1. Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease characterized by inflammation, demyelination, and neurodegeneration, which impact the central nervous system (CNS). This condition majorly affects young adults and can lead to several neurological symptoms, such as cognitive, sensory, and motor impairments [
1]. In detail, the pathophysiology of MS involves the immune-mediated destruction of myelin, resulting in a progressive decline in motor function, which can significantly impact patients’ daily activities and quality of life [
2,
3]. Motor impairments in MS patients include muscle weakness, loss of muscle control, abnormal muscle tone, ataxia, and fatigue [
1,
4]. Notably, motor dysfunction also affects psychosocial aspects of patients’ lives. For instance, gait impairments can lead to increased risk of falls, social isolation, and decreased participation in community activities, all of which contribute to a lower quality of life [
2,
5]. From this perspective, improving motor function of MS patients is a crucial goal of the therapeutic treatments used in clinical practice. To this aim, current therapies aimed at enhancing motor functions in MS patients encompass pharmacological treatments, physical rehabilitation, and neuromodulation techniques. Pharmacological therapies primarily aim to reduce the frequency of relapses and slow disease progression. Importantly, these medications do not directly enhance motor function, but they can help preserve neurological function over time, indirectly enhancing motor capabilities [
6]. Physical rehabilitation comprises exercise programs tailored to individual capabilities to improve muscle strength, balance, and overall physical fitness [
7,
8]. Neuromodulation techniques, including transcranial magnetic stimulation (TMS) and vagus nerve stimulation (VNS), are emerging as promising tools for motor recovery. TMS has been utilized to enhance cortical excitability and facilitate motor learning [
9], whereas VNS has shown potential in promoting neuroplasticity, which may help in motor function recovery after demyelination [
10]. Among the neuromodulation techniques, transcutaneous electrical nerve stimulation (TENS) is a non-invasive technique that delivers low-voltage electrical currents through the skin to stimulate peripheral nerves, which can modulate pain perception and promote neuroplastic changes in the CNS [
11]. Notably, the effectiveness of TENS to enhance outcomes across the International Classification of Functioning (ICF) domains [
12] of body structure and function (e.g., clinical Modified Ashworth Scale, MAS) and activity (e.g., walking and moving) has been underscored by several recent systematic reviews [
13,
14,
15]. Regarding the TENS application for MS treatment, studies have shown that TENS can effectively reduce spasticity, improve motor function, and enhance patients’ quality of life [
16]. Despite the promising findings, the efficacy of TENS in MS treatment remains a topic of debate. In fact, some studies demonstrated that while TENS may be beneficial for certain symptoms, its effectiveness can vary among individuals [
16]. This variability calls for further research to establish standardized protocols and identify which patient populations may benefit most from TENS therapy.
The Mollii Suit (Exoneural Network AB, Danderyd, Sweden) is a full-body garment equipped with 58 electrodes, which deliver low-frequency electrical stimulation to selected muscle groups. It is administered to treat spasticity, motor impairments, and chronic pain of neurological origin associated with pathologies such as cerebral palsy, multiple sclerosis, stroke, and acquired brain or spinal cord injuries. The device aims to reduce spasticity and pain while supporting improvements in mobility, flexibility, and range of motion [
17]. The incorporation of TENS into a wearable format has several benefits for rehabilitation objectives. The electrodes affixed to the suit’s body fabric enable accurate targeting of certain muscle groups. This enables the design of personalized treatment to address the individual’s unique needs. Furthermore, the wearable capabilities of EMS allow patients to participate in treatment while executing functional tasks and motions. The latter feature enables users to engage in activities of daily living and functional duties, hence increasing the likelihood of regular and frequent utilization, which is crucial for achieving optimum therapeutic results [
18]. Importantly, it should be noted that studies investigating the CNS and autonomic nervous system (ANS) activities in response to the EMS treatment are lacking. Monitoring both the CNS and the ANS responses allows for the evaluation of the effects of the treatment on the neuroplasticity and the psychophysiological condition of the patient, reflecting the acceptance of the garment by the users. To this goal, a multimodal approach able to measure brain activity and peripheral biosignals influenced by the ANS is fundamental.
In order to evaluate the effects of the EMS on the CNS during and after the stimulation, it could be crucial to monitor brain activity through portable neuroimaging techniques. To this aim, electroencephalography (EEG) is a powerful tool for evaluating brain plasticity. EEG is a non-invasive, portable scalp-located neuroimaging technique able to measure the electrical activity of the brain through electrodes placed on the scalp. It is characterized by a high temporal resolution, which allows for the detection of rapid changes in brain activity and can be employed to explore the mechanisms of neuroplasticity. For instance, studies have demonstrated that specific EEG patterns, such as alpha (α) and gamma (γ) oscillations, are associated with different states of cortical excitability and can predict the outcomes of plasticity-inducing interventions [
19,
20]. Importantly, a novel approach for EEG data analysis increasingly recognized as a valuable tool for assessing brain plasticity relies on the evaluation of the EEG microstates, which are quasi-stable patterns of electrical activity in the brain that last approximately 60 to 120 milliseconds [
21]. The EEG microstates aim to decompose the continuous EEG signal into a finite number of discrete microstates that reflect coherent neuronal activity [
22]. Microstates are typically classified into four canonical classes, each associated with different functional networks in the brain. For instance, microstate A is associated with the salience network, while microstate B is related to the default mode network [
23,
24]. Hence, some features of the microstates, such as occurrence and duration, can provide insights into the underlying neural mechanisms of cognitive processes, including attention, memory, and emotional regulation [
25,
26]. Notably, alterations in the dynamics of these microstates can be indicative of several neurological and psychiatric conditions, such as schizophrenia, depression, and Alzheimer’s disease [
24,
27,
28].
Investigating the physiological responses to the treatment is a crucial aspect to consider when assessing the effectiveness of the EMS. Importantly, TENS has been shown to influence the balance between sympathetic (SNS) and parasympathetic (PNS) nervous system activity [
29] and to induce pain relief [
30]. In addition, changes in autonomic responses can indicate how well patients are coping with the treatment, helping the clinicians in adjusting treatment parameters to enhance patient comfort and adherence [
31]. To this aim, the employment of wearable and contactless technologies able to monitor physiological signals related to the ANS activity could offer information regarding the effectiveness and acceptance of the EMS-based treatment [
32]. Among the variety of methods available to this goal, the heart rate variability (HRV) is a well-established parameter to assess the ANS activity, also providing information regarding the balance between SNS and PNS [
33,
34]. Information regarding the HRV is usually obtained through electrocardiography (ECG) and photoplethysmography [
35], particularly when the sensors are embedded in wearable devices such as smartwatches, smart jewelry, and smart t-shirts [
36].
