The Therapeutic Scope of Orofacial Mesenchymal Stem Cells
Abstract
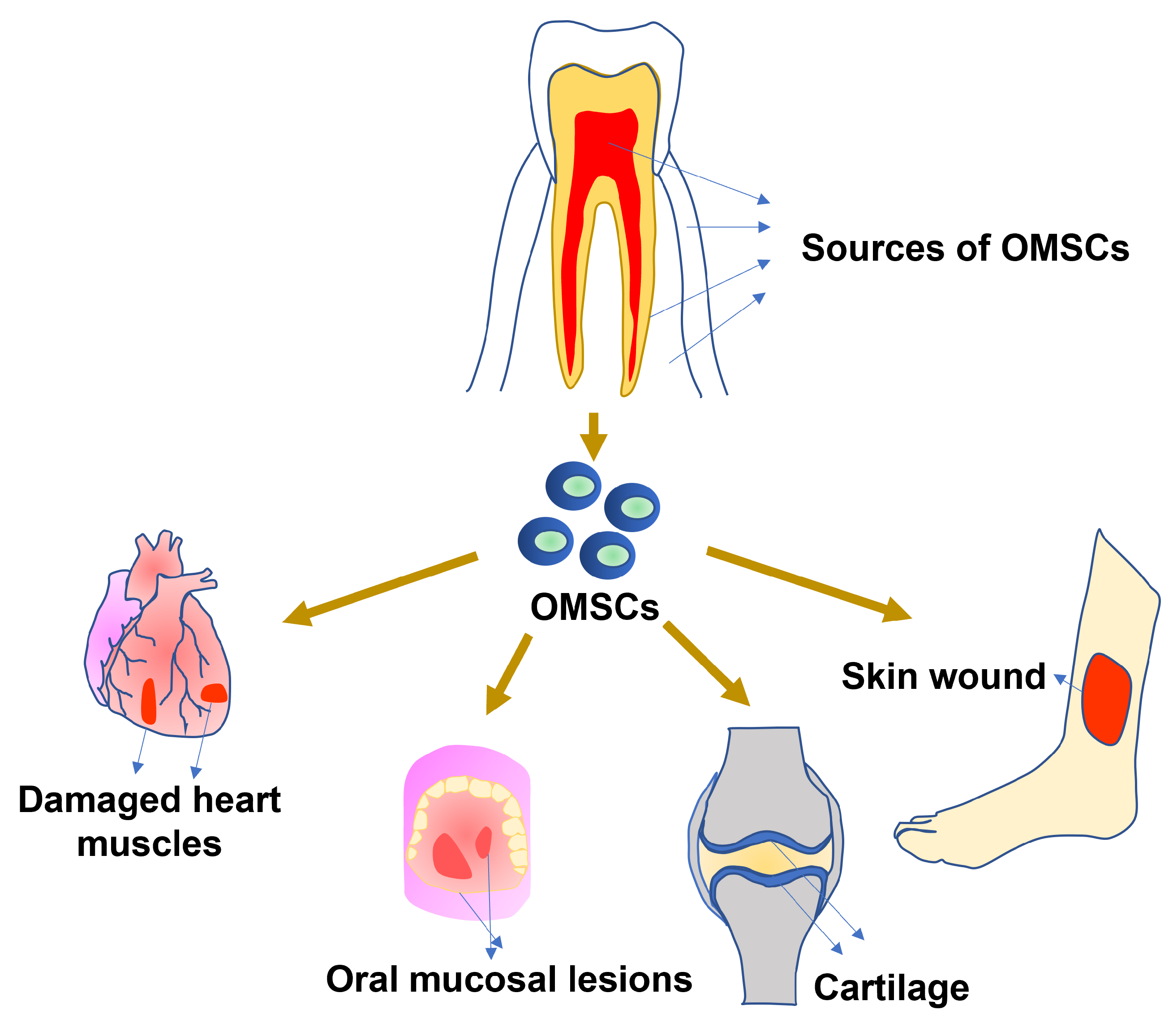
1. Introduction
2. OMSCs in Craniofacial Bone Tissue Regeneration
2.1. Conventional Approaches to Bone Repair
2.2. Tissue Engineering Strategies for Bone Regeneration
2.2.1. The Role of Scaffolds
2.2.2. Role of Growth Factors in Vascularized Bone
2.2.3. Preclinical Evaluation of Bone Reconstruction in Animal Models
3. OMSC for Other Tissue Regeneration
3.1. Regeneration of Soft Tissues
3.1.1. Cartilage Tissue
3.1.2. Cardiac Tissue
3.1.3. Muscle Tissue
3.1.4. Retinal and Neural Regeneration
3.1.5. Dentin-Pulp Complex
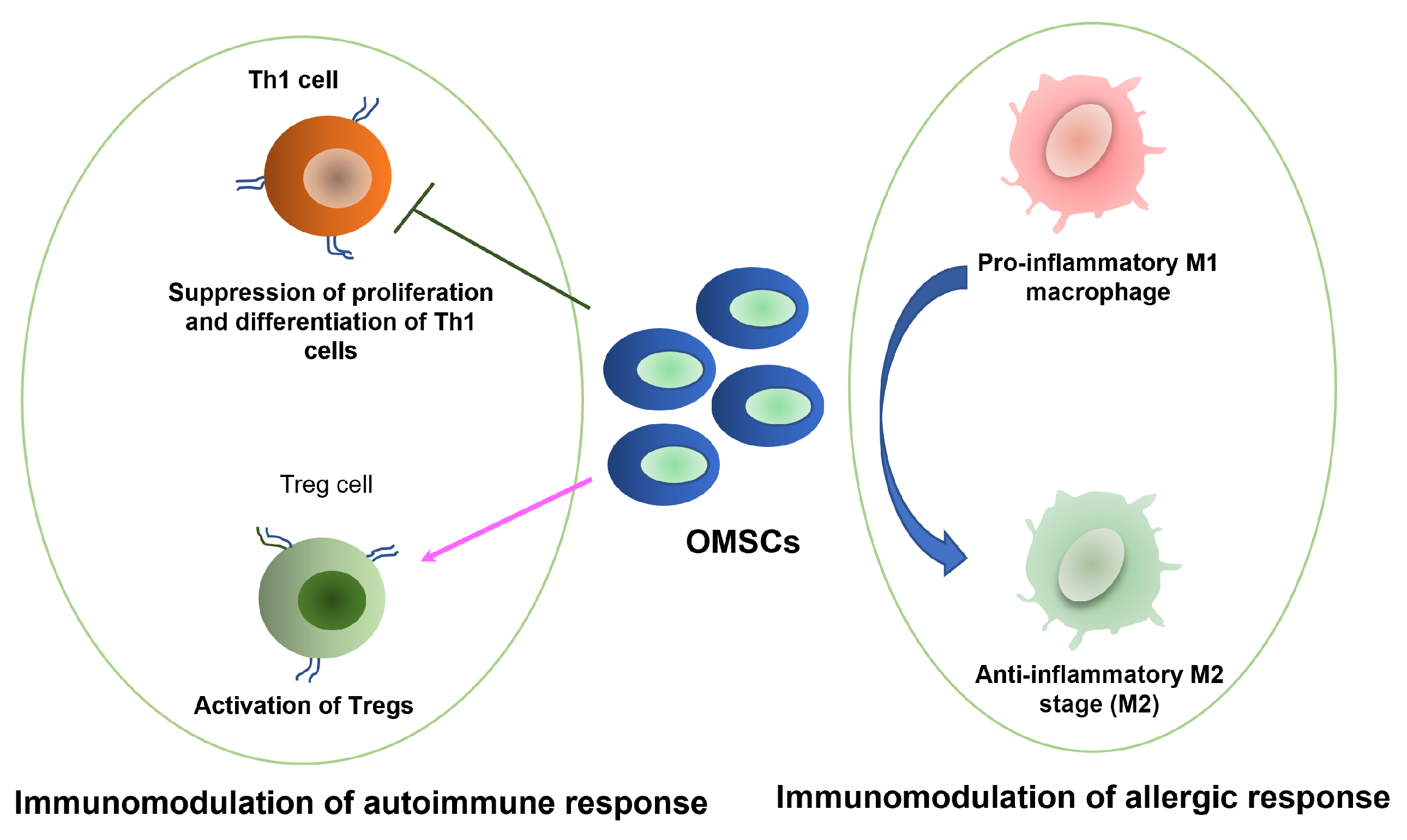
3.2. Immunomodulatory Functions of OMSCs
3.2.1. Application in Wound Healing, Allergy, and Inflammation
3.2.2. Application in Autoimmune Disorders
4. Challenges, Limitations, and Reasons for Conflicting Data
4.1. Intrinsic Biological Constraints
4.2. Methodological and Translational Challenges
4.3. Potential Reasons for Conflicting Data
4.3.1. Animal Models
4.3.2. Cell Isolation and Culture Techniques
4.3.3. Scaffold Properties and Delivery Systems
4.3.4. Exosome Preparation
4.3.5. Study Design and Reporting
4.4. Limitations of Existing Technologies
5. Enabling Future Technologies and Directions
5.1. Emerging Biological Applications
5.2. 3D Bioprinting and Advanced Scaffolds
5.3. The Role of Artificial Intelligence in Regenerative Medicine
5.4. Microfluidic Systems in Bone Tissue Engineering
5.5. Cell-Free Therapies: The Potential of Exosomes
5.6. Strategies for Clinical-Scale OMSCs Production
5.7. Designing Multicenter Studies for Clinical Translation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef] [PubMed]
- Kaundal, U.; Bagai, U.; Rakha, A. Immunomodulatory plasticity of mesenchymal stem cells: A potential key to successful solid organ transplantation. J. Transl. Med. 2018, 16, 31. [Google Scholar] [CrossRef]
- Suma, G.; Arora, M.; Lakhanpal, M. Stem cell therapy: A novel treatment approach for oral mucosal lesions. J. Pharm. Bioallied Sci. 2015, 7, 2. [Google Scholar] [CrossRef]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal Stem Cells: Revisiting History, Concepts, and Assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Kaltschmidt, C.; Widera, D. Adult Craniofacial Stem Cells: Sources and Relation to the Neural Crest. Stem Cell Rev. Rep. 2012, 8, 658–671. [Google Scholar] [CrossRef]
- Zaky, S.; Cancedda, R. Engineering Craniofacial Structures: Facing the Challenge. J. Dent. Res. 2009, 88, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xing, Y.; Jia, L.; Ji, Y.; Zhao, B.; Wen, Y.; Xu, X. An In Vitro Comparative Study of Multisource Derived Human Mesenchymal Stem Cells for Bone Tissue Engineering. Stem Cells Dev. 2018, 27, 1634–1645. [Google Scholar] [CrossRef]
- Wu, V.; Helder, M.N.; Bravenboer, N.; Ten Bruggenkate, C.M.; Jin, J.; Klein-Nulend, J.; Schulten, E.A.J.M. Bone Tissue Regeneration in the Oral and Maxillofacial Region: A Review on the Application of Stem Cells and New Strategies to Improve Vascularization. Stem Cells Int. 2019, 2019, 6279721. [Google Scholar] [CrossRef]
- Fishero, B.; Kohli, N.; Das, A.; Christophel, J.; Cui, Q. Current Concepts of Bone Tissue Engineering for Craniofacial Bone Defect Repair. Craniomaxillofac. Trauma Reconstr. 2015, 8, 23–30. [Google Scholar] [CrossRef]
- Strem, B.M.; Hicok, K.C.; Zhu, M.; Wulur, I.; Alfonso, Z.; Schreiber, R.E.; Fraser, J.K.; Hedrick, M.H. Multipotential differentiation of adipose tissue-derived stem cells. Keio J. Med. 2005, 54, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.G.; Maghaireh, H.; Mangano, F.G. Stem Cells in Dentistry: Types of Intra- and Extraoral Tissue-Derived Stem Cells and Clinical Applications. Stem Cells Int. 2018, 2018, 4313610. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Seagroves, J.T.; Chen, C.; Shah, K.; Aghaloo, T.; Wu, B.M.; Bencharit, S.; Moshaverinia, A. Dental and orofacial mesenchymal stem cells in craniofacial regeneration: The prosthodontist’s point of view. J. Prosthet. Dent. 2017, 118, 455–461. [Google Scholar] [CrossRef]
