Ocular Biometry and Refractive Prediction in Short Eyes: A Comparison of Two Swept-Source Optical Coherence Tomography-Based Biometers
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Study Participants
2.3. Statistical Analysis
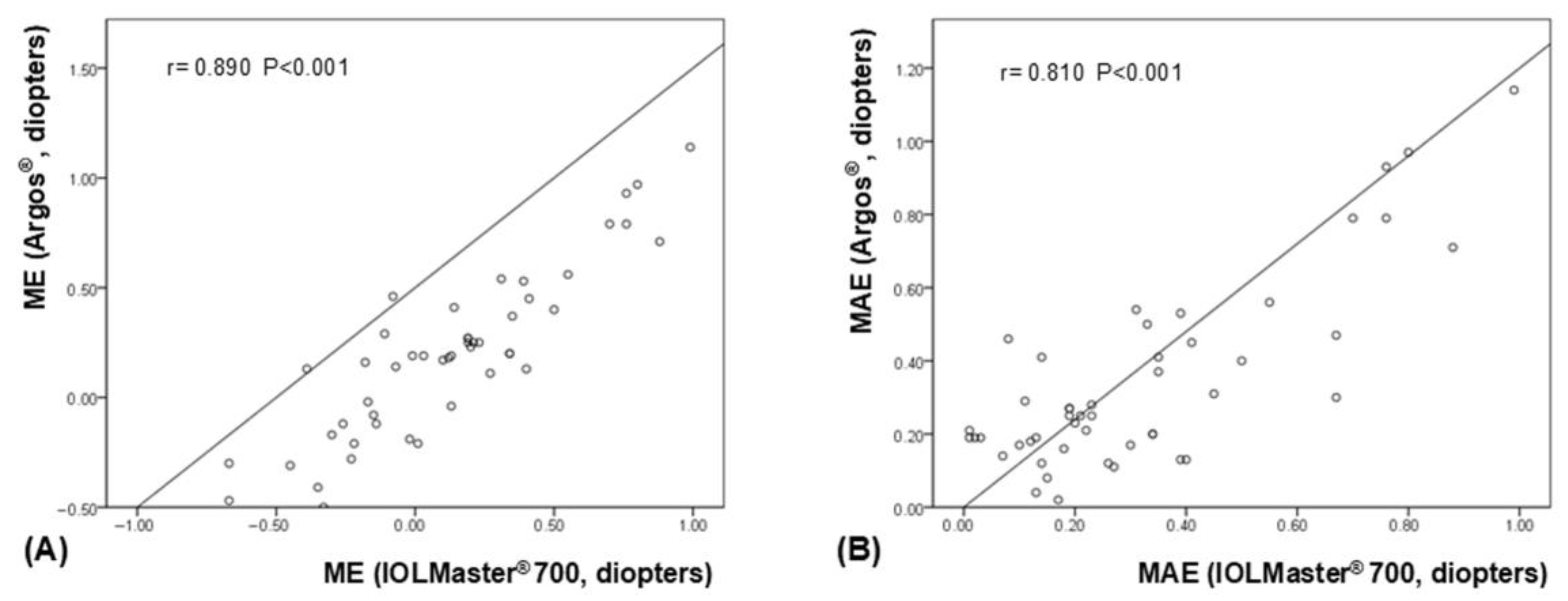
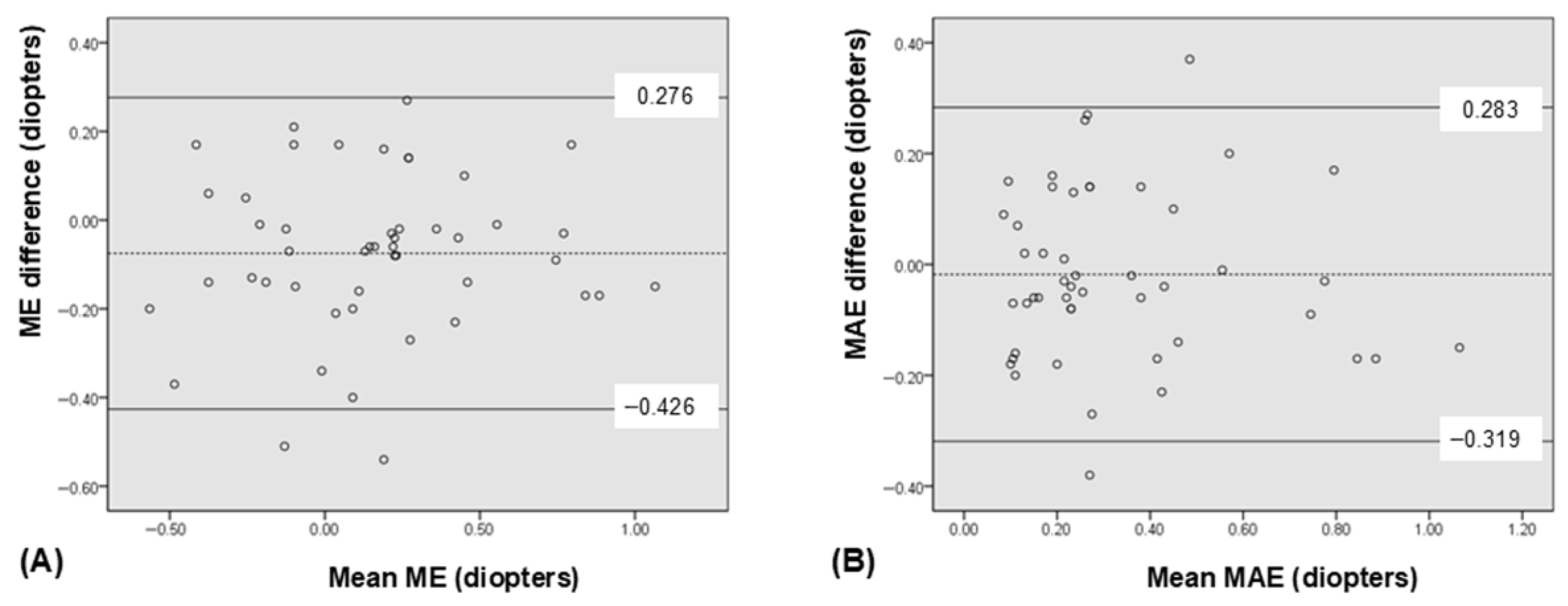
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, H.; Kim, T.-I.; Seo, K.Y.; Jun, I. Clinical Results of Cataract Surgery Using the Primus-HD® Intraocular Lens. Ann. Optom. Contact Lens 2023, 22, 98–104. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, Y.K. Assessing Refractive Stability after Cataract Surgery in Axial Myopes: One-piece and Three-piece Intraocular Lenses. J. Korean Ophthalmol. Soc. 2022, 63, 150–159. [Google Scholar] [CrossRef]
- Yang, C.M.; Lim, D.H.; Kim, H.J.; Chung, T.Y. Comparison of two swept-source optical coherence tomography biometers and a partial coherence interferometer. PLoS ONE 2019, 14, e0223114. [Google Scholar] [CrossRef]
- Fenzl, R.E.; Gills, J.P.; Cherchio, M. Refractive and visual outcome of hyperopic cataract cases operated on before and after implementation of the Holladay II formula. Ophthalmology 1998, 105, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dai, G.; Pazo, E.E.; Xu, L.; Wu, X.; Zhang, H.; Lin, T.; He, W. Accuracy of intraocular lens calculation formulas in cataract patients with steep corneal curvature. PLoS ONE 2020, 15, e0241630. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhang, J.; Han, X.; Huang, Y.; Huang, R.; Ye, J.; Wen, L.; Qiu, X.; Chen, X.; Tan, X.; et al. Intraocular lens calculation formula selection for short eyes: Based on axial length and anterior chamber depth. BMC Ophthalmol. 2025, 25, 21. [Google Scholar] [CrossRef]
- Flikier, D. IOL Power Calculation in the Short Eye. In Intraocular Lens Calculations; Aramberri, J., Hoffer, K.J., Olsen, T., Savini, G., Shammas, H.J., Eds.; Springer International Publishing: Cham, Switzerland, 2024. [Google Scholar]
- Shammas, H.J.; Ortiz, S.; Shammas, M.C.; Kim, S.H.; Chong, C. Biometry measurements using a new large-coherence-length swept-source optical coherence tomographer. J. Cataract Refract. Surg. 2016, 42, 50–61. [Google Scholar] [CrossRef]