Another relevant tool for ANS activity assessment is infrared thermography (IRT). This non-invasive imaging technique measures surface temperature, which is related to physiological blood flow modulations and autonomic regulation [
37,
38]. In detail, the ANS influences skin temperature through its control of blood vessel dilation and constriction. Therefore, changes in skin temperature can be suggestive of ANS activity, providing, in this context, information on the physiological responses to therapeutic interventions [
37]. Notably, IRT can provide real-time feedback on the physiological effects of therapy, which can enhance patient engagement and acceptance of treatment [
39]. Importantly, in the literature no studies combining EEG microstate analysis, HRV, and IRT in EMS-treated patients with MS are reported.
In this perspective, the aim of this study is to assess modifications in the ANS and CNS activity in response to a session with the EMS and after one month of treatment through HRV, IRT, and EEG on a patient affected by MS. Specifically, the patient exhibited reduced functional mobility and spasticity, thus clinical tests widely used in MS to provide meaningful information on lower-limb strength and altered muscle tone have been employed. Additionally, since MS is frequently associated with alterations in cortical excitability and network dynamics [
40], the EEG was applied to monitor the brain activity.
Finally, considering the high prevalence of autonomic dysfunction in MS [
41], HRV and IRT were used to provide a non-invasive measure of ANS activity.
2. Materials and Methods
2.1. Study Design and Participant
The present study consists of a case report investigating the acute and subacute effects of a treatment session with the EXOPULSE Mollii suit on a multiple sclerosis patient. The participant was a 43-year-old woman with secondary progressive multiple sclerosis, diagnosed in 2006. The patient began treatment with Ocrelizumab in September 2018 and completed the treatment in December 2023. Ocrelizumab is a humanized recombinant monoclonal antibody targeting CD20, which selectively binds to CD20-expressing B cells. CD20 is a surface antigen found on pre-B cells, mature B cells, and memory B cells, but it is not expressed on lymphoid stem cells or plasma cells. The mechanism of action involves immunomodulation through the reduction in the number and function of CD20-positive B cells.
The initial dose, approximately two years prior, was 600 mg, administered as two separate intravenous infusions: a first infusion of 300 mg followed by a second infusion of 300 mg two weeks later. Subsequent doses were administered as a single 600 mg intravenous infusion every six months. The treatment was monitored every six months through neurological evaluations, follow-up MRI scans, and periodic blood tests. The patient did not report or experience any adverse events or side effects.
In addition, the patient began treatment with amantadine 100 mg in 2014. Amantadine is an antiviral used in the prevention and early treatment of influenza A virus infections; it also improves mild disabilities caused by bradykinesia, as well as tremor and rigidity in Parkinson’s disease. The patient is currently still undergoing treatment with amantadine.
During the neuro-suit stimulation protocol, the patient was followed up with close neurological monitoring and underwent regular brain and spinal cord MRI scans with and without contrast agent, blood tests, neurological assessments, and evaluation scales. The disease had been declared stable for approximately two years at the time of enrollment, and her EDSS score had remained at 7 for about two years. Furthermore, the patient receives intravesical botulinum toxin injections for neurogenic bladder management. She is alert, conscious, oriented, and cooperative. Postural changes and transfers are possible with adaptations. Standing is feasible with support; ambulation is possible with moderate assistance and a cane for very short distances (10 m). For longer distances, the patient uses a wheelchair. The patient exhibited a paraparetic/ataxic gait with a cautious, small-stepped pattern. The Romberg test was positive. During the finger-to-nose test, the patient exhibits a tendency toward retropulsion. Bilateral single-leg stance is not achievable. Coordination assessment reveals left-sided paresis in the upper limbs, accompanied by an impaired pincer grasp. Muscle tone is increased in the left pectoralis major and biceps brachii, while muscle trophism is reduced but remains within normal limits. A mild weakness is also observed on the right side. Deep tendon reflexes are hyperreflexive, and sensory examination indicates distal hypoesthesia and paresthesia.
In the lower limbs, there is severe left-sided paresis with distal plegia affecting the tibialis anterior, extensor digitorum longus, and gastrocnemius–soleus complex, with a Medical Research Council (MRC) muscle grade of 1/5. Right-sided paresis is also present. Spastic hypertonia is noted in the rectus femoris and biceps femoris, with positive Duncan–Ely test and Tardieu signs, as well as in the triceps surae, evidenced by a positive Silverlskiold test bilaterally. Inexhaustible clonus and marked muscle atrophy are observed. Deep tendon reflexes are hyperreflexive, particularly in the patellar and Achilles reflexes. The right ankle is edematous, with increased thermotactile sensitivity and a measured circumference difference of +1 cm.
Additional findings include the absence of dysphagia; however, there is a delayed onset of the swallowing reflex following COVID-19 infection. The patient has a neurogenic bladder requiring self-catheterization and a neurogenic bowel managed with Movicol therapy. There have been no recent falls. While there is no dyspnea, the patient reports experiencing easy fatigability.
The participant signed a written consent form, and she could withdraw from the experiment at any time. The current study was conducted in accordance with the ethical standards recognized by the Declaration of Helsinki.
