Characterization of La2O3 Nanoparticles and Their Effects on Bacteria, Vero and MG63 Cells, and Zebrafish Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of La2O3 NPs Dispersion
2.2.2. Characterization Using Fluorescent Spectrophotometer
2.2.3. Characterization of Particle Size and Zeta Potential
2.2.4. Characterization of La2O3NPs by Raman Spectroscopy
2.2.5. Characterization of La2O3 NPs by X-Ray Diffraction (XRD)
2.2.6. Characterization of La2O3 NPs by Thermogravimetric Analysis (TGA)
2.2.7. Characterization of La2O3 NPs by Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.2.8. Characterization of La2O3 NPs by Transmission Electron Microscopy (TEM)
2.3. Antibacterial Assay
2.4. Antioxidant Assay
2.4.1. Enzymatic Glutathione Reductase Activity of La2O3 NPs
2.4.2. Non-Enzymatic Antioxidant Activity by 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
2.5. Cell Viability Assays (In Vitro Assay for Cytotoxicity Activity: MTT Assay)
2.6. Zebrafish Husbandry and Embryo Culture
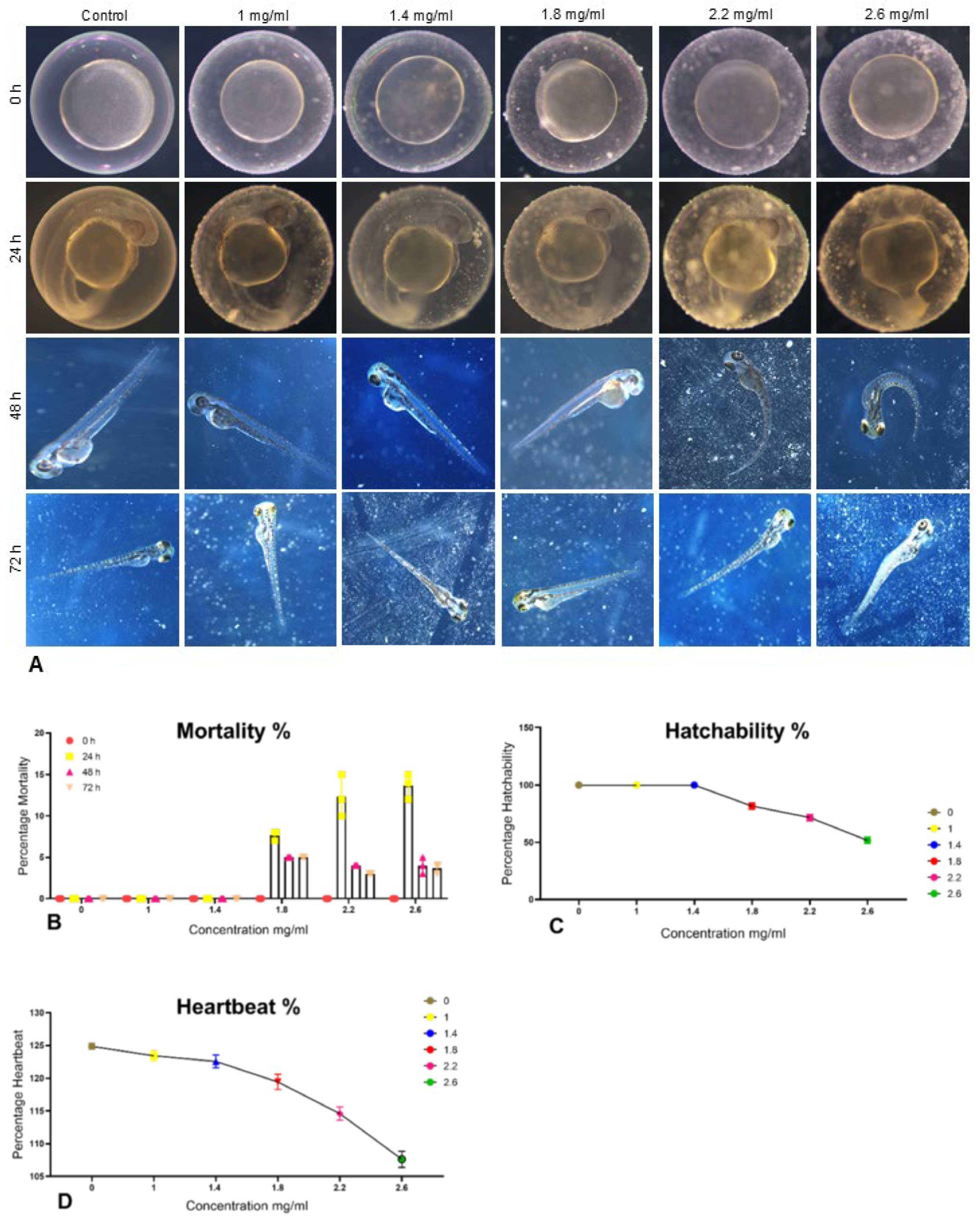
2.6.1. Teratogenicity Assay
2.6.2. Percentage Mortality
2.6.3. Percentage Hatchability
2.6.4. Percentage Heartbeat
3. Results and Discussion
3.1. Physico-Chemical Characterizations
3.1.1. Solubility of La2O3 NPs
3.1.2. Characterization of La2O3 NPs Using a Fluorescent Spectrophotometer
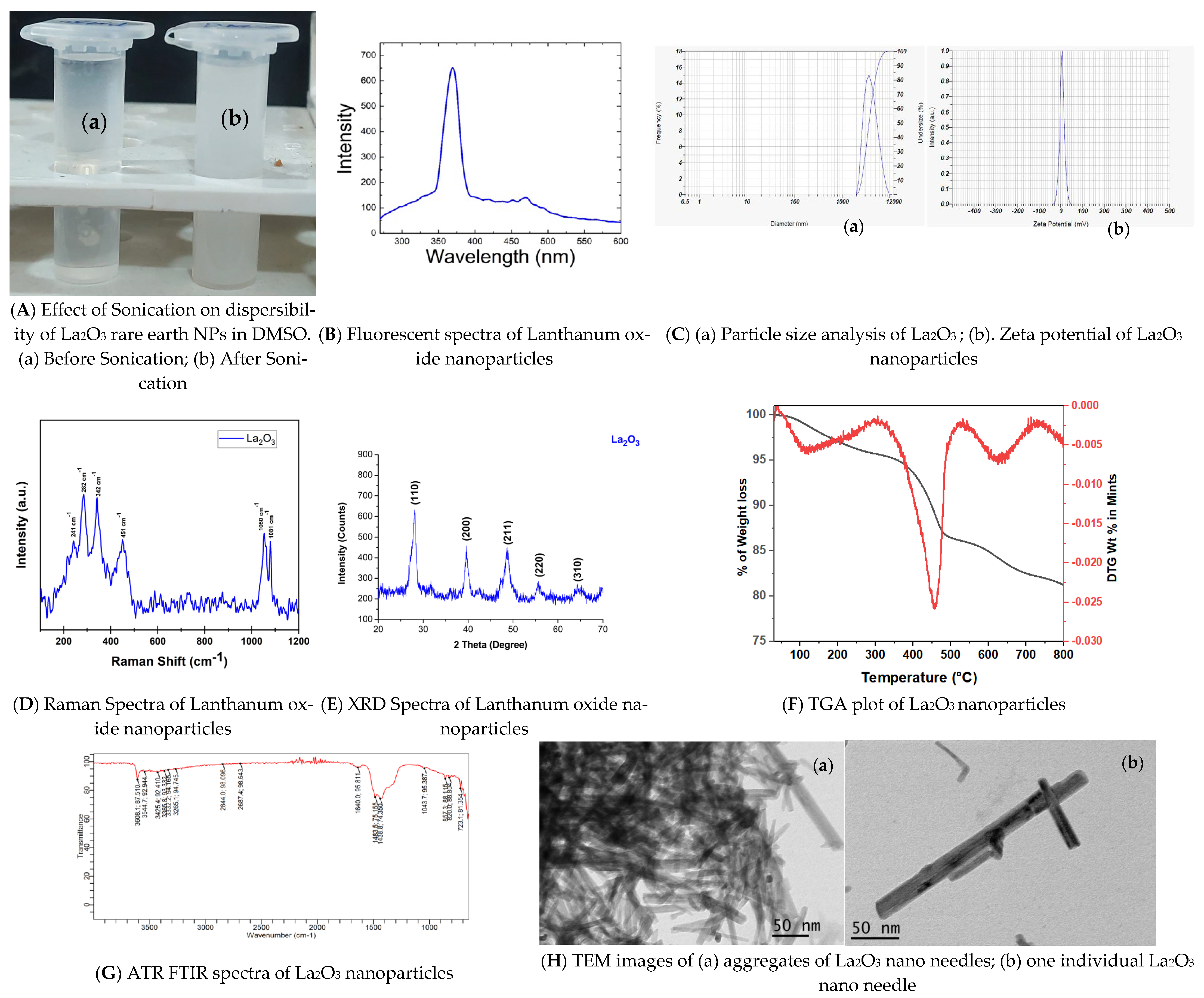
3.1.3. Particle Size Analysis and Zeta Potential of La2O3 NPs
3.1.4. Characterization of La2O3NPs by Raman Spectroscopy
3.1.5. Characterization of La2O3 NPs by XRD
3.1.6. Characterization of La2O3 NPs by TGA
3.1.7. ATR-FTIR of La2O3 NPs by ATR-FTIR
3.1.8. Characterization of La2O3 NPs by Transmission Electron Microscopy
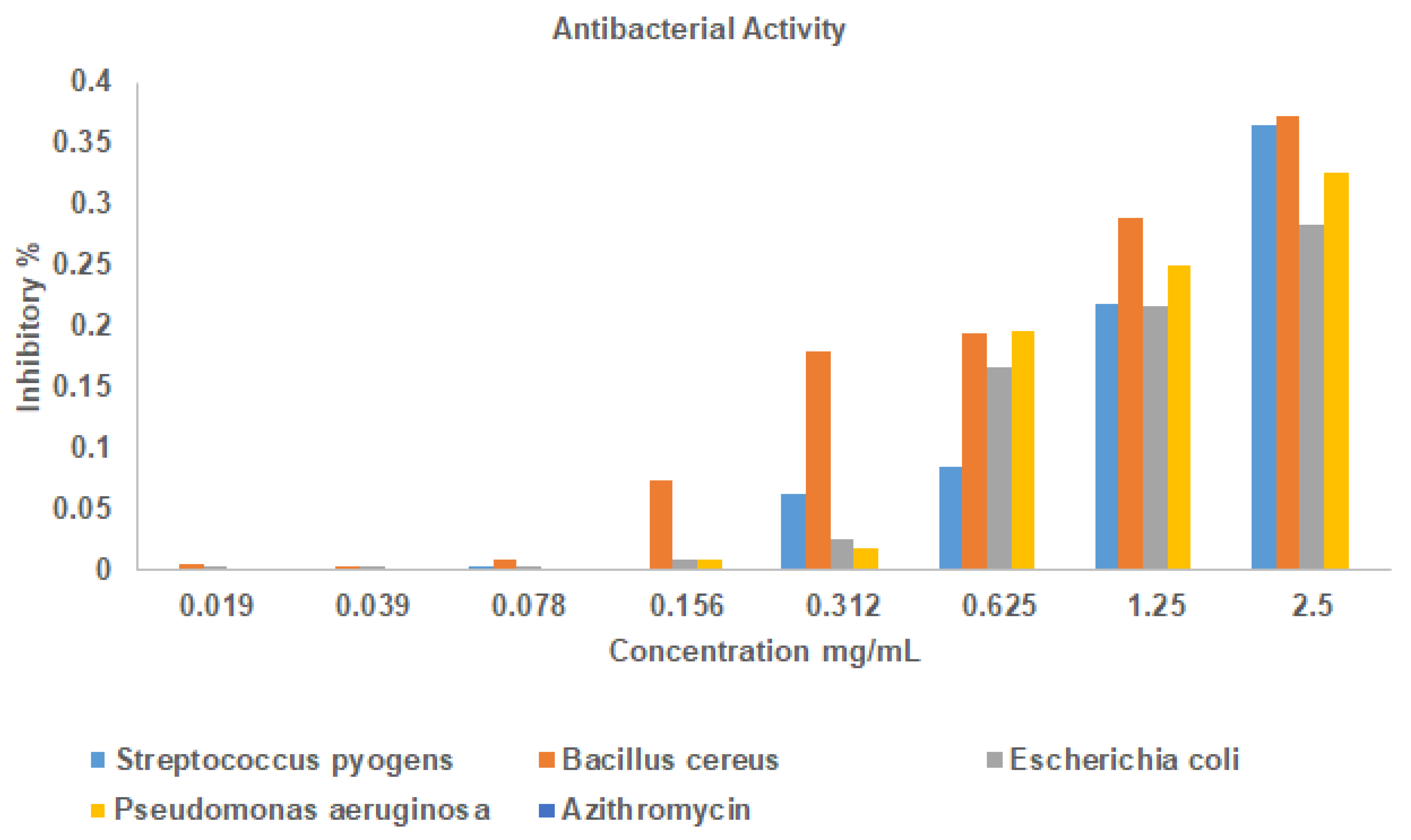
3.2. Antibacterial Activity of La2O3 NPs
3.3. Antioxidant Activity of La2O3 NPs
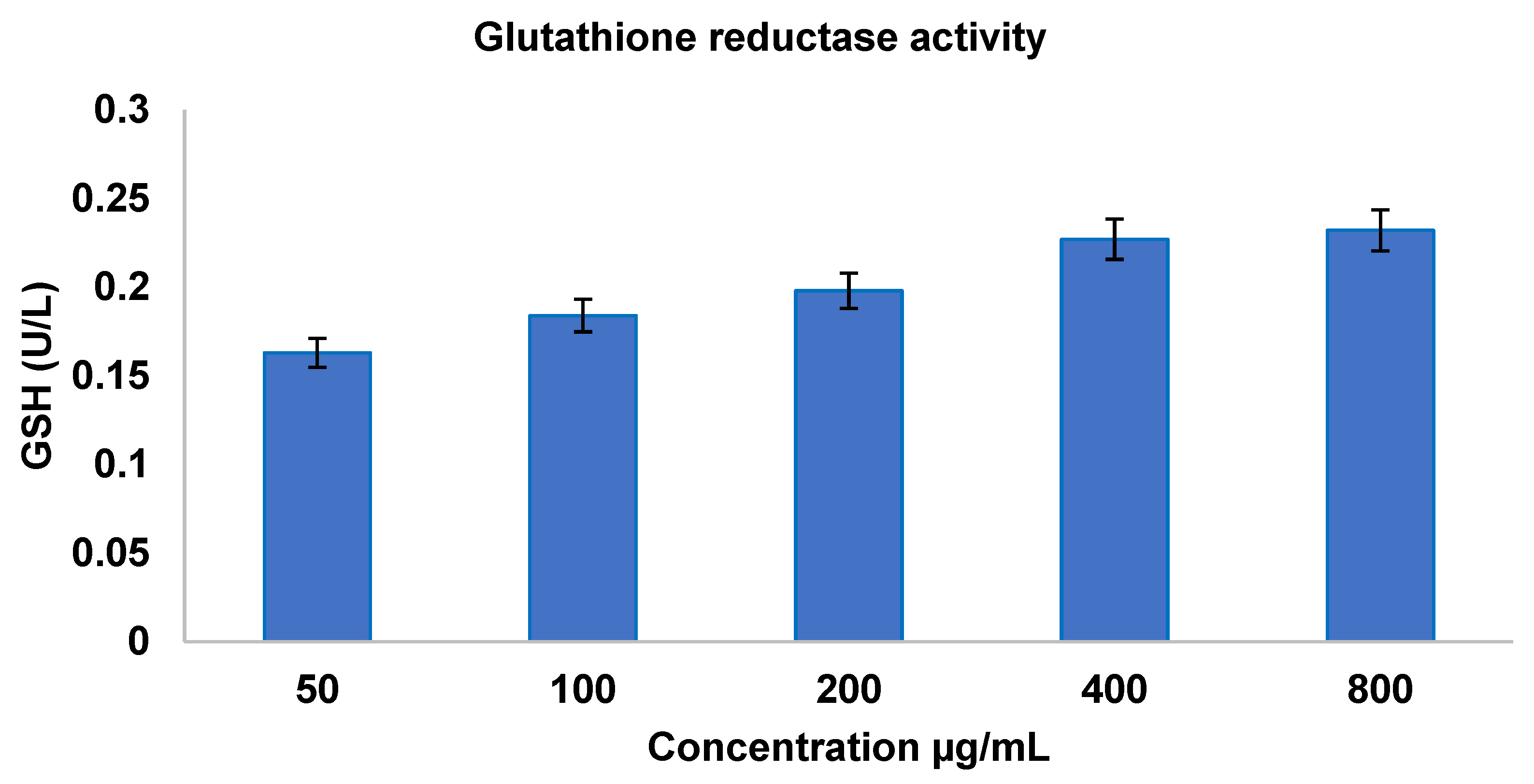
3.3.1. Antioxidant Activity—Enzymatic Assay Glutathione Reductase Activity
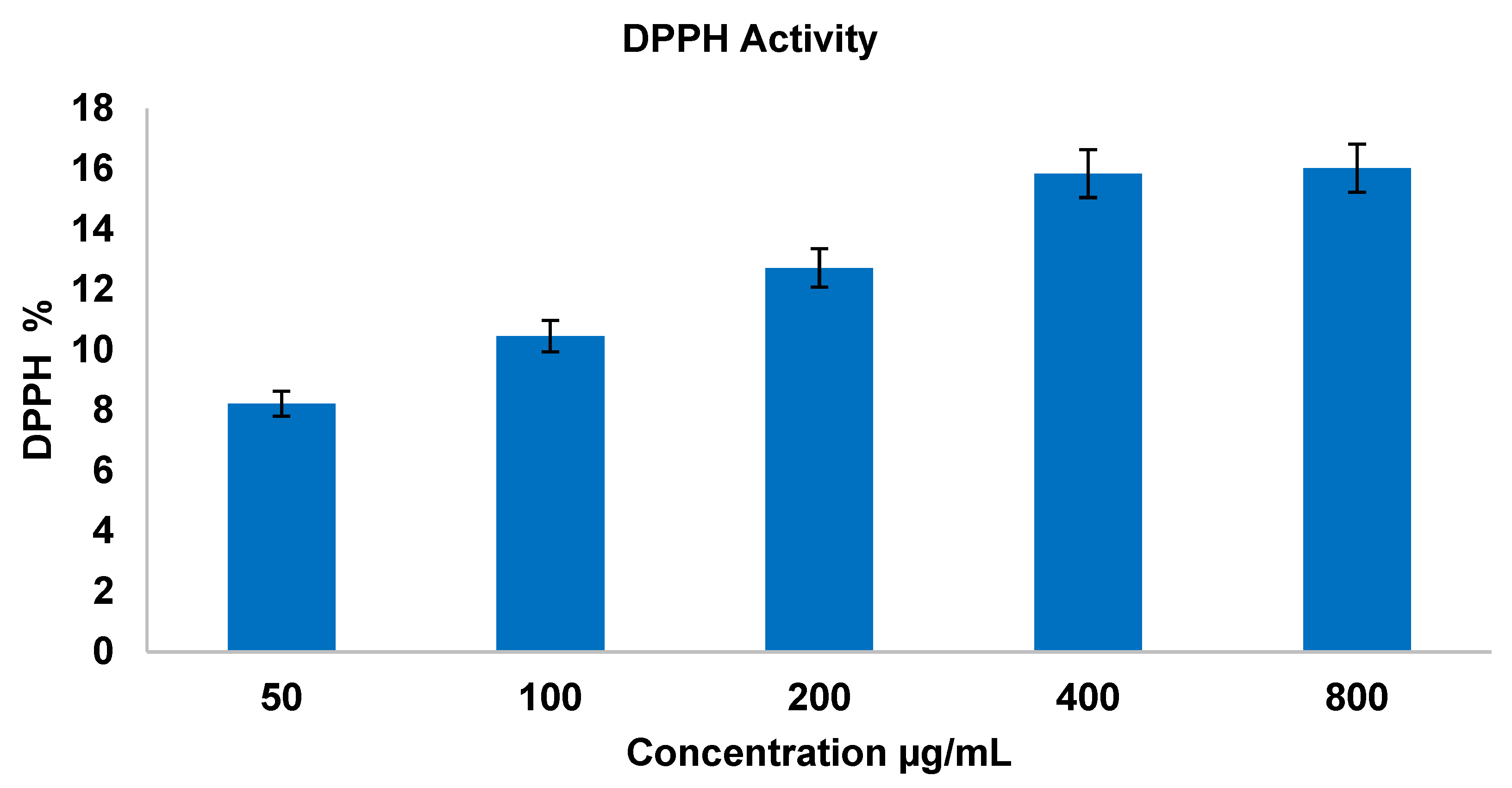
3.3.2. Antioxidant Activity—Non-Enzymatic Assay 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
3.4. Morphological Evaluation by Optical Microscopy
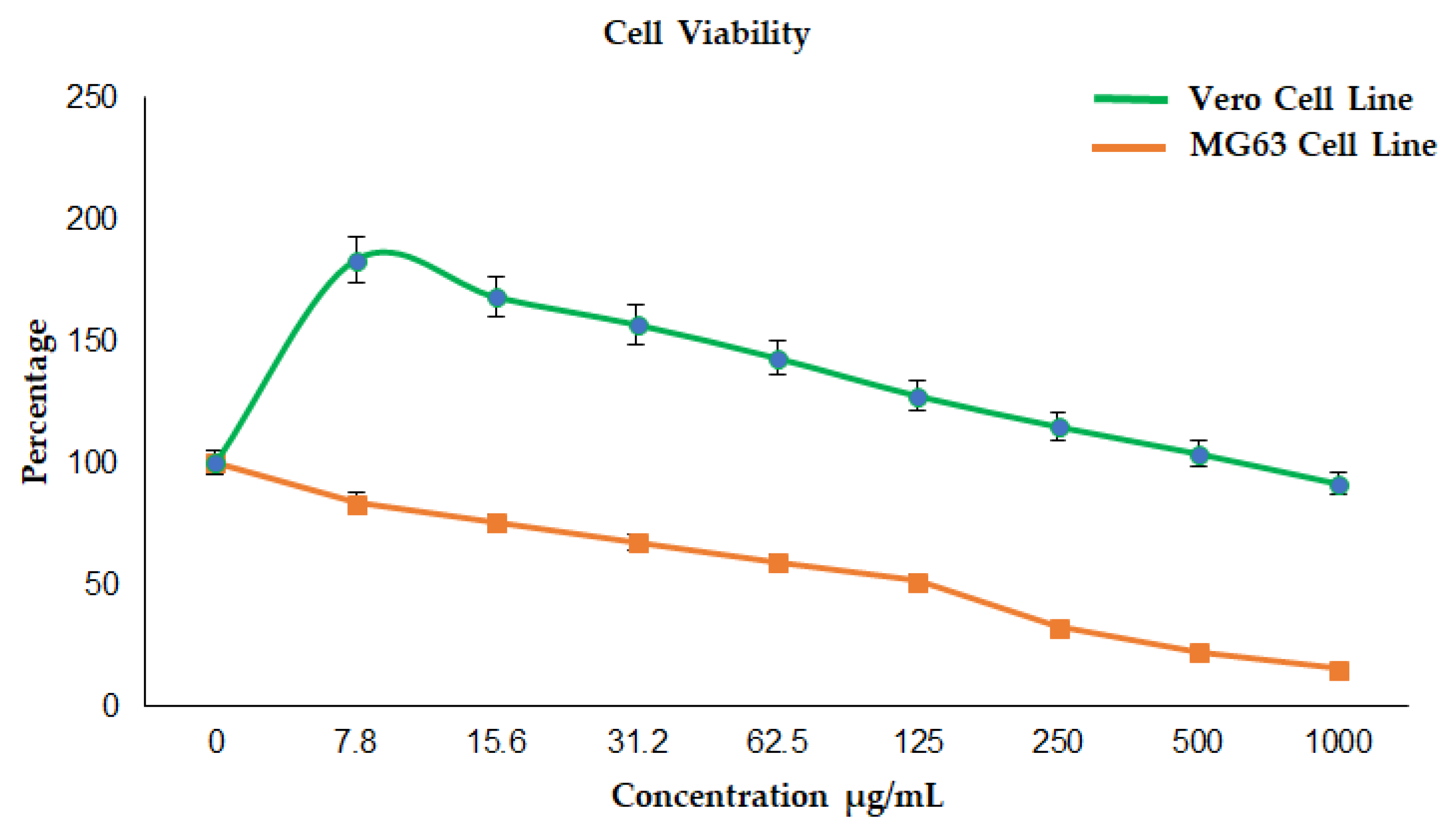
3.4.1. Cytotoxicity Effect of La2O3 NPs on Vero Cell Line
3.4.2. Anticancer Effect of La2O3 NPs on the MG 63 Cell Line
3.5. Teratogenicity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay |
| XRD | X-ray diffractometery |
| TGA | Thermogravimetric analysis |
| ATR-FTIR | Attenuated total reflectance Fourier transform infrared spectroscopy |
| TEM | Transmission electron microscopy |
| TM | Trademark |
| NPs | Nanoparticles |