- Nemeth, G.; Modis, L., Jr. Ocular measurements of a swept-source biometer: Repeatability data and comparison with an optical low-coherence interferometry biometer. J. Cataract Refract. Surg. 2019, 45, 789–797. [Google Scholar] [CrossRef]
- Omoto, M.K.; Torii, H.; Masui, S.; Ayaki, M.; Tsubota, K.; Negishi, K. Ocular biometry and refractive outcomes using two swept-source optical coherence tomography-based biometers with segmental or equivalent refractive indices. Sci. Rep. 2019, 9, 6557. [Google Scholar] [CrossRef]
- Higashiyama, T.; Mori, H.; Nakajima, F.; Ohji, M. Comparison of a new biometer using swept-source optical coherence tomography and a conventional biometer using partial coherence interferometry. PLoS ONE 2018, 13, e0196401. [Google Scholar] [CrossRef]
- Oh, H.Y.; Choi, J.H.; Chung, Y.K. Comparison of Ocular Biometry Using Ultrasound and Swept-source Optical Coherence Tomography in Severe Cataract. Ann. Optom. Contact Lens 2020, 19, 1–4. [Google Scholar]
- Yang, J.Y.; Kim, H.K.; Kim, S.S. Axial length measurements: Comparison of a new swept-source optical coherence tomography–based biometer and partial coherence interferometry in myopia. J. Cataract. Refract. Surg. 2017, 43, 328–332. [Google Scholar] [CrossRef]
- Rementería-Capelo, L.A.; Contreras, I.; García-Pérez, J.L.; Ruiz-Alcocer, J. Refractive Accuracy of a Novel Swept-Source OCT in Patients With Short and Long Eyes. J. Ophthalmol. 2025, 2025, 9987580. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, D.; Weikert, M.P.; Koch, D.D. Calculation of Axial Length Using a Single Group Refractive Index versus Using Different Refractive Indices for Each Ocular Segment: Theoretical Study and Refractive Outcomes. Ophthalmology 2019, 126, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Lee, H.J.; Lee, S.-Y.; Kim, J.Y.; Ma, D.J.; Jeong, J.H. Comparison of Clinical Outcomes between Swept-source Optical Coherence Tomography Biometer and Partial Coherence Interferometer. J. Korean Ophthalmol. Soc. 2020, 61, 905–910. [Google Scholar] [CrossRef]