- Kim, D.; Lee, A.E.; Xu, Q.; Zhang, Q.; Le, A.D. Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine - A Comprehensive Review. Front. Immunol. 2021, 12, 667221. [Google Scholar] [CrossRef]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 2020, 11, 2041731420943839. [Google Scholar] [CrossRef]
- Hosseini, V.; Maroufi, N.F.; Saghati, S.; Asadi, N.; Darabi, M.; Ahmad, S.N.S.; Hosseinkhani, H.; Rahbarghazi, R. Current progress in hepatic tissue regeneration by tissue engineering. J. Transl. Med. 2019, 17, 383. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Xu, X.; Chen, C.; Ansari, S.; Zadeh, H.H.; Snead, M.L.; Shi, S. Application of stem cells derived from the periodontal ligament or gingival tissue sources for tendon tissue regeneration. Biomaterials 2014, 35, 2642–2650. [Google Scholar] [CrossRef]
- Alarcón-Apablaza, J.; Prieto, R.; Rojas, M.; Fuentes, R. Potential of Oral Cavity Stem Cells for Bone Regeneration: A Scoping Review. Cells 2023, 12, 1392. [Google Scholar] [CrossRef] [PubMed]
- Kandalam, U.; Kawai, T.; Ravindran, G.; Brockman, R.; Romero, J.; Munro, M.; Ortiz, J.; Heidari, A.; Thomas, R.; Kuriakose, S.; et al. Predifferentiated Gingival Stem Cell-Induced Bone Regeneration in Rat Alveolar Bone Defect Model. Tissue Eng. Part A 2021, 27, 424–436. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Wang, X.; Xing, H.; Zhang, G.; Wu, X.; Zou, X.; Feng, L.; Wang, D.; Li, M.; Zhao, J.; Du, J.; et al. Restoration of a Critical Mandibular Bone Defect Using Human Alveolar Bone-Derived Stem Cells and Porous Nano-HA/Collagen/PLA Scaffold. Stem Cells Int. 2016, 2016, 8741641. [Google Scholar] [CrossRef]
- Rehman, A.; Nigam, A.; Laino, L.; Russo, D.; Todisco, C.; Esposito, G.; Svolacchia, F.; Giuzio, F.; Desiderio, V.; Ferraro, G. Mesenchymal Stem Cells in Soft Tissue Regenerative Medicine: A Comprehensive Review. Medicina 2023, 59, 1449. [Google Scholar] [CrossRef]
- Iwata, T.; Yamato, M.; Zhang, Z.; Mukobata, S.; Washio, K.; Ando, T.; Feijen, J.; Okano, T.; Ishikawa, I. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J. Clin. Periodontol. 2010, 37, 1088–1099. [Google Scholar] [CrossRef]
- Shi, S.; Robey, P.; Gronthos, S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone 2001, 29, 532–539. [Google Scholar] [CrossRef]
- Dannan, A. Dental-derived Stem Cells and whole Tooth Regeneration: An Overview. J. Clin. Med. Res. 2009, 1, 63. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Shakesheff, K.M.; White, L.J. Dental pulp stem cells: Function isolation and applications in regenerative medicine: Function isolation and applications of dental pulp stem cells. J. Tissue Eng. Regen. Med. 2015, 9, 1205–1216. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Cortez De SantAnna, J.P.; Frisene, I.; Gazarini, J.P.; Gomes Pinheiro, C.C.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Franco Bueno, D. Systematic Review of Human Dental Pulp Stem Cells for Cartilage Regeneration. Tissue Eng. Part B Rev. 2020, 26, 1–12. [Google Scholar] [CrossRef]
- Couble, M.L.; Farges, J.C.; Bleicher, F.; Perrat-Mabillon, B.; Boudeulle, M.; Magloire, H. Odontoblast Differentiation of Human Dental Pulp Cells in Explant Cultures. Calcif. Tissue Int. 2000, 66, 129–138. [Google Scholar] [CrossRef]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Becchetti, E.; Marchionni, C.; Alviano, F.; Fossati, V.; et al. Multipotent Mesenchymal Stem Cells with Immunosuppressive Activity Can Be Easily Isolated from Dental Pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef]
- Ji, L.; Bao, L.; Gu, Z.; Zhou, Q.; Liang, Y.; Zheng, Y.; Xu, Y.; Zhang, X.; Feng, X. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol. Res. 2019, 67, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Yamada, Y.; Katagiri, W.; Sugito, T.; Ito, K.; Ueda, M. Stem Cell Proliferation Pathways Comparison between Human Exfoliated Deciduous Teeth and Dental Pulp Stem Cells by Gene Expression Profile from Promising Dental Pulp. J. Endod. 2009, 35, 1536–1542. [Google Scholar] [CrossRef]