| BPM | Beats per minute |
| ATCC | American Type Culture Collection |
| LC | Lethal concentration |
| MRI | Magnetic resonance imaging |
| DNA | Deoxyribonucleic acid |
| ROS | Radical oxygen species/reactive oxygen species |
| LAK Cells | Lymphokine-activated killer cells |
| DMSO | Dimethyl sulphoxide |
| NCCS | National Centre for Cell Sciences |
| UK | United Kingdom |
| EDTA | Ethylenediaminetetraacetic acid |
| GSSG | Glutathione disulfide |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate Hydrogen |
| UV | Ultraviolet |
| GSH | Reduced glutathione |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| BHT | Butylated Hydroxy Toluene |
| RSA | Radical scavenging activity |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| IC | Inhibitory concentration |
| CCSEA | Committee for the Control and Supervision of Experiments on Animals |
| OECD | Organisation for Economic Co-operation and Development |
| ICDD | International Centre for Diffraction Data |
| OD | Outer diameter/optical density |
| MIC | Minimum inhibitory concentration |
| OM | Optical microscope |
| FBS | Foetal bovine serum |
| NOEC | No Observed Effect Concentration |
References
- Huq, M.A. Extracellular Synthesis of Bioactive Silver Nanoparticles Using Brevibacillus Sp. MAHUQ-41 and Their Potential Application Against Drug-Resistant Bacterial Pathogens Listeria Monocytogenes and Yersinia Enterocolitica. J. Funct. Biomater. 2025, 16, 241. [Google Scholar] [CrossRef]
- Huq, M.A.; Rana, M.R.; Samad, A.; Rahman, M.S.; Rahman, M.M.; Ashrafudoulla, M.; Akter, S.; Park, J.-W. Green Synthesis, Characterization, and Potential Antibacterial and Anticancer Applications of Gold Nanoparticles: Current Status and Future Prospects. Biomedicines 2025, 13, 1184. [Google Scholar] [CrossRef]
- Dabhane, H.; Ghotekar, S.; Tambade, P.; Medhane, V. Plant mediated green synthesis of lanthanum oxide (La2O3) nanoparticles: A review. J. Med. Nanomater. Chem. 2020, 2, 291–299. [Google Scholar]
- Thompson, K.H.; Orvig, C. Editorial: Lanthanide Compounds for Therapeutic and Diagnostic Applications. Chem. Soc. Rev. 2006, 35, 499. [Google Scholar] [CrossRef]
- Veerasingam, M.; Murugesan, B.; Mahalingam, S. Ionic Liquid Mediated Morphologically Improved Lanthanum Oxide Nanoparticles by Andrographis Paniculata Leaves Extract and Its Biomedical Applications. J. Rare Earths 2020, 38, 281–291. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Z.-H.; Yu, F.-M.; Chen, H.-B. Structure and Properties of—Nanocomposite Films for Biomedical Applications. Bioinorg. Chem. Appl. 2011, 2011, 853048. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; Jue, T.R.; McDonald, K.L. Cytotoxic Lanthanum Oxide Nanoparticles Sensitize Glioblastoma Cells to Radiation Therapy and Temozolomide: An In Vitro Rationale for Translational Studies. Sci. Rep. 2020, 10, 18156. [Google Scholar] [CrossRef]