- Porwolik, M.; Porwolik, A.; Mrukwa-Kominek, E. Evaluation of Selected Biometric Parameters in Cataract Patients-A Comparison between Argos® and IOLMaster 700®: Two Swept-Source Optical Coherence Tomography-Based Biometers. Med. 2024, 60, 1057. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, F.; Matarazzo, F.; Findl, O.; Maurino, V. Comparative analysis of 2 swept-source optical coherence tomography biometers. J. Cataract Refract. Surg. 2019, 45, 1124–1129. [Google Scholar] [CrossRef]
- Shammas, H.J.; Shammas, M.C.; Jivrajka, R.V.; Cooke, D.L.; Potvin, R. Effects on IOL Power Calculation and Expected Clinical Outcomes of Axial Length Measurements Based on Multiple vs Single Refractive Indices. Clin. Ophthalmol. 2020, 14, 1511–1519. [Google Scholar] [CrossRef]
- Tamaoki, A.; Kojima, T.; Hasegawa, A.; Yamamoto, M.; Kaga, T.; Tanaka, K.; Ichikawa, K. Clinical Evaluation of a New Swept-Source Optical Coherence Biometer That Uses Individual Refractive Indices to Measure Axial Length in Cataract Patients. Ophthalmic Res. 2019, 62, 11–23. [Google Scholar] [CrossRef]
- Melles, R.B.; Holladay, J.T.; Chang, W.J. Accuracy of Intraocular Lens Calculation Formulas. Ophthalmology 2018, 125, 169–178. [Google Scholar] [CrossRef]
- Sudhakar, S.; Hill, D.C.; King, T.S.; Scott, I.U.; Mishra, G.; Ernst, B.B.; Pantanelli, S.M. Intraoperative aberrometry versus preoperative biometry for intraocular lens power selection in short eyes. J. Cataract Refract. Surg. 2019, 45, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cho, S.Y.; Yang, J.W.; Kim, C.S.; Lee, Y.C. The correlation of differences in the ocular component values with the degree of myopic anisometropia. Korean J. Ophthalmol. 2013, 27, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T. Sources of error in intraocular lens power calculation. J. Cataract Refract. Surg. 1992, 18, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Hipólito-Fernandes, D.; Luís, M.E.; Serras-Pereira, R.; Gil, P.; Maduro, V.; Feijão, J.; Alves, N. Anterior chamber depth, lens thickness and intraocular lens calculation formula accuracy: Nine formulas comparison. Br. J. Ophthalmol. 2022, 106, 349–355. [Google Scholar] [CrossRef]
- Multack, S.; Plummer, N.; Marneris, A.; Hall, B. A Retrospective Trial Comparing Prediction Accuracy of Three Biometers in Short, Medium, and Long Eyes. Clin. Ophthalmol. 2025, 19, 577–583. [Google Scholar] [CrossRef]