- Ng, T.K.; Yung, J.S.Y.; Choy, K.W.; Cao, D.; Leung, C.K.S.; Cheung, H.S.; Pang, C.P. Transdifferentiation of periodontal ligament-derived stem cells into retinal ganglion-like cells and its microRNA signature. Sci. Rep. 2015, 5, 16429. [Google Scholar] [CrossRef]
- Cen, L.P.; Ng, T.K.; Liang, J.J.; Zhuang, X.; Yao, X.; Yam, G.H.F.; Chen, H.; Cheung, H.S.; Zhang, M.; Pang, C.P. Human Periodontal Ligament-Derived Stem Cells Promote Retinal Ganglion Cell Survival and Axon Regeneration After Optic Nerve Injury. Stem Cells 2018, 36, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Caplan, A.I. Stem Cells in Dental Pulp of Deciduous Teeth. Tissue Eng. Part B Rev. 2012, 18, 129–138. [Google Scholar] [CrossRef]
- Rosa, V.; Dubey, N.; Islam, I.; Min, K.S.; Nör, J.E. Pluripotency of Stem Cells from Human Exfoliated Deciduous Teeth for Tissue Engineering. Stem Cells Int. 2016, 2016, 5957806. [Google Scholar] [CrossRef]
- Mohd Nor, N.H.; Mansor, N.I.; Mohd Kashim, M.I.A.; Mokhtar, M.H.; Mohd Hatta, F.A. From Teeth to Therapy: A Review of Therapeutic Potential within the Secretome of Stem Cells from Human Exfoliated Deciduous Teeth. Int. J. Mol. Sci. 2023, 24, 11763. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Shibata, R.; Yamamoto, N.; Nishikawa, M.; Hibi, H.; Tanigawa, T.; Ueda, M.; Murohara, T.; Yamamoto, A. Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci. Rep. 2015, 5, 16295. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Mark Bartold, P.; Batouli, S.; Brahim, J.; Young, M.; Gehron Robey, P.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Sun, H.H.; Lu, H.; Yu, Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012, 33, 6320–6344. [Google Scholar] [CrossRef]
- Ding, G.; Liu, Y.; Wang, W.; Wei, F.; Liu, D.; Fan, Z.; An, Y.; Zhang, C.; Wang, S. Allogeneic Periodontal Ligament Stem Cell Therapy for Periodontitis in Swine. Stem Cells 2010, 28, 1829–1838. [Google Scholar] [CrossRef]
- Alamdari, G.; Majidinia, M. Diagnostic and therapeutic potential of oral cavity–derived exosomes in oral and maxillofacial tissue engineering: Current advances and future perspectives. In Naunyn-Schmiedeberg’s Archives of Pharmacology; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal Stem Cells Derived from Human Gingiva Are Capable of Immunomodulatory Functions and Ameliorate Inflammation-Related Tissue Destruction in Experimental Colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nguyen, P.D.; Shi, S.; Burrell, J.C.; Xu, Q.; Cullen, K.D.; Le, A.D. Neural Crest Stem-Like Cells Non-genetically Induced from Human Gingiva-Derived Mesenchymal Stem Cells Promote Facial Nerve Regeneration in Rats. Mol. Neurobiol. 2018, 55, 6965–6983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Su, W.R.; Shi, S.H.; Wilder-Smith, P.; Xiang, A.P.; Wong, A.; Nguyen, A.L.; Kwon, C.W.; Le, A.D. Human Gingiva-Derived Mesenchymal Stem Cells Elicit Polarization of M2 Macrophages and Enhance Cutaneous Wound Healing. Stem Cells 2010, 28, 1856–1868. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Y.; Cui, D.; Pan, Y.; Zheng, L.; Wan, M. Dental Tissue-Derived Human Mesenchymal Stem Cells and Their Potential in Therapeutic Application. Stem Cells Int. 2020, 2020, 8864572. [Google Scholar] [CrossRef]
- Yildirim, S.; Zibandeh, N.; Genc, D.; Ozcan, E.M.; Goker, K.; Akkoc, T. The Comparison of the Immunologic Properties of Stem Cells Isolated from Human Exfoliated Deciduous Teeth, Dental Pulp, and Dental Follicles. Stem Cells Int. 2016, 2016, 4682875. [Google Scholar] [CrossRef]
- Sowmya, S.; Chennazhi, K.P.; Arzate, H.; Jayachandran, P.; Nair, S.V.; Jayakumar, R. Periodontal Specific Differentiation of Dental Follicle Stem Cells into Osteoblast, Fibroblast, and Cementoblast. Tissue Eng. Part C Methods 2015, 21, 1044–1058. [Google Scholar] [CrossRef]