2.2. Intervention
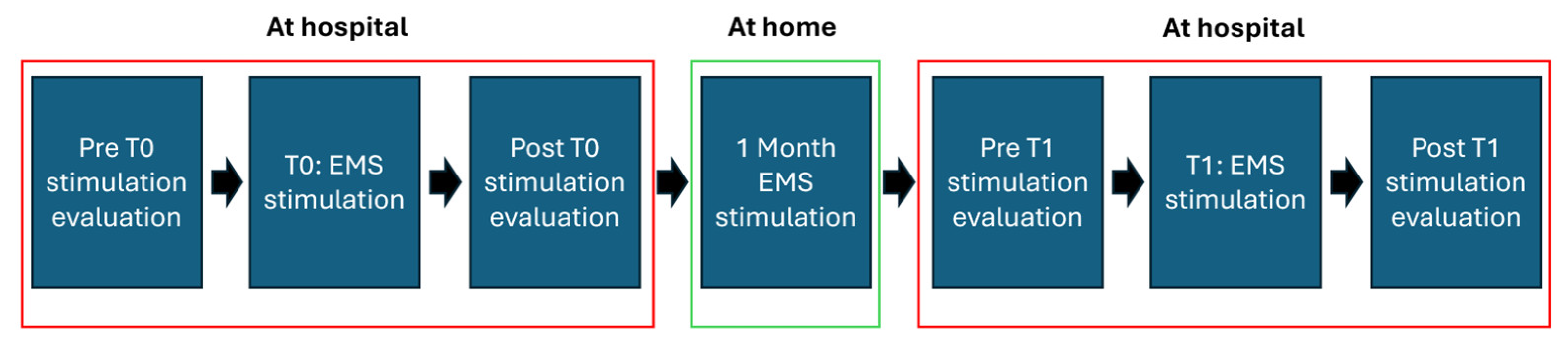
The experiment was composed of two separate sessions 1 month apart. During the first session, the motor function evaluation was performed both before and after the stimulation, whereas in the second session the evaluation was performed only after the EMS treatment. Notably, the motor function evaluation was performed at the same time of day (at 11 a.m.) for both sessions. In the month between the first and second sessions, the patient underwent EMS sessions three times per week, for a total of 12 EMS sessions. The stimulation lasted 60 min, activating all 58 electrodes and using a stimulation pattern tailored for the peculiar patient. During the stimulation, the patient was positioned supine on a bed. This study design allowed for the evaluation of the acute effects of the stimulation during the first session and sub-acute effects during the second session. The timeline of the study is described in
Figure 1.
2.3. EMS Configuration
The suit was provided to the patient following a physiatric assessment, during which indications and contraindications were evaluated. It was granted through a free loan-for-use agreement. The free rental period took place between July and September 2022. The suit was provided by the Reggio Emilia branch of Otto Bock Soluzioni Ortopediche SRL US (Reggio Emilia, Italy). The serial numbers were as follows:
Jacket, size W/XL—model and serial number: 100-1-12575
Trousers, size W/XL—model and serial number: 100-1-12604
Control unit: 100-4-1331
The software used to program the suit was “Molliisoft”, version 1.2. The stimulation pattern was tailored based on the patient’s spasticity profile. The parameters of the stimulation were fixed based on initial response and tolerance and monitored to ensure safety, therapeutic efficacy, consistency during the one-month treatment period, and avoid inadvertent changes by the patient. Notably, the EMS does not disclose a formal open-source algorithm to tailor the treatment to the patients, but its clinical use is guided by a semi-standardized protocol based on initial assessment, initial parameter setting, customization based on response, and locking parameters.
The electrical stimulation parameters were carefully individualized by the clinical team at T0 based on the patient’s characteristics: diagnosis, symptom severity, age, and muscle mass of the targeted muscle group undergoing functional stimulation.
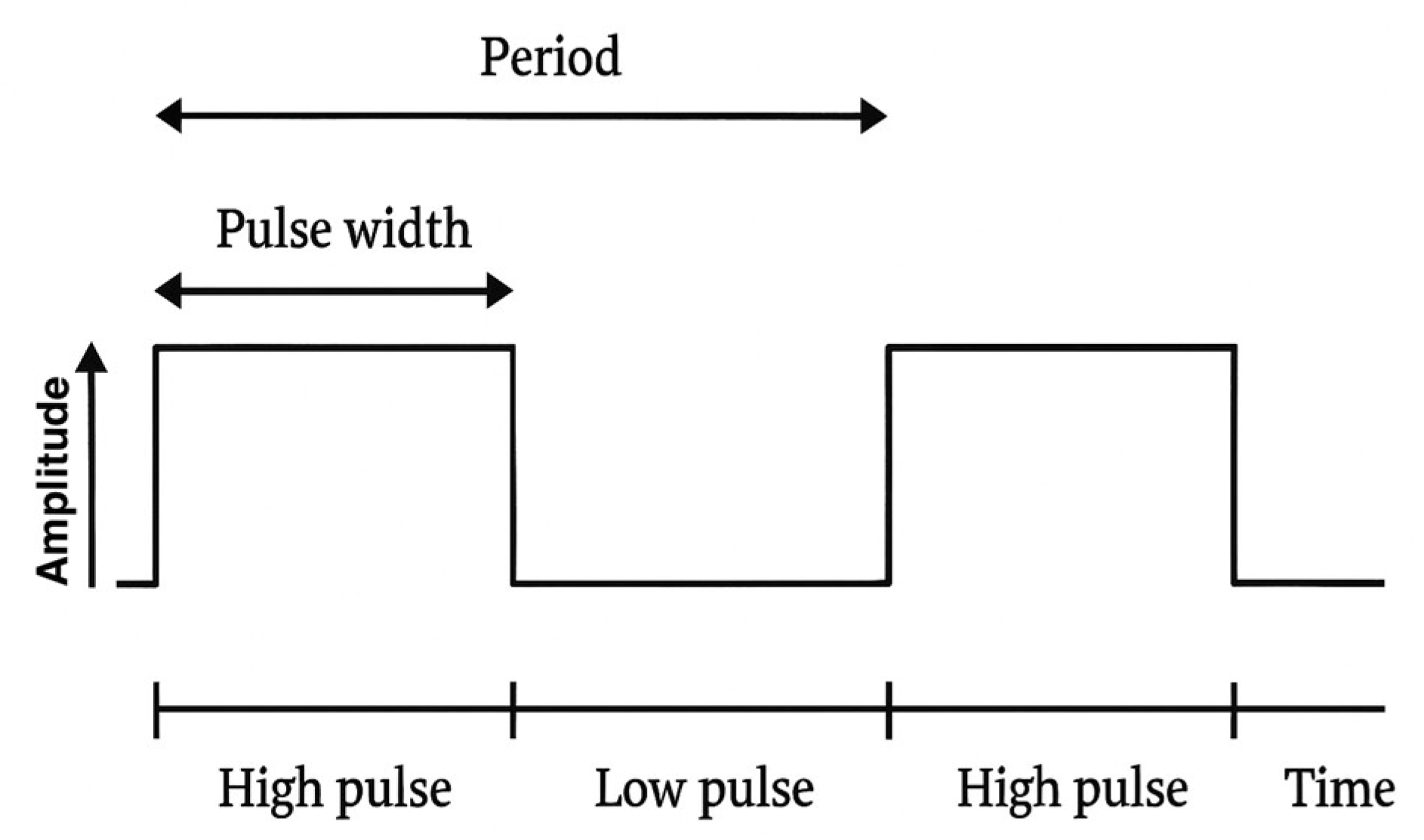
The stimulation is set at 20 V, 20 Hz, while the pulse widths, expressed in microseconds, vary. In the presented scheme, these vary from 25 (point 1) to 175 (point 30) microseconds.