- Teo, R.D.; Termini, J.; Gray, H.B. Lanthanides: Applications in Cancer Diagnosis and Therapy. J. Med. Chem. 2016, 59, 6012–6024. [Google Scholar] [CrossRef]
- Zhang, Q.; O’Brien, S.; Grimm, J. Biomedical Applications of Lanthanide Nanomaterials, for Imaging, Sensing and Therapy. Nanotheranostics 2022, 6, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Traykova, M.; Saso, L.; Kostova, I. Involvement of Lanthanides in the Free Radicals Homeostasis. Curr. Top. Med. Chem. 2014, 14, 2508–2519. [Google Scholar] [CrossRef]
- Almukhlafi, H.; Ali, D.; Almutairi, B.; Yaseen, K.N.; Alyami, N.; Almeer, R.; Alkahtani, S.; Alarifi, S. Role of Oxidative Stress in La2O3 Nanoparticle-Induced Cytotoxicity and Apoptosis in CHANG and HuH-7 Cells. Int. J. Nanomed. 2021, 16, 3487–3496. [Google Scholar] [CrossRef] [PubMed]
- Alverca, E.; Andrade, M.; Dias, E.; Sam Bento, F.; Batoréu, M.C.C.; Jordan, P.; Silva, M.J.; Pereira, P. Morphological and Ultrastructural Effects of Microcystin-LR from Microcystis Aeruginosa Extract on a Kidney Cell Line. Toxicon 2009, 54, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, C.; Abid-Essefi, S.; Bouslimi, A.; El Golli, E.; Bacha, H. Cytotoxicity and Related Effects of T-2 Toxin on Cultured Vero Cells. Toxicon 2006, 48, 343–352. [Google Scholar] [CrossRef]
- Fernández Freire, P.; Labrador, V.; Pérez Martín, J.M.; Hazen, M.J. Cytotoxic Effects in Mammalian Vero Cells Exposed to Pentachlorophenol. Toxicology 2005, 210, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Poli, P.; Aline De Mello, M.; Buschini, A.; Mortara, R.A.; Northfleet De Albuquerque, C.; Da Silva, S.; Rossi, C.; Zucchi, T.M.A.D. Cytotoxic and Genotoxic Effects of Megazol, an Anti-Chagas’ Disease Drug, Assessed by Different Short-Term Tests. Biochem. Pharmacol. 2002, 64, 1617–1627. [Google Scholar] [CrossRef]
- Rehman, Y.; Copet, C.; Morlando, A.; Huang, X.F.; Konstantinov, K. Investigation of ROS Scavenging Properties and In Vitro Cytotoxicity of Oxygen-Deficient La2O3-x Nanostructure Synthesized by Spray Pyrolysis Method. J. Nanostruct. Chem. 2020, 10, 347–361. [Google Scholar] [CrossRef]
- Menezes, C.; Alverca, E.; Dias, E.; Sam-Bento, F.; Pereira, P. Involvement of Endoplasmic Reticulum and Autophagy in Microcystin-LR Toxicity in Vero-E6 and HepG2 Cell Lines. Toxicol. Vitr. 2013, 27, 138–148. [Google Scholar] [CrossRef]