- Multack, S.; Plummer, N.; Smits, G.; Hall, B. Randomized Trial Comparing Prediction Accuracy of Two Swept Source Optical Coherence Tomography Biometers. Clin. Ophthalmol. 2023, 17, 2423–2428. [Google Scholar] [CrossRef]
- Shrivastava, A.K.; Behera, P.; Kumar, B.; Nanda, S. Precision of intraocular lens power prediction in eyes shorter than 22 mm: An analysis of 6 formulas. J. Cataract Refract. Surg. 2018, 44, 1317–1320. [Google Scholar] [CrossRef]
- Day, A.C.; Foster, P.J.; Stevens, J.D. Accuracy of intraocular lens power calculations in eyes with axial length <22.00 mm. Clin. Exp. Ophthalmol. 2012, 40, 855–862. [Google Scholar] [CrossRef]
- Amornpetchsathaporn, A.; Chantra, S.; Annopawong, K.; Seresirikachorn, K.; Kongsomboon, K.; Wanichwecharungruang, B. Ocular Biometry Profile and Its Associations with Systemic and Demographic Factors in Thai Cataract Patients. Clin. Ophthalmol. 2025, 19, 1155–1166. [Google Scholar] [CrossRef]
- De Bernardo, M.; Zeppa, L.; Zeppa, L.; Cornetta, P.; Vitiello, L.; Rosa, N. Biometric Parameters and Corneal Astigmatism: Differences Between Male and Female Eyes. Clin. Ophthalmol. 2020, 14, 571–580. [Google Scholar] [CrossRef]
- Richter, G.M.; Wang, M.; Jiang, X.; Wu, S.; Wang, D.; Torres, M.; Choudhury, F.; Varma, R.; for the Chinese American Eye Study Group. Ocular Determinants of Refractive Error and Its Age- and Sex-Related Variations in the Chinese American Eye Study. JAMA Ophthalmol. 2017, 135, 724–732. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.; Lee, S.J.; Han, S.B.; Kong, Y.T.; Yang, H.K.; Hyon, J.Y. Age-related differences in ocular biometry in adult Korean population. BMC Ophthalmol. 2016, 16, 146. [Google Scholar] [CrossRef]
- Solomon, R.; Tamilarasi, S.; Sachdev, G.; Dandapani, R. Accuracy of Barrett versus third-generation intraocular lens formula across all axial lengths. Oman J. Ophthalmol. 2022, 15, 290–294. [Google Scholar] [CrossRef]
- Ma, Y.; Lin, Y.; Li, Y.; Hu, Z.; Qiu, K. Accuracy of new intraocular lens calculation formulas in Chinese eyes with short axial lengths. Front Med 2023, 10, 1257873. [Google Scholar] [CrossRef]