- Genc, D.; Zibandeh, N.; Uluc, K.; Kahraman Koytak, P.; Gokalp, M.; Tanridag, T.; Akkoc, T. Dental Follicle Mesenchymal Stem Cells Enhance CD4+Foxp3+ Regulatory T Cells in the Lymphocytes of Amyotrophic Lateral Sclerosis Patients. Clin. Exp. Health Sci. 2017, 7, 85–90. [Google Scholar] [CrossRef]
- Kanao, S.; Ogura, N.; Takahashi, K.; Ito, K.; Suemitsu, M.; Kuyama, K.; Kondoh, T. Capacity of Human Dental Follicle Cells to Differentiate into Neural Cells In Vitro. Stem Cells Int. 2017, 2017, 8371326. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, H.; Li, G.; Yu, J.; Fang, F.; Qiu, W. Dental-derived mesenchymal stem cell sheets: A prospective tissue engineering for regenerative medicine. Stem Cell Res. Ther. 2022, 13, 38. [Google Scholar] [CrossRef]
- Zibandeh, N.; Genc, D.; Duran, Y.; Banzragch, M.; Sokwala, S. Human dental follicle mesenchymal stem cells alleviate T cell response in inflamed tissue of Crohn’s patients. Turk. J. Gastroenterol. 2020, 31, 400–409. [Google Scholar] [CrossRef]
- Ulusoy, C.; Zibandeh, N.; Yıldırım, S.; Trakas, N.; Zisimopoulou, P.; Küçükerden, M.; Tașlı, H.; Tzartos, S.; Göker, K.; Tüzün, E.; et al. Dental follicle mesenchymal stem cell administration ameliorates muscle weakness in MuSK-immunized mice. J. Neuroinflamm. 2015, 12, 231. [Google Scholar] [CrossRef]
- Matsubara, T.; Suardita, K.; Ishii, M.; Sugiyama, M.; Igarashi, A.; Oda, R.; Nishimura, M.; Saito, M.; Nakagawa, K.; Yamanaka, K.; et al. Alveolar Bone Marrow as a Cell Source for Regenerative Medicine: Differences Between Alveolar and Iliac Bone Marrow Stromal Cells. J. Bone Miner. Res. 2005, 20, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Dou, H.; Tian, B.; Li, L.; Jin, L.; Zhang, Z.; Hu, L. Bone regeneration capacities of alveolar bone mesenchymal stem cells sheet in rabbit calvarial bone defect. J. Tissue Eng. 2020, 11, 2041731420930379. [Google Scholar] [CrossRef]
- Ghaderi, H.; Razmkhah, M.; Kiany, F.; Chenari, N.; Haghshenas, M.R. Comparison of Osteogenic and Chondrogenic Differentiation Ability of Buccal Fat Pad Derived Mesenchymal Stem Cells and Gingival Derived Cells. J. Dent. 2018, 19, 124. [Google Scholar]
- Meshram, M.; Anchlia, S.; Shah, H.; Vyas, S.; Dhuvad, J.; Sagarka, L. Buccal Fat Pad-Derived Stem Cells for Repair of Maxillofacial Bony Defects. J. Maxillofac. Oral Surg. 2019, 18, 112–123. [Google Scholar] [CrossRef]
- Soudi, A.; Yazdanian, M.; Ranjbar, R.; Tebyanian, H.; Yazdanian, A.; Tahmasebi, E.; Keshvad, A.; Seifalian, A. Role and application of stem cells in dental regeneration: A comprehensive overview. Excli J. 2021, 20, 454. [Google Scholar] [CrossRef] [PubMed]
- Gorjup, E.; Danner, S.; Rotter, N.; Habermann, J.; Brassat, U.; Brummendorf, T.H.; Wien, S.; Meyerhans, A.; Wollenberg, B.; Kruse, C.; et al. Glandular tissue from human pancreas and salivary gland yields similar stem cell populations. Eur. J. Cell Biol. 2009, 88, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, C.; Barazzuol, L.; Coppes, R.P. The evolving definition of salivary gland stem cells. NPJ Regen. Med. 2021, 6, 4. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Kucia, M.; Jadczyk, T.; Greco, N.J.; Wojakowski, W.; Tendera, M.; Ratajczak, J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012, 26, 1166–1173. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Shi, S.; et al. Mesenchymal Stem Cell-Mediated Functional Tooth Regeneration in Swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Ikeda, E.; Hirose, M.; Kotobuki, N.; Shimaoka, H.; Tadokoro, M.; Maeda, M.; Hayashi, Y.; Kirita, T.; Ohgushi, H. Osteogenic differentiation of human dental papilla mesenchymal cells. Biochem. Biophys. Res. Commun. 2006, 342, 1257–1262. [Google Scholar] [CrossRef]