- Puerari, R.C.; Ferrari, E.; de Cezar, M.G.; Gonçalves, R.A.; Simioni, C.; Ouriques, L.C.; Vicentini, D.S.; Matias, W.G. Investigation of Toxicological Effects of Amorphous Silica Nanostructures with Amine-Functionalized Surfaces on Vero Cells. Chemosphere 2019, 214, 679–687. [Google Scholar] [CrossRef]
- Pakrashi, S.; Dalai, S.; Sabat, D.; Singh, S.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of Al2O3 Nanoparticles at Low Exposure Levels to a Freshwater Bacterial Isolate. Chem. Res. Toxicol. 2011, 24, 1899–1904. [Google Scholar] [CrossRef]
- Ma, Y.; He, X.; Zhang, P.; Zhang, Z.; Guo, Z.; Tai, R.; Xu, Z.; Zhang, L.; Ding, Y.; Zhao, Y.; et al. Phytotoxicity and Biotransformation of La2O3 Nanoparticles in a Terrestrial Plant Cucumber (Cucumis sativus). Nanotoxicology 2011, 5, 743–753. [Google Scholar] [CrossRef]
- Balusamy, B.; Taştan, B.E.; Ergen, S.F.; Uyar, T.; Tekinay, T. Toxicity of Lanthanum Oxide (La2O3) Nanoparticles in Aquatic Environments. Environ. Sci. Process. Impacts 2015, 17, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.I.; Joo, Y.H.; Pak, P.J.; Kim, J.S.; Chung, N. Different Shapes of Al2O3 Particles Induce Differential Cytotoxicity via a Mechanism Involving Lysosomal Destabilization and Reactive Oxygen Species Generation. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 433–442. [Google Scholar] [CrossRef]
- Drahansky, M.; Paridah, M.; Moradbak, A.; Mohamed, A.; Owolabi, F.A.; Asniza, M. We Are IntechOpen, the World’ s Leading Publisher of Open Access Books Built by Scientists, for Scientists TOP 1%. Intech 2016, 11, 13. [Google Scholar]
- Li, Y.; Tian, X.; Lu, Z.; Yang, C.; Yang, G.; Zhou, X.; Yao, H.; Zhu, Z.; Xi, Z.; Yang, X. Mechanism for α-MnO2 Nanowire-Lnduced Cytotoxicity in Hela Cells. J. Nanosci. Nanotechnol. 2010, 10, 397–404. [Google Scholar] [CrossRef]
- Sashchenko, L.P.; Lukyanova, T.I.; Kabanova, O.D.; Mirkina, I.; Yatskin, O.N.; Pongor, S.; Gnuchev, N.V. Different Pathways of the Release of Cytotoxic Proteins in LAK Cells. Immunol. Lett. 1996, 53, 25–29. [Google Scholar] [CrossRef]
- Lottering, M.D.K.M.; Seegers, J.C. Differential cytotoxic effects of gamma-linolenic acid on MG-63 and HeLa cells. Prostaglandins Leukot Essent Fat. Acids 1994, 51, 109–120. [Google Scholar]
- Del Fattore, A.; Luciano, R.; Saracino, R.; Battafarano, G.; Rizzo, C.; Pascucci, L.; Alessandri, G.; Pessina, A.; Perrotta, A.; Fierabracci, A.; et al. Differential Effects of Extracellular Vesicles Secreted by Mesenchymal Stem Cells from Different Sources on Glioblastoma Cells. Expert Opin. Biol. Ther. 2015, 15, 495–504. [Google Scholar] [CrossRef]