| Male (n, (Eyes)) | Female (n, (Eyes)) | |
|---|---|---|
| 40–49 years | 1 (1 eyes) | |
| 50–59 years | 1 (1 eyes) | |
| 60–69 years | 3 (5 eyes) | 8 (13 eyes) |
| 70–79 years | 1 (2 eyes) | 10 (18 eyes) |
| 80 years or older | 5 (8 eyes) | |
| Total | 4 (7 eyes) | 25 (41 eyes) |
| Parameters | IOL Master® 700 | ARGOS | Mean Difference | 95% CI * | p Value † | ICC ‡ |
|---|---|---|---|---|---|---|
| AL (mm) | 21.65 ± 0.24 | 21.70 ± 0.23 | −0.05 | −0.07, −0.03 | <0.001 | 0.975 |
| ACD (mm) | 2.65 ± 0.34 | 2.79 ± 0.33 | −0.14 | −0.15, −0.13 | <0.001 | 0.957 |
| K (diopters) | 46.33 ± 0.81 | 46.32 ± 0.82 | +0.01 | −0.04, +0.07 | 0.585 | 0.988 |
| LT (mm) | 4.88 ± 0.39 | 4.89 ± 0.38 | −0.01 | −0.02, +0.00 | 0.224 | 0.994 |
| Parameters | IOL Master® 700 | ARGOS® | Mean Difference | 95% CI * | p Value |
|---|---|---|---|---|---|
| ME † (diopters, D) | +0.12 ± 0.39 | +0.20 ± 0.38 | −0.08 | −0.13, −0.02 | 0.006 § |
| MAE ‡ (D) | 0.32 ± 0.25 | 0.34 ± 0.25 | −0.02 | −0.06, +0.03 | 0.423 § |
| Eyes within ± 0.50 D | 77.1% (37/48) | 75.0% (36/48) | 0.811 ∥ | ||
| Eyes within ± 1.00 D | 100% (48/48) | 97.9% (47/48) | 0.315 ∥ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, J.; Han, S.B. Ocular Biometry and Refractive Prediction in Short Eyes: A Comparison of Two Swept-Source Optical Coherence Tomography-Based Biometers. Bioengineering 2025, 12, 983. https://doi.org/10.3390/bioengineering12090983
Seong J, Han SB. Ocular Biometry and Refractive Prediction in Short Eyes: A Comparison of Two Swept-Source Optical Coherence Tomography-Based Biometers. Bioengineering. 2025; 12(9):983. https://doi.org/10.3390/bioengineering12090983
Chicago/Turabian StyleSeong, Jiyun, and Sang Beom Han. 2025. "Ocular Biometry and Refractive Prediction in Short Eyes: A Comparison of Two Swept-Source Optical Coherence Tomography-Based Biometers" Bioengineering 12, no. 9: 983. https://doi.org/10.3390/bioengineering12090983
APA StyleSeong, J., & Han, S. B. (2025). Ocular Biometry and Refractive Prediction in Short Eyes: A Comparison of Two Swept-Source Optical Coherence Tomography-Based Biometers. Bioengineering, 12(9), 983. https://doi.org/10.3390/bioengineering12090983







