak, M.; Fairaq, R.; Kirat, O.M.; Bin Helayel, H. Management of Acute Corneal Hydrops Using Compression Sutures and Intracameral Air Injection. Am. J. Case Rep. 2024, 25, e944517. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, A.; Gianniou, C.; Hashemi, K.; Kymionis, G. Intraoperative anterior optical coherence tomography-guided synechiolysis in a post-penetrating keratoplasty patient with peripheral corneal opacification. Ther. Clin. Risk Manag. 2018, 14, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.T.-C.; Betzler, B.K.; Lim, S.Y.; Ang, B.C.H. Anterior segment imaging in minimally invasive glaucoma surgery—A systematic review. Acta Ophthalmol. 2022, 100, e617–e634. [Google Scholar] [CrossRef]
- Moreno, I.P.; Altares, L.G.; Martin-Gutierrez, M.P.; Morote, I.J.-A. Intraoperative optical coherence tomography in glaucoma surgery with Ex-PRESS® implant. Eur. J. Ophthalmol. 2025, 35, NP16–NP18. [Google Scholar]
- Bondalapati, S.; Ambati, B. Intraocular foreign body removal: A novel technique using intraoperative imaging. Int. Ophthalmol. 2017, 37, 749–752. [Google Scholar] [CrossRef]
- Chaniyara, M.H.; Bafna, R.; Urkude, J.; Sharma, N. Rescuing the host Descemet’s membrane in full-thickness traumatic wound dehiscence in deep anterior lamellar keratoplasty: Intraoperative optical coherence tomography (iOCT)-guided technique. BMJ Case Rep. 2017, 2017, bcr-2017–221495. [Google Scholar] [CrossRef]
- Keller, B.; Draelos, M.; Zhou, K.; Qian, R.; Kuo, A.N.; Konidaris, G.; Hauser, K.; Izatt, J.A. Optical Coherence Tomography-Guided Robotic Ophthalmic Microsurgery via Reinforcement Learning from Demonstration. IEEE Trans. Robot. Publ. IEEE Robot. Autom. Soc. 2020, 36, 1207–1218. [Google Scholar] [CrossRef]
| Study Type | Sample Size | Procedure | iOCT Role | |
|---|---|---|---|---|
| Pasricha et al. [17] | Case series | 2 patients | DSAEK | Graft insertion, unfolding, tamponade, and attachment verification |
| Sharma et al. [19] | Prospective interventional case series | 25 eyes | DMEK | Visualizing areas of PAS, retained DM tags, roll configuration and orientation, interface fluid and peripheral folds |
| Moramarco et al. [29] | Case series | 6 eyes | Corneal hydrops drainage | Controlled drainage of the stromal dome with a 23-gauge sclerotome, with dynamic monitoring of fluid outflow |
| Siebelmann et al. [30] | Case series | 2 patients | Corneal hydrops drainage | iOCT-guided puncture and drainage of intrastromal fluid pockets combined with anterior chamber sulfur hexafluoride-fill and pre-descemetic sutures |
| Kaur et al. [31] | Prospective interventional case series | 7 patients | Corneal hydrops drainage | Assess the morphological features of the hydrops, titrate the magnitude of suture tightness and confirm the depth of suture placement |
| Petrovic et al. [33] | Case report | 1 patient | Synechiolysis | Detect non-clinically visible synechiae and confirm complete synechiolysis |
| Platas Moreno et al. [35] | Case report | 1 patient | Ex-PRESS® implant | Locate the correct placement of implants |
| Bondalapati et al. [36] | Case report | 1 patient | Intraocular foreign body removal | Localization of the foreign body and confirmation that there was no remaining foreign body |
| Chaniyara et al. [37] | Case report | 1 patient | Traumatic wound dehiscence repair in DALK | Visualization of donor tissue separated from host Descemet’s membrane, peripheral iridocorneal touch and distorted graft–host junction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
di Geronimo, N.; Moramarco, A.; Romano, V.; Mete, M.; Fontana, L. Overcoming Opacity: The Role of Intraoperative OCT in Complex Corneal and Anterior Segment Surgery. Bioengineering 2026, 13, 15. https://doi.org/10.3390/bioengineering13010015
di Geronimo N, Moramarco A, Romano V, Mete M, Fontana L. Overcoming Opacity: The Role of Intraoperative OCT in Complex Corneal and Anterior Segment Surgery. Bioengineering. 2026; 13(1):15. https://doi.org/10.3390/bioengineering13010015
Chicago/Turabian Styledi Geronimo, Natalie, Antonio Moramarco, Vito Romano, Maurizio Mete, and Luigi Fontana. 2026. "Overcoming Opacity: The Role of Intraoperative OCT in Complex Corneal and Anterior Segment Surgery" Bioengineering 13, no. 1: 15. https://doi.org/10.3390/bioengineering13010015
APA Styledi Geronimo, N., Moramarco, A., Romano, V., Mete, M., & Fontana, L. (2026). Overcoming Opacity: The Role of Intraoperative OCT in Complex Corneal and Anterior Segment Surgery. Bioengineering, 13(1), 15. https://doi.org/10.3390/bioengineering13010015