- Matsuyama, S.; Iwadate, M.; Kondo, M.; Saitoh, M.; Hanyu, A.; Shimizu, K.; Aburatani, H.; Mishima, H.K.; Imamura, T.; Miyazono, K.; et al. SB-431542 and Gleevec Inhibit Transforming Growth Factor-Beta-Induced Proliferation of Human Osteosarcoma Cells. Cancer Res. 2003, 63, 7791–7798. [Google Scholar]
- Ke, L.D.; Shi, Y.-X.; Yung, W.K.A. VEGF(121), VEGF(165) Overexpression Enhances Tumorigenicity in U251 MG but Not in NG-1 Glioma Cells. Cancer Res. 2002, 62, 1854–1861. [Google Scholar]
- Giacomotto, J.; Ségalat, L. High-throughput Screening and Small Animal Models, Where Are We? Br. J. Pharmacol. 2010, 160, 204–216. [Google Scholar] [CrossRef]
- Massarsky, A.; Dupuis, L.; Taylor, J.; Eisa-Beygi, S.; Strek, L.; Trudeau, V.L.; Moon, T.W. Assessment of Nanosilver Toxicity during Zebrafish (Danio Rerio) Development. Chemosphere 2013, 92, 59–66. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Osibote, O.A. Green Synthesis of Metal Oxide Nanoparticles, and Their Various Applications. J. Hazard. Mater. Adv. 2024, 13, 100401. [Google Scholar] [CrossRef]
- d’Amora, M.; Schmidt, T.J.N.; Konstantinidou, S.; Raffa, V.; De Angelis, F.; Tantussi, F. Effects of Metal Oxide Nanoparticles in Zebrafish. Oxidative Med. Cell. Longev. 2022, 2022, 3313016. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, L.; Li, Q.X.; Liang, H.; Qin, H.; Masutani, S.; Yoza, B. Toxicity of Lanthanum Oxide Nanoparticles to the Fungus Moniliella Wahieum Y12T Isolated from Biodiesel. Chemosphere 2018, 199, 495–501. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth, and Its Application in the In Vitro Antibacterial Screening of Phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.M.; Spooner, R.J. Glutathione Reductase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1983; Volume 3, pp. 258–265. [Google Scholar]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicryl- Hydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Mitra, I.; Mukherjee, S.; Reddy Venkata, P.B.; Dasgupta, S.; Jagadeesh Bose, C.K.; Mukherjee, S.; Linert, W.; Moi, S.C. Benzimidazole Based Pt(II) Complexes with Better Normal Cell Viability than Cisplatin: Synthesis, Substitution Behavior, Cytotoxicity, DNA Binding and DFT Study. RSC Adv. 2016, 6, 76600–76613. [Google Scholar] [CrossRef]
- Roy, I.; Magesh, K.T.; Sathyakumar, M.; Sivachandran, A.; Purushothaman, D.; Aravindhan, R. Evaluation of Wound Healing Property of the Ethanolic Extract of Glycyrrhiza Glabra on Vero Cell Lines Using In Vitro Scratch Assay Test. J. Pharm. Bioallied Sci. 2023, 15, S630–S635. [Google Scholar] [CrossRef]