Each point increases the stimulation by 5 microseconds (point 2 = 30 microseconds; point 3 = 35 microseconds, and so on). The waveform used in the stimulation protocol is reported in
Figure 2.
The stimulation applied to the patient aims to improve foot pronation–supination through significant activation of the tibialis anterior muscles, considering the muscular tension (95 microseconds on the right, 110 microseconds on the left). Similarly, hyperextension of the knee was addressed through stimulation of the muscle group responsible for knee flexion (65 microseconds on the right, 75 microseconds on the left), and hip flexion was facilitated by stimulating the hip flexors (65 microseconds bilaterally). Additionally, targeted stimulation was applied to the hip abductors (90 microseconds on the right and 105 microseconds on the left). All these stimulations aim to improve gait pattern, reduce extensor synergy, and strengthen balance in both static and dynamic conditions.
Regarding the upper limbs, due to reduced endurance and hand functionality, with greater impairment on the left side, stimulation was introduced for the scapular girdle (35 microseconds in all segments), the triceps to support elbow control (50 microseconds on the right, 60 microseconds on the left), and the muscles responsible for wrist flexion (65 microseconds on the right and 80 microseconds on the left).
According to the stimulation protocol for this diagnosis, stimulation was applied to stabilize posture at the level of the abdominal muscles (50 microseconds, bilaterally) and the paravertebral muscles (60 microseconds, bilaterally).
Finally, a light multi-site stimulation (25 microseconds) was applied to all segments not involved in functional support stimulation, following the general stimulation protocol with the EMS, to enhance the overall relaxation effect. The EMS worn by the participant and the graphical scheme of the electrical stimulation delivered by the device are reported in
Figure 3A and
Figure 3B, respectively. The selected muscle groups and stimulation settings (pulse width) employed in the EMS for the intervention are reported in
Table 1.
2.4. Clinical Scales
The following clinical scales were evaluated for the patient. Notably, all variables were evaluated in basal conditions (i.e., prior to the intervention) and after the intervention.
Sit to Stand (STS): it assesses lower limb strength, balance, and endurance by measuring the time or number of repetitions a person takes to move from a sitting to a standing position.
Timed Up and Go (TUG): it is a test used to assess mobility, balance, walking ability, and fall risk. During the test, the participant starts seated, stands up, walks a short distance (typically 3 m), turns, returns, and sits down again while the time is recorded.
Clonus Test: it is a neurological examination used to assess involuntary, rhythmic, and repetitive muscle contractions, typically in the lower limbs, to evaluate upper motor neuron lesions.
Pendulum Test: it is used to evaluate spasticity by observing the passive swinging motion of the lower limb after an initial impulse.
VAS (Visual Analog Scale): it is a subjective measure of pain intensity, usually represented as a 10 cm line where one end represents “no pain” and the other represents “worst pain imaginable.” The patient has to mark a point on the line to indicate their perceived pain level.
Modified Ashworth Scale (MAS) Tone Test: it is a clinical tool used to assess muscle spasticity by measuring resistance during passive soft-tissue stretching.
Penn Spasm Frequency Scale: it is a scale used to assess the frequency and severity of muscle spasms in individuals with neurological conditions, particularly spinal cord injuries.
Modified Fatigue Impact Test Scales (MFIS): it is a questionnaire-based assessment that measures the impact of fatigue on physical, cognitive, and psychosocial functioning in individuals with neurological disorders, particularly MS.
2.5. Instrumental Measures
Instrumental measures were taken during the motor function evaluation as well as during the stimulation during both the first and second session. The EEG data were recorded through a 9-channels device (Encephalan Mini AP-10 system). The impedance between scalp and electrodes was checked before each recording in order to collect signals with good quality. The sample frequency was 250 Hz. The electrodes were placed in accordance with the 10–20 standard, covering the whole head.
The same device was used to measure the 1-lead ECG, with a sample frequency of 250 Hz.
The IRT signals were acquired through FLIR SC660 thermal infrared camera (Teledyne FLIR, Wilsonville, OR, USA). It is characterized by a 640 × 480 bolometer FPA, a sensitivity/noise equivalent temperature difference of 30 mK at 30 °C, and a field of view of 24° × 18°. The camera was positioned 60 cm from the subject and the sampling rate was set to 10 Hz. Importantly, the camera was calibrated using a blackbody to reduce optical artefacts and any sensor response drift or shift. To mitigate thermoregulatory influences, IRT measurements were performed in compliance with thermal measurement standards [
42]. In particular, the recordings were carried out in a thermoneutral environment to minimize thermoregulatory-induced alterations. Additionally, the patient was given a 15-min acclimation period prior to the session to allow thermal stabilization with the surrounding environment [
43]. Notably, to minimize potential influences of circadian rhythm, all sessions were conducted at a consistent time of day [
44].
2.6. Data Analysis
Regarding the EEG data, a band-pass filter with cut-off frequencies of 1 Hz and 80 Hz, in conjunction with a notch filter at 50 Hz, was used (zero-lag 2nd order Butterworth digital filters). EEG epochs that were saturated or distorted were discarded by visual assessment. Additionally, cardiac and ocular artifacts, together with muscular activity contaminations, were eliminated using a semiautomatic technique grounded on independent component analysis (ICA) [
45]. Specifically, blind source separation is mainly performed through ICA, since brain and artifactual independent components (ICs) exhibit recognizable patterns. Relying on deep-learning ability of self-extracting the features of interest, a CNN for off-line, automatic artifact identification through ICs developed in [
45] was employed.