- Yang, H.; Cao, Y.; Zhang, J.; Liang, Y.; Su, X.; Zhang, C.; Liu, H.; Han, X.; Ge, L.; Fan, Z. DLX5 and HOXC8 enhance the chondrogenic differentiation potential of stem cells from apical papilla via LINC01013. Stem Cell Res. Ther. 2020, 11, 271. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Sun, L.; Guo, W.; Tian, W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J. Neural Eng. 2017, 14, 026005. [Google Scholar] [CrossRef]
- Basabrain, M.S.; Zaeneldin, A.; Bijle, M.N.; Zhang, C. Dental stem cell sphere formation and potential for neural regeneration: A scoping review. Heliyon 2024, 10, e40262. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: Implication in disease and tissue regeneration. World J. Stem Cells 2019, 11, 604–617. [Google Scholar] [CrossRef]
- Dunn, C.A.; Jin, Q.; Taba, M.; Franceschi, R.T.; Bruce Rutherford, R.; Giannobile, W.V. BMP gene delivery for alveolar bone engineering at dental implant defects. Mol. Ther. 2005, 11, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, M.K.; Park, J.H.; Won, J.E.; Kim, T.H.; Kim, H.W. Performance of Novel Nanofibrous Biopolymer Membrane for Guided Bone Regeneration within Rat Mandibular Defect. In Vivo 2011, 25, 589. [Google Scholar] [PubMed]
- Waite, P.D.; Waite, D.E. Bone grafting for the alveolar cleft defect. Semin. Orthod. 1996, 2, 192–196. [Google Scholar] [CrossRef]
- Costantino, P.D.; Hiltzik, D.; Govindaraj, S.; Moche, J. Bone Healing and Bone Substitutes. Facial Plast. Surg. 2002, 18, 013–026. [Google Scholar] [CrossRef]
- Chamieh, F.; Collignon, A.M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 6, 38814. [Google Scholar] [CrossRef]
- Elsalanty, M.E.; Genecov, D.G. Bone Grafts in Craniofacial Surgery. Craniomaxillofac. Trauma Reconstr. 2009, 2, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.; Faraz, F.; Lamba, A.K.; Tandon, S.; Kamal, Z.; Madni, Z.K. Effect of Demineralization Time on the Release of Bone Morphogenetic Protein in Indigenously Prepared Demineralized Freeze-Dried Bone Allografts: A Comparative In Vitro Study. Cureus 2025, 17, e84037. [Google Scholar] [CrossRef]
- Kamadjaja, D.B.; Satriyo, H.; Setyawan, A.; Lesmaya, Y.D.; Safril, J.W.; Sumarta, N.P.M.; Rizqiawan, A.; Danudiningrat, C.P.; Tran, T.T. Analyses of Bone Regeneration Capacity of Freeze-Dried Bovine Bone and Combined Deproteinized-Demineralized Bovine Bone Particles in Mandibular Defects: The Potential Application of Biological Forms of Bovine-Bone Filler. Eur. J. Dent. 2022, 16, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Bigham, A.S.; Dehghani, S.N.; Shafiei, Z.; Torabi Nezhad, S. Xenogenic demineralized bone matrix and fresh autogenous cortical bone effects on experimental bone healing: Radiological, histopathological and biomechanical evaluation. J. Orthop. Traumatol. Off. J. Ital. Soc. Orthop. Traumatol. 2008, 9, 73–80. [Google Scholar] [CrossRef]
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 2007, 28, 4240–4250. [Google Scholar] [CrossRef]
- Chenard, K.E.; Teven, C.M.; He, T.C.; Reid, R.R. Bone Morphogenetic Proteins in Craniofacial Surgery: Current Techniques, Clinical Experiences, and the Future of Personalized Stem Cell Therapy. J. Biomed. Biotechnol. 2012, 2012, 601549. [Google Scholar] [CrossRef]
- Laurencin, C.T.; El-Amin, S.F. Xenotransplantation in Orthopaedic Surgery. J. Am. Acad. Orthop. Surg. 2008, 16, 4–8. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Oh, T.; Rahman, M.M.; Lim, J.H.; Park, M.S.; Kim, D.Y.; Yoon, J.h.; Kim, W.H.; Kikuchi, M.; Tanaka, J.; Koyama, Y.; et al. Guided bone regeneration with beta-tricalcium phosphate and poly L-lactide-co-glycolide-co-epsilon-caprolactone membrane in partial defects of canine humerus. J. Vet. Sci. 2006, 7, 73. [Google Scholar] [CrossRef]