- Kumar, B.; Tharumasivam, S.V.; Boominathan, V.; Perumal, E.; Dhandapani, P.; Kaliyaperumal, K.; Ar-umugam, S.; Subramanian, K.; Renuga, P.S.; Shakthivel, V.; et al. A Pilot Study on Nanotherapy of Momordica charantia against Trimethyltin Chloride-Induced Neu-rotoxicity in Danio rerio (Zebrafish). J. Nanomater. 2020, 2021, 2180638. [Google Scholar] [CrossRef]
- Ambrová, M.; Jurišová, J. Solubilities of Lanthanum Oxide in Fluoride Melts. Thermochim. Acta 2006, 443, 105–108. [Google Scholar] [CrossRef]
- Judd, B.R. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Janani, B.J.; Syed, A.; Allela, O.Q.B.; Kareem, R.A.; Abed, R.E.; Ali Al-Nuaimi, A.M.; Athab, Z.H.; AL-Shwaiman, H.A.; Subramaniam, M.; Wong, L.S. Biosynthesis of Lanthanum Oxide-Cerium Phosphate as Luminescent Materials Using a Marine Soft Coral for Cytotoxic, Photocatalytic and Photometric Applications. J. Lumin. 2024, 275, 120822. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.H.; Mao, A.; Qi, X. Photoluminescence of Sm3+ and Eu3+ Co-Doped La2O3–Ga2O3 Glasses Fabricated by an Aerodynamic Levitation Furnace. Ceram. Int. 2024, 50, 38784–38791. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Dhanakodi, K.; Shanmugasundaram, K.; Surendhiran, S. Synthesis and Characterization of Lanthanum Oxide Nanoparticles: A Study on the Effects of Surfactants. Mater. Today Proc. 2021, 47, 901–906. [Google Scholar] [CrossRef]
- Eswaran, A.; Thirumalainambi, M.; Subramaniam, R.; Annadurai, G. Highly Selective CO2 Sensing Response of Lanthanum Oxide Nanoparticle Electrodes at Ambient Temperature. Nanoscale Adv. 2023, 5, 3761–3770. [Google Scholar] [CrossRef]
- Sizochenko, N.; Mikolajczyk, A.; Syzochenko, M.; Puzyn, T.; Leszczynski, J. Zeta Potentials (ζ) of Metal Oxide Nanoparticles: A Meta-Analysis of Experimental Data and a Predictive Neural Networks Modeling. NanoImpact 2021, 22, 100317. [Google Scholar] [CrossRef] [PubMed]
- Irshad, K.A.; Anees, P.; Rajitha, R.; Ravindran, T.R.; Srihari, V.; Kalavathi, S.; Shekar, N.V.C. Anomalous Lattice Compression in the Hexagonal La2O3—A High Pressure X-Ray Diffraction, Raman Spectroscopy and First Principle Study. J. Alloys Compd. 2020, 822, 153657. [Google Scholar] [CrossRef]
- Ismail, W.; Belal, A.; Abdo, W.; El-Shaer, A. Investigating the Physical and Electrical Properties of La2O3 via Annealing of La(OH)3. Sci. Rep. 2024, 14, 7716. [Google Scholar] [CrossRef]
- Jbeli, R.; Boukhachem, A.; Saadallah, F.; Alleg, S.; Amlouk, M.; Ezzaouïa, H. Synthesis and Physical Properties of Fe Doped La2 O3 Thin Films Grown by Spray Pyrolysis for Photocatalytic Applications. Mater. Res. Express 2019, 6, 066414. [Google Scholar] [CrossRef]