The pre-processed EEG data were analyzed over five pertinent frequency bands (θ-band: 3.5–8.2 Hz, α-band: 7.4–13 Hz, β-band: 13–30 Hz, δ-band: 1–4 Hz, γ-band: 26–40 Hz), and the power temporal envelopes were evaluated as the absolute values of their Hilbert transform.
In addition, the microstates segmentation was performed. The global field power (GFP) was computed as reported in Equation (1) [
46]:
where
n is the number of channels and
u is the amplitude in µV at time
t.
The time points corresponding to local maxima of GFP were identified, and the most representative scalp topographies were extracted using a k-means clustering algorithm. The clustering procedure was performed across a range of 2 to 20 clusters, and the optimal number of clusters was determined using the Krzanowski–Lai criterion [
47]. This analysis revealed that the optimal number of microstates was k = 4. Subsequently, the patient’s EEG data were segmented according to these four prototypical microstate maps by applying a temporal smoothing procedure with a 12-millisecond window size [
48]. As a result, a time series vector was generated, labeling each time point with one of the four identified microstates.
To characterize each of the four microstates, the following metrics were computed [
49]:
The ECG data processing included band-pass filtering the signals with cut-off frequencies ranging from 1 to 40 Hz. The R-peaks were detected, and the RR interval was assessed, enabling the analysis of both time- and frequency-domain measures. The following metrics have been calculated in detail:
The Standard Deviation of Normal Heartbeat Intervals (SDNN): it quantifies the overall variability in heart rate by measuring the standard deviation of the intervals between successive normal heartbeats (NN intervals). It indicates the activation of both the sympathetic and parasympathetic nervous systems.
The Root Mean Square of Successive Differences (RMSSD): it assesses short-term changes in heart rate.
The Low-Frequency Power (LF, 0.04–0.15 Hz): it signifies a combination of sympathetic and parasympathetic activity, while it is often linked to sympathetic dominance.
The High-Frequency Power (HF, 0.15–0.40 Hz): it indicates parasympathetic (vagal) activity and correlates with relaxation, deep respiration, and recuperation.
The Low-Frequency to High-Frequency Ratio (LF/HF): it serves as a measure of autonomic equilibrium, with elevated values signifying heightened sympathetic dominance and diminished values reflecting enhanced parasympathetic activity.
For the analysis of the infrared thermography (IRT) signals, the quality of the thermal recordings was initially verified through visual inspection, and no recordings were excluded. Six regions of interest (ROIs) were defined: the nose tip, left and right nostrils, chin, perioral region, and corrugator area. The localization of these ROIs across video frames was achieved by tracking the nose tip using a dedicated algorithm [
50]. In instances where the tracking algorithm failed—typically due to substantial head movement—the affected segments were corrected by interpolating values based on the mean of six samples preceding and following the motion artifact. This tracking procedure allowed for the extraction of the temporal dynamics of temperature within the selected ROIs. The following metrics were evaluated for each ROI:
Mean Temperature (T_avg): Represents the average thermal value over time, indicating baseline skin temperature.
Standard Deviation (T_std): Measures the variability of the temperature signal, reflecting fluctuations in thermal activity.
Kurtosis (T_kurt): Quantifies the peakedness of the temperature distribution, highlighting the presence of extreme values.
Skewness (T_skew): Assesses the asymmetry of the temperature distribution, indicating directional bias in thermal shifts.
Sample Entropy (T_SampEn): Evaluates the complexity and regularity of the thermal signal, with lower values suggesting more predictable patterns.
Low-Frequency Power (T_LF): Captures slow oscillatory components in the thermal signal, often associated with sympathetic activity.
High-Frequency Power (T_HF): Reflects faster fluctuations in the signal, generally linked to parasympathetic modulation.
LF/HF Ratio (T_LF/HF): Indicates the balance between sympathetic and parasympathetic influences on thermal regulation.
4. Discussion
4.1. Functional Improvements
The present study is a novel case report study on the use of the EMS suit in an MS patient procedure that has never been carried out in a scientific setting. The clinical scales assessed before (T0) and after (T1) one month of EMS in a MS patient indicate notable functional improvements. The STS time decreased from 8 s to 6 s, suggesting enhanced lower limb strength and mobility. However, it should be highlighted that the TUG test remained stable, indicating that overall mobility and gait speed did not improve significantly. Regarding the spasticity-related tests, positive outcomes were observed in response to the EMS treatment. Specifically, the Clonus Test evaluated on the right lower limb shifted from “inexhaustible” to “absent” in response to the intervention, while the left lower limb improved from “inexhaustible” to “exhaustible.” These changes indicate a reduction in involuntary muscle contractions and hyperreflexia, suggesting that the EMS may have contributed to neuromuscular relaxation. Similarly, the Pendulum Test showed a decrease in the oscillation count from 5 to 3 for the right side and from 4 to 3 for the left side, supporting the trend of improved muscle tone regulation. Although these findings suggest that the EMS may be effective in reducing spasticity and improving functional lower limb performance in MS patients, further research is warranted to determine the long-term sustainability of these effects and to explore whether combining EMS with physical therapy could lead to more comprehensive mobility improvements.
Regarding the fatigue perception, the MFIS scale went from 43 to 40, demonstrating an improvement in this aspect. It is worth noting that the MFIS scale consists of three subscales (physical subscale, cognitive subscale, and psychosocial subscale), and the subscale that predominantly improved was the physical fatigue subscale, highlighting the impact of TENS on muscle and thus fatigue. Importantly, TENS is usually administered in reduced muscle groups, whereas the EMS is able to stimulate more body districts simultaneously. Hence, the marked perception of reduction in physical fatigue (rather than the other two components, cognitive and psychosocial) could be related to the simultaneous stimulation of numerous muscle groups involved in the perception of muscle fatigue [
51,
52].
It should be noted that no minimal clinically important difference has been firmly established for these tests in the MS population, hence the improvement observed could be considered potentially relevant, but the validation of MS-specific minimal clinically important difference thresholds is necessary.