- Eickholz, P.; Kim, T.; Holle, R. Guided tissue regeneration with non-resorbable and biodegradable barriers: 6 months results. J. Clin. Periodontol. 1997, 24, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Maeda, H. Recent Developments of Functional Scaffolds for Craniomaxillofacial Bone Tissue Engineering Applications. Sci. World J. 2013, 2013, 863157. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.M.; Takita, H.; Kohgo, T.; Atsumi, K.; Itoh, H.; Kuboki, Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J. Biomed. Mater. Res. 2000, 51, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Barralet, J.; Grover, L.; Gaunt, T.; Wright, A.; Gibson, I. Preparation of macroporous calcium phosphate cement tissue engineering scaffold. Biomaterials 2002, 23, 3063–3072. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Zhu, Y.; Wang, L.; Tomsia, A.P.; Mao, C. Phage Nanofibers Induce Vascularized Osteogenesis in 3D Printed Bone Scaffolds. Adv. Mater. 2014, 26, 4961–4966. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ito, K.; Nakamura, S.; Ueda, M.; Nagasaka, T. Promising Cell-Based Therapy for Bone Regeneration Using Stem Cells from Deciduous Teeth, Dental Pulp, and Bone Marrow. Cell Transplant. 2011, 20, 1003–1013. [Google Scholar] [CrossRef]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef]
- Putranti, N.A.R.; Kunimatsu, R.; Rikitake, K.; Hiraki, T.; Nakajima, K.; Abe, T.; Tsuka, Y.; Sakata, S.; Nakatani, A.; Nikawa, H.; et al. Combination of Carbonate Hydroxyapatite and Stem Cells from Human Deciduous Teeth Promotes Bone Regeneration by Enhancing BMP-2, VEGF and CD31 Expression in Immunodeficient Mice. Cells 2022, 11, 1914. [Google Scholar] [CrossRef]
- Prahasanti, C.; Subrata, L.H.; Saskianti, T.; Suardita, K.; Ernawati, D.S. Combined Hydroxyapatite Scaffold and Stem Cell from Human Exfoliated Deciduous Teeth Modulating Alveolar Bone Regeneration via Regulating Receptor Activator of Nuclear Factor-Kb and Osteoprotegerin System. Iran. J. Med. Sci. 2019, 44, 415–421. [Google Scholar] [CrossRef]
- Dai, P.; Qi, G.; Zhu, M.; Du, Q.; Wang, K.; Gao, Y.; Li, M.; Feng, X.; Zhang, X. Periodontal ligament stem cell tissue engineering scaffolds can guide and promote canine periodontal tissue regeneration. Front. Vet. Sci. 2024, 11, 1465879. [Google Scholar] [CrossRef]
- Ji, S.; Guvendiren, M. 3D Printed Wavy Scaffolds Enhance Mesenchymal Stem Cell Osteogenesis. Micromachines 2019, 11, 31. [Google Scholar] [CrossRef]
- Omerkić, S.; Omerkić Dautović, D. Opportunities and challenges of dental stem cells in transforming healthcare through regenerative medicine. Discov. Med. 2025, 2, 126. [Google Scholar] [CrossRef]
- Tomar, G.B.; Srivastava, R.K.; Gupta, N.; Barhanpurkar, A.P.; Pote, S.T.; Jhaveri, H.M.; Mishra, G.C.; Wani, M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010, 393, 377–383. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Chen, C.; Akiyama, K.; Xu, X.; Chee, W.W.L.; Schricker, S.R.; Shi, S. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.; Oreffo, R. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cells Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef]
- Saran, U.; Gemini Piperni, S.; Chatterjee, S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 2014, 561, 109–117. [Google Scholar] [CrossRef]
- Almubarak, S.; Nethercott, H.; Freeberg, M.; Beaudon, C.; Jha, A.; Jackson, W.; Marcucio, R.; Miclau, T.; Healy, K.; Bahney, C. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2016, 83, 197–209. [Google Scholar] [CrossRef]
- Huang, J.; Han, Q.; Cai, M.; Zhu, J.; Li, L.; Yu, L.; Wang, Z.; Fan, G.; Zhu, Y.; Lu, J.; et al. Effect of Angiogenesis in Bone Tissue Engineering. Ann. Biomed. Eng. 2022, 50, 898–913. [Google Scholar] [CrossRef] [PubMed]
- Altyar, A.E.; El-Sayed, A.; Abdeen, A.; Piscopo, M.; Mousa, S.A.; Najda, A.; Abdel-Daim, M.M. Future regenerative medicine developments and their therapeutic applications. Biomed. Pharmacother. 2023, 158, 114131. [Google Scholar] [CrossRef]
- Stegen, S.; Van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Portal-Núñez, S. Role of angiogenesis on bone formation. Histol. Histopathol. 2012, 27, 559–566. [Google Scholar] [CrossRef]