- Orera, A.; Larraz, G.; Sanjuán, M.L. Spectroscopic Study of the Competition between Dehydration and Carbonation Effects in La2O3-Based Materials. J. Eur. Ceram. Soc. 2013, 33, 2103–2110. [Google Scholar] [CrossRef]
- Pathan, A.A.; Desai, K.R.; Bhasin, C.P. Synthesis of La2O3 Nanoparticles Using Glutaric Acid and Propylene Glycol for Future CMOS Applications. Int. J. Nano. Chem. 2017, 3, 21–25. [Google Scholar] [CrossRef]
- Hanif, Z.; Jabeen, N.; Anwaar, S.; Aftab, A.; Hussain, S.Z.; Anwar, T.; Qureshi, H.; Munazir, M.; Zaman, W.; Soufan, W. Synthesis and Characterization of Lanthanum Oxide Nanoparticles Using Citrus Aurantium and Their Effects on Citrus Limon Germination and Callogenesis. Sci. Rep. 2024, 14, 21737. [Google Scholar] [CrossRef]
- Mohamed Riyas, Z.; Ramesh Prabhu, M.; Sankaranarayanan, K. Hydrothermal Synthesis of La2O3–ZnO Nanocomposites as Electrode Material for Asymmetric Supercapacitor Applications. J. Mater. Sci. Mater. Electron. 2023, 34, 1612. [Google Scholar] [CrossRef]
- Riyas, Z.M.; Prabhu, M.R. Microwave Irradiation Effect of La2O3-CeO2 Nanocomposites as a Potential Electrode Material for Asymmetric Supercapacitor. Ionics 2024, 30, 5737–5754. [Google Scholar] [CrossRef]
- Adi, W.A.; Wardiyati, S.; Dewi, S.H. Nanoneedles of Lanthanum Oxide (La2O3): A Novel Functional Material for Microwave Absorber Material. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012066. [Google Scholar] [CrossRef]
- Zheng, H.; Ji, Z.; Roy, K.R.; Gao, M.; Pan, Y.; Cai, X.; Wang, L.; Li, W.; Chang, C.H.; Kaweeteerawat, C.; et al. Engineered Graphene Oxide Nanocomposite Capable of Preventing the Evolution of Antimicrobial Resistance. ACS Nano 2019, 13, 11488–11499. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver Nanoparticles as Antimicrobial Agent: A Case Study on E. Coli as a Model for Gram-Negative Bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Shakoor, N.; Hussain, T.; Azeem, I.; Zhou, P.; Zhang, P.; Hao, Y.; Rinklebe, J.; Rui, Y. Bio-Interaction of Nano and Bulk Lanthanum and Ytterbium Oxides in Soil System: Biochemical, Genetic, and Histopathological Effects on Eisenia Fetida. J. Hazard. Mater. 2021, 415, 125574. [Google Scholar] [CrossRef] [PubMed]
- Chakka, S.V.; Thanjavur, N.; Lee, S.; Kim, S. Synthesis and Characterization of Lanthanum-Doped Curcumin-Functionalized Antimicrobial Copper Oxide Nanoparticles. J. Rare Earths 2023, 41, 1606–1615. [Google Scholar] [CrossRef]