4.2. CNS-Related Changes
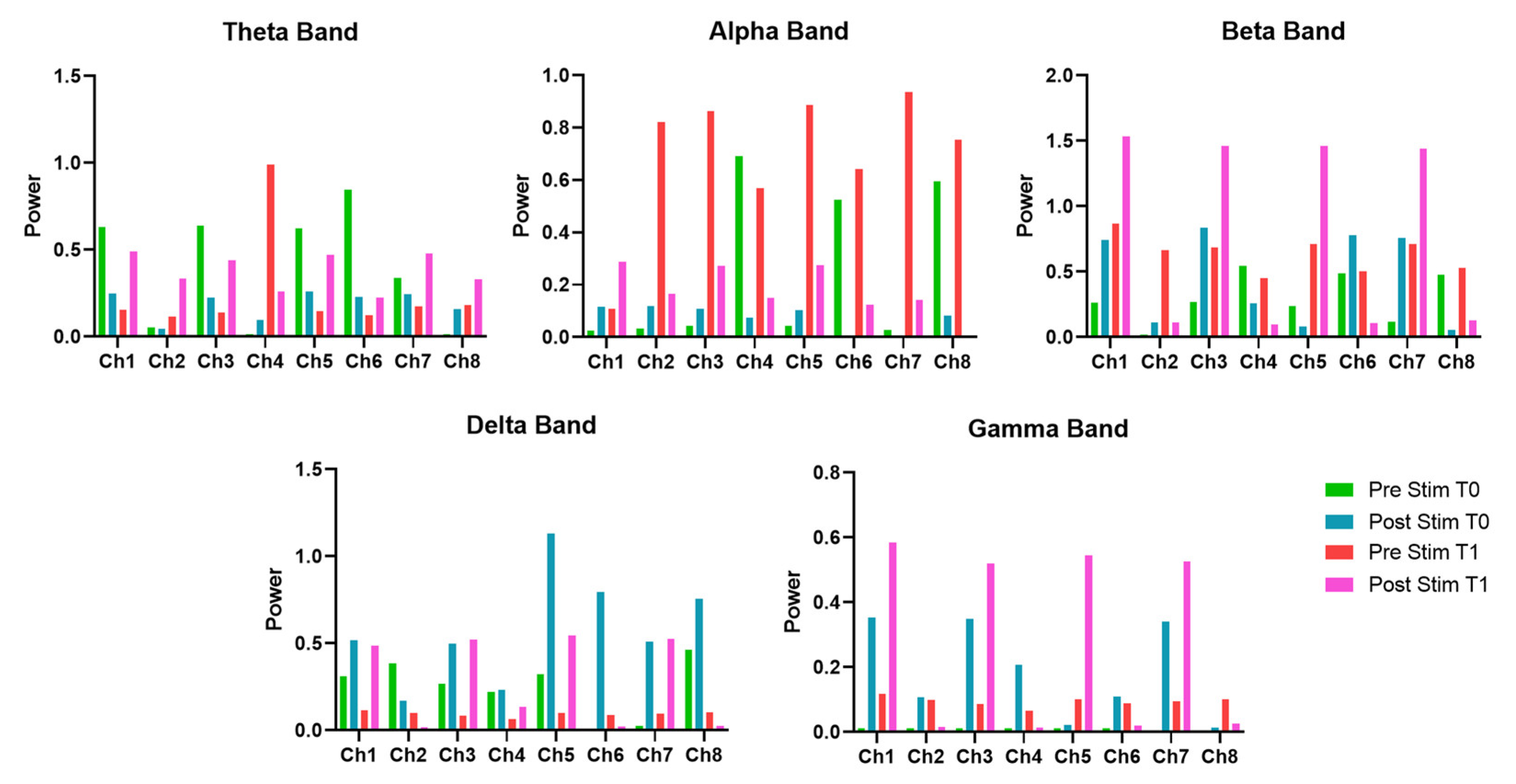
The EEG band analysis before and after the EMS treatment provides insights into its impact on neural activity. Regarding the θ-band power, which is often associated with cognitive processing, memory, and relaxation, it showed a non-uniform response across channels. Specifically, at T0, the post-stimulation measurement generally showed a decrease in θ-band power on most channels, suggesting an immediate reduction in slow-wave activity, which could indicate increased cortical activation, thus reflecting a shift toward a more alert neural state. Notably, at T1, pre-stimulation values were lower compared to T0, suggesting a long-term reduction in baseline θ-band power. However, post-stimulation values showed an increase, indicating that EMS may facilitate a neuroplastic response over time. These results suggest that short-term EMS application reduces θ-band activity, potentially promoting wakefulness and cognitive engagement, while long-term application might modulate the brain’s baseline state, making it more responsive to stimulation.
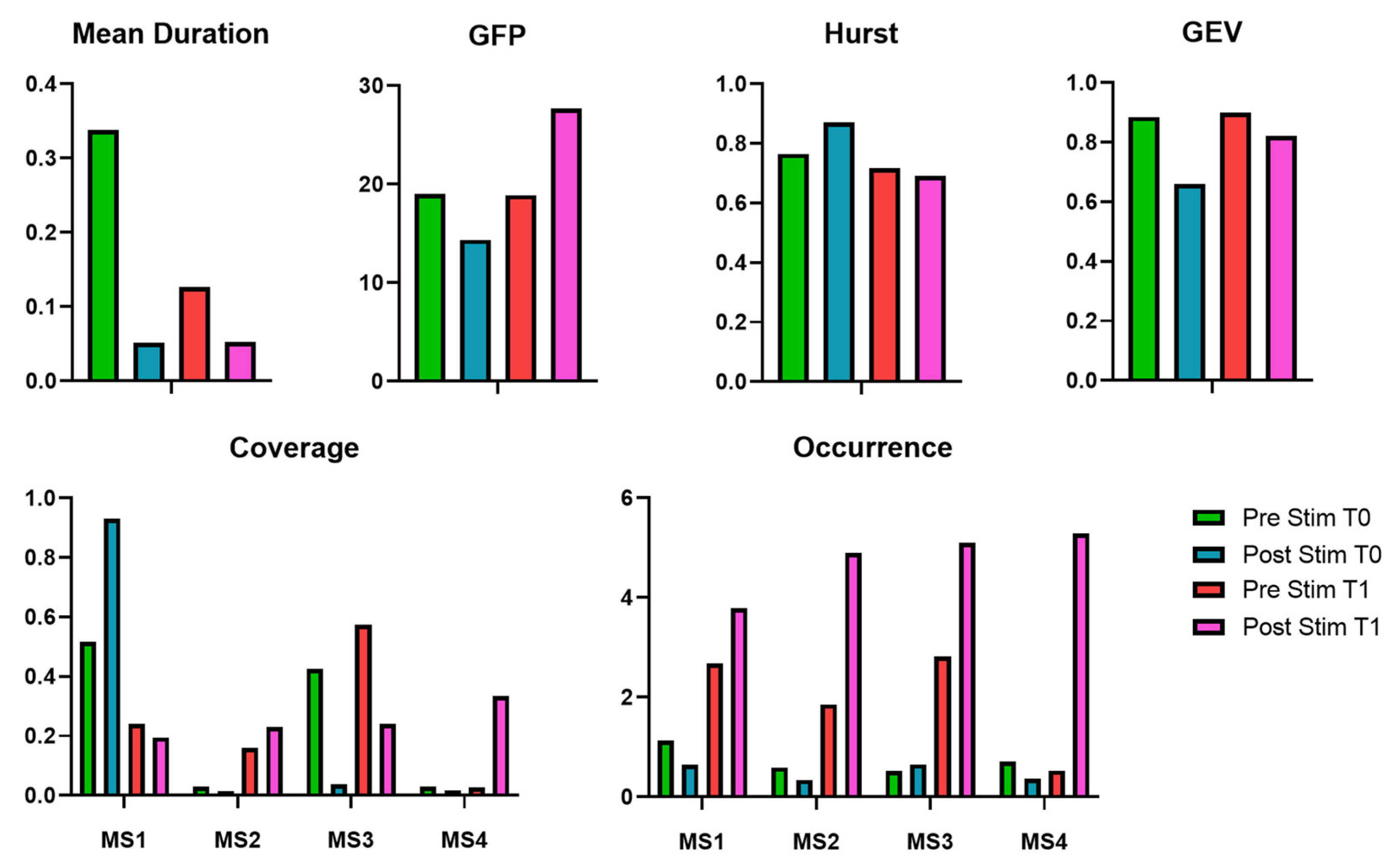
In addition, the EEG microstate analysis highlights modifications in brain dynamics over both short- and long-term applications. In the short term, immediately after stimulation at T0, the mean duration of microstates decreased from 0.3381 to 0.0516, suggesting a reduction in the stability of microstate sequences. This modification underscores an increased rate of state-switching, reflecting heightened neural reactivity or modifications in cognitive processing. At the same time, GFP reduction associated with the EMS stimulation suggests a short-term reduction in overall brain activation. However, the Hurst exponent’s increase suggests a shift toward more persistent and stable neural dynamics. It is worth highlighting that GEV decreased from 0.8855 to 0.6604, reflecting a reduced ability of microstate templates to explain overall brain activity, which could suggest increased neural complexity or a transient state of reorganization.