- Li, A.; Sasaki, J.I.; Abe, G.L.; Katata, C.; Sakai, H.; Imazato, S. Vascularization of a Bone Organoid Using Dental Pulp Stem Cells. Stem Cells Int. 2023, 2023, 5367887. [Google Scholar] [CrossRef]
- Burkus, J.K.; Transfeldt, E.E.; Kitchel, S.H.; Watkins, R.G.; Balderston, R.A. Clinical and Radiographic Outcomes of Anterior Lumbar Interbody Fusion Using Recombinant Human Bone Morphogenetic Protein-2. Spine 2002, 27, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.D.; Baffes, G.C.; Wolfe, M.W.; Sampath, T.K.; Rueger, D.C.; Whitecloud, T.S. The effect of recombinant human osteogenic protein-1 on healing of large segmental bone defects. J. Bone Jt. Surg. 1994, 76, 827–838. [Google Scholar] [CrossRef]
- Sampath, T.K.; Maliakal, J.C.; Hauschka, P.V.; Jones, W.K.; Sasak, H.; Tucker, R.F.; White, K.H.; Coughlin, J.E.; Tucker, M.M.; Pang, R.H. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J. Biol. Chem. 1992, 267, 20352–20362. [Google Scholar] [CrossRef]
- Carano, R.A.; Filvaroff, E.H. Angiogenesis and bone repair. Drug Discov. Today 2003, 8, 980–989. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Liu, K.; Gao, F. Hydrogel scaffolds in bone regeneration: Their promising roles in angiogenesis. Front. Pharmacol. 2023, 14, 1050954. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Wang, Y.; Liu, Z.; Li, Z.; Li, J.; Chen, Q.; Meng, Q.; Shu, W.W.; Wu, J.; et al. Endothelialized microvessels fabricated by microfluidics facilitate osteogenic differentiation and promote bone repair. Acta Biomater. 2022, 142, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef]
- Montesano, R.; Vassalli, J.D.; Baird, A.; Guillemin, R.; Orci, L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 7297–7301. [Google Scholar] [CrossRef]
- Globus, R.K.; Patterson-Buckendahl, P.; Gospodarowicz, D. Regulation of Bovine Bone Cell Proliferation by Fibroblast Growth Factor and Transforming Growth Factorβ. Endocrinology 1988, 123, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Kawaguchi, H.; Hanada, K.; Aoyama, I.; Hiyama, Y.; Nakamura, T.; Kuzutani, K.; Tamura, M.; Kurokawa, T.; Nakamura, K. Single local injection of recombinant fibroblast growth factor-2 stimulates healing of segmental bone defects in rabbits. J. Orthop. Res. 1998, 16, 654–659. [Google Scholar] [CrossRef]
- Hollinger, J.O.; Onikepe, A.O.; MacKrell, J.; Einhorn, T.; Bradica, G.; Lynch, S.; Hart, C.E. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet-derived growth factor-bb and an injectable beta-tricalcium phosphate/collagen matrix. J. Orthop. Res. 2008, 26, 83–90. [Google Scholar] [CrossRef]
- Kaigler, D.; Avila, G.; Wisner-Lynch, L.; Nevins, M.L.; Nevins, M.; Rasperini, G.; Lynch, S.E.; Giannobile, W.V. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin. Biol. Ther. 2011, 11, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Deuel, T.F.; Senior, R.M.; Huang, J.S.; Griffin, G.L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J. Clin. Investig. 1982, 69, 1046–1049. [Google Scholar] [CrossRef]
- Guo, P.; Hu, B.; Gu, W.; Xu, L.; Wang, D.; Huang, H.J.S.; Cavenee, W.K.; Cheng, S.Y. Platelet-Derived Growth Factor-B Enhances Glioma Angiogenesis by Stimulating Vascular Endothelial Growth Factor Expression in Tumor Endothelia and by Promoting Pericyte Recruitment. Am. J. Pathol. 2003, 162, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Deckers, M.M.L.; Van Bezooijen, R.L.; Van Der Horst, G.; Hoogendam, J.; Van Der Bent, C.; Papapoulos, S.E.; Löwik, C.W.G.M. Bone Morphogenetic Proteins Stimulate Angiogenesis through Osteoblast-Derived Vascular Endothelial Growth Factor A. Endocrinology 2002, 143, 1545–1553. [Google Scholar] [CrossRef]
- Yeh, L.C.C.; Lee, J.C. Osteogenic protein-1 increases gene expression of vascular endothelial growth factor in primary cultures of fetal rat calvaria cells. Mol. Cell. Endocrinol. 1999, 153, 113–124. [Google Scholar] [CrossRef]
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef][Green Version]