- Adams, T.; Anwar, R.; Mfarej, M.; Rundatz, T.; Coyle, M.; McLaughlin, J. Nutritional Stress of Cultured Vero Cells Causes Altered Growth and Morphology as Seen in Neoplastic Transformation. Am. J. Undergrad. Res. 2015, 12, 63–75. [Google Scholar] [CrossRef]
- Sangour, M.H.; Ali, I.M.; Atwan, Z.W.; Al Ali, A.A.A.L.A. Effect of Ag Nanoparticles on Viability of MCF-7 and Vero Cell Lines and Gene Expression of Apoptotic Genes. Egypt. J. Med. Hum. Genet. 2021, 22, 9. [Google Scholar] [CrossRef]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of Nano-Scale TiO2, ZnO and Their Bulk Counterparts on Zebrafish: Acute Toxicity, Oxidative Stress and Oxidative Damage. Sci. Total Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, J.; Meng, H.; Yin, Y.; Zhen, H.; Zheng, X.; Shi, H.; Wu, X.; Zu, Y.; Wang, B.; et al. Rare Earth Elements Lanthanum and Praseodymium Adversely Affect Neural and Cardiovascular Development in Zebrafish (Danio Rerio). Environ. Sci. Technol. 2021, 55, 1155–1166. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Z.; Bai, W.; Zhang, L.; He, X.; Ma, Y.; Liu, Y.; Chai, Z. Effects of Rare Earth Elements La and Yb on the Morphological and Functional Development of Zebrafish Embryos. J. Environ. Sci. 2012, 24, 209–213. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishore, J.; Dunuwilla, T.S.; Raja, V.K.B.; Abraham Louis, S.; Boopathy, L.K.; Saravanan, D.; Zhvania, M.; Gupta, M. Characterization of La2O3 Nanoparticles and Their Effects on Bacteria, Vero and MG63 Cells, and Zebrafish Development. Bioengineering 2025, 12, 995. https://doi.org/10.3390/bioengineering12090995
Kishore J, Dunuwilla TS, Raja VKB, Abraham Louis S, Boopathy LK, Saravanan D, Zhvania M, Gupta M. Characterization of La2O3 Nanoparticles and Their Effects on Bacteria, Vero and MG63 Cells, and Zebrafish Development. Bioengineering. 2025; 12(9):995. https://doi.org/10.3390/bioengineering12090995
Chicago/Turabian StyleKishore, Jugal, Tharaka Srinatha Dunuwilla, Venkatagiri Krishnamoorthy Bupesh Raja, Stanley Abraham Louis, Lokesh Kumar Boopathy, Durai Saravanan, Mzia Zhvania, and Manoj Gupta. 2025. "Characterization of La2O3 Nanoparticles and Their Effects on Bacteria, Vero and MG63 Cells, and Zebrafish Development" Bioengineering 12, no. 9: 995. https://doi.org/10.3390/bioengineering12090995
APA StyleKishore, J., Dunuwilla, T. S., Raja, V. K. B., Abraham Louis, S., Boopathy, L. K., Saravanan, D., Zhvania, M., & Gupta, M. (2025). Characterization of La2O3 Nanoparticles and Their Effects on Bacteria, Vero and MG63 Cells, and Zebrafish Development. Bioengineering, 12(9), 995. https://doi.org/10.3390/bioengineering12090995