Overall, the results indicate that short-term EMS application leads to a disruption of microstate stability, increasing state-switching and reducing the predictability of brain activity. This effect likely represents acute neural plasticity and reorganization. Over time, however, brain activity appears to adapt to the repeated stimulation, as reflected in the increased GFP and a return to a more diverse microstate dynamic. The observed increase in GFP at T1 post-stimulation suggests that prolonged TENS application may enhance overall neural excitability, which could potentially support cognitive function improvements in MS patients. These findings highlight the capability of EMS to modify the CNS activation patterns, but further research is necessary to determine whether these neurophysiological changes correlate with functional cognitive improvements and whether optimizing stimulation protocols could improve the long-term duration of these benefits.
4.3. ANS-Related Changes
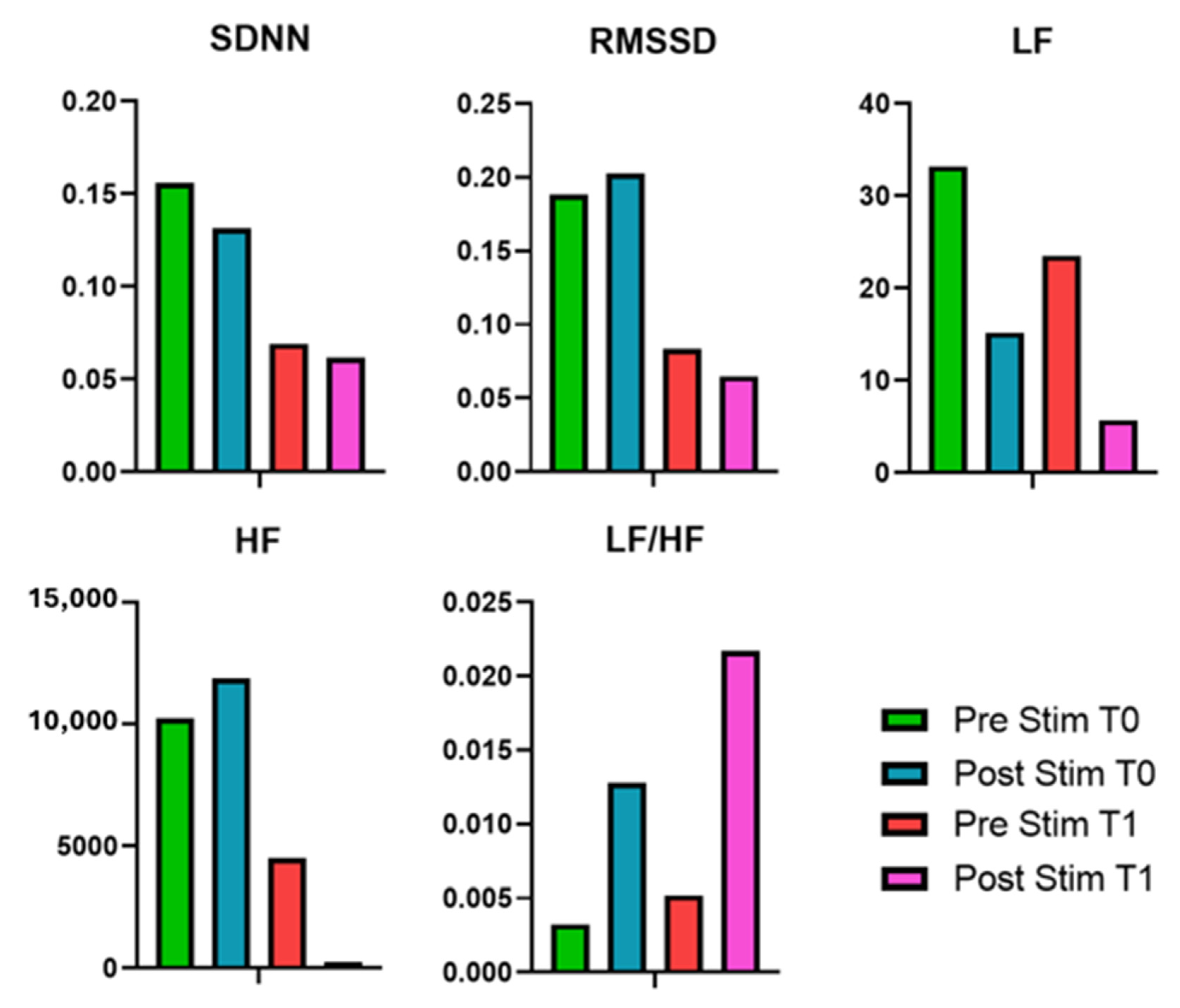
Regarding the HRV metrics, the application of EMS over one month led to notable changes in HRV features. In detail, at T0, post-stimulation measurements showed an increase in RMSSD and HF power, suggesting an acute enhancement of PNS activity. At T1, baseline HRV values declined, suggesting a possible decrease in vagal tone. LF power showed a substantial increase post-stimulation at T0, followed by a further increase at T1 pre-stimulation, and by a significant reduction in post-stimulation. Conversely, HF power initially increased slightly at T0 post-stimulation but then dropped at T1 post-stimulation. These results suggest that while EMS may initially enhance autonomic flexibility, prolonged application could lead to compensatory shifts or autonomic fatigue. Hence, further investigation is needed to clarify the long-term effects of TENS on ANS function in MS patients, particularly regarding potential benefits over extended periods of stimulation.