- Maes, C. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J. Clin. Investig. 2006, 116, 1230–1242. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Dunning, S.P.; Bunn, H.F. Regulation of the Erythropoietin Gene: Evidence That the Oxygen Sensor Is a Heme Protein. Science 1988, 242, 1412–1415. [Google Scholar] [CrossRef]
- Steinbrech, D.S.; Mehrara, B.J.; Saadeh, P.B.; Greenwald, J.A.; Spector, J.A.; Gittes, G.K.; Longaker, M.T. VEGF expression in an osteoblast-like cell line is regulated by a hypoxia response mechanism. Am. J. -Physiol.-Cell Physiol. 2000, 278, C853–C860. [Google Scholar] [CrossRef]
- Sharma, M.B.; Limaye, L.S.; Kale, V.P. Mimicking the functional hematopoietic stem cell niche in vitro: Recapitulation of marrow physiology by hydrogel-based three-dimensional cultures of mesenchymal stromal cells. Haematologica 2012, 97, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.; Suri, C.; Smith, K.; McClain, J.; Sato, T.N.; Yancopoulos, G.D.; McDonald, D.M. Leakage-Resistant Blood Vessels in Mice Transgenically Overexpressing Angiopoietin-1. Science 1999, 286, 2511–2514. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zou, T.; Yao, Q.; Zhang, Y.; Zhao, Y.; Zhang, C. Hypoxia-mimicking cobalt-doped multi-walled carbon nanotube nanocomposites enhance the angiogenic capacity of stem cells from apical papilla. Mater. Sci. Eng. C 2021, 120, 111797. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, P.; Jiang, G.; Zhang, M.; Yu, F.; Dong, X.; Wang, L.; Chen, Y.; Zhang, W.; Qi, Y.; et al. A novel magnesium ion-incorporating dual-crosslinked hydrogel to improve bone scaffold-mediated osteogenesis and angiogenesis. Mater. Sci. Eng. C 2021, 121, 111868. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, B.; Yang, H.; Han, Y.; Wu, Q.; Dai, K.; Zheng, Y. Biodegradable ZnLiCa ternary alloys for critical-sized bone defect regeneration at load-bearing sites: In vitro and in vivo studies. Bioact. Mater. 2021, 6, 3999–4013. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, G.; Ren, S.; Zhang, X.; Li, C.; Wu, W.; Wang, H.; Liu, H.; Zhou, H.; Chen, Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020, 11, 38. [Google Scholar] [CrossRef]
- Yang, H.; Shin, S.; Ahn, J.; Choi, Y.; Kim, K.H.; Chung, C.J. Local Injection of Pulp Cells Enhances Wound Healing during the Initial Proliferative Phase through the Stimulation of Host Angiogenesis. J. Endod. 2013, 39, 788–794. [Google Scholar] [CrossRef]
- Huang, G.T.J.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/Progenitor Cell–Mediated De Novo Regeneration of Dental Pulp with Newly Deposited Continuous Layer of Dentin in an In Vivo Model. Tissue Eng. Part A 2010, 16, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Bento, L.; Zhang, Z.; Imai, A.; Nör, F.; Dong, Z.; Shi, S.; Araujo, F.; Nör, J. Endothelial Differentiation of SHED Requires MEK1/ERK Signaling. J. Dent. Res. 2013, 92, 51–57. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Chen, C.; Xu, X.; Akiyama, K.; Ansari, S.; Zadeh, H.H.; Shi, S. Bone Regeneration Potential of Stem Cells Derived from Periodontal Ligament or Gingival Tissue Sources Encapsulated in RGD-Modified Alginate Scaffold. Tissue Eng. Part A 2013, 20, 611–621. [Google Scholar] [CrossRef]
- Kempen, D.H.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Lafage-Proust, M.H.; Plouët, J.; Bloomfield, S.; Alexandre, C.; Vico, L. Increase of Both Angiogenesis and Bone Mass in Response to Exercise Depends on VEGF. J. Bone Miner. Res. 2004, 19, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Kaigler, D.; Rice, K.G.; Krebsbach, P.H.; Mooney, D.J. Combined Angiogenic and Osteogenic Factor Delivery Enhances Bone Marrow Stromal Cell-Driven Bone Regeneration. J. Bone Miner. Res. 2005, 20, 848–857. [Google Scholar] [CrossRef]
- Herford, A.S.; Stoffella, E.; Tandon, R. Reconstruction of Mandibular Defects Using Bone Morphogenic Protein: Can Growth Factors Replace the Need