The IRT data analysis reveals notable changes in thermal distribution secondary to the EMS treatment. T_avg at the nose tip decreased between the post-stimulation measurements at T0 and T1. This drop in temperature suggests a potential alteration in blood flow regulation, possibly due to autonomic nervous system modulation. Since the nose tip is particularly sensitive to SNS dependent vasoconstriction, this reduction may reflect increased SNS activity or a shift in thermoregulatory balance over time.
Additionally, T_std increased from T0 to T1 on the Nose Tip, suggesting a greater thermal dispersion, which could imply an irregular or more dynamic vasomotor response after one month of EMS application. Similarly, T_kurt and T_skew increased after 1 month of treatment, indicating modifications of the temperature distribution over time.
These findings indicate that EMS may influence facial temperature patterns, possibly by affecting SNS control over microcirculatory dynamics. The immediate post-stimulation effects at T0 showed relatively stable temperature dispersion, whereas over time, at T1, temperature variability increased, along with changes in distribution characteristics. This could reflect an adaptation of the ANS to repeated stimulation, leading to altered vascular responses. Further investigation is necessary to determine whether these thermographic changes correlate with broader autonomic adjustments and whether they hold clinical significance for individuals with neurological conditions such as MS.
4.4. Neurophysiological Correlates of Functional Improvements
This case report suggests that EMS was associated with reduced spasticity, as indicated by the Clonus and Pendulum Tests. These functional effects align with reports that TENS can improve strength and gait performance in people with MS and related populations, including case series using direct-current neuromuscular stimulation [
53]. However, it should be highlighted that the TENS literature in MS shows mixed results. In fact, Miller et al., 2007 found not significant effect regarding the capability of TENS to reduce spasticity in MS patients [
54]. Notably, this evidence may help explain why TUG remained unchanged for the investigated patient.
At the CNS level, the EEG θ-band reductions and altered microstate dynamics are supported by studies demonstrating that TENS modulates sensorimotor cortical excitability and oscillatory brain activity [
55], while microstate metrics like duration, GFP, and GEV have been linked to shifts in large-scale brain network dynamics and plasticity [
28,
56]. These CNS-related modifications can be associated with the assessed improvement in functional lower limb performance and reduction in perception of physical fatigue.
The ANS-related findings are consistent with evidence that TENS can modify autonomic activity [
57,
58]. Moreover, the HRV findings are consistent with the IRT results. In fact, facial thermographic responses reflect ANS vasoconstriction modulating facial blood flow and autonomic balance [
59]. In fact, an increased sympathetic activity is shown by the HRV metrics at T1 with respect to T0. Accordingly, the increased thermal variability observed in IRT at T1 likely reflects a long-term adaptation of sympathetic vasomotor control. The nose tip, highly sensitive to sympathetic-mediated vasoconstriction [
59], showed greater dispersion and irregularity of temperature distribution after repeated stimulation, suggesting a more dynamic vascular response. Moreover, the reduction of SampEn of the temperature time course has been associated with increased sympathetic activity [
60].
Finally, these findings demonstrate that EMS influences cortical excitability and autonomic regulation, which may foster specific functional gains such as improved lower-limb strength and reduced spasticity.
4.5. Strengths and Limitations
This case report has several strengths. First, it adopts a multimodal strategy, combining functional scales (STS, TUG, spasticity tests, fatigue questionnaires) with neurophysiological measures (EEG, HRV, and IRT). This integrated approach provides a more comprehensive insight of how EMS may influence motor function, cortical dynamics, and autonomic regulation in MS. Second, the study applied a longitudinal design, evaluating both immediate (T0) and one-month (T1) responses, allowing for distinguishing between acute and adaptive effects of stimulation. Finally, the detailed reporting of stimulation parameters and outcome measures enhances reproducibility of the findings.
Nonetheless, important limitations must be acknowledged. Findings are based on a single patient, limiting generalizability across the heterogeneous MS population. Moreover, the absence of a control condition prevents us from assessing eventual placebo effects or natural symptom fluctuations. Furthermore, evaluator blinding was not feasible in this single-case design. This limitation is particularly relevant for subjective measures such as spasticity scales and fatigue questionnaires, which may be more susceptible to observer bias. Additionally, follow-up was restricted to one month, hence further studies enlarging the evaluation period should be performed. Finally, the results are protocol-specific, thus it should be investigated whether different EMS parameters would yield more effective outcomes. Importantly, it should be noted that these findings are hypothesis-generating, thus future studies including randomized controlled and crossover protocols with extended follow-up and evaluator blinding should be implemented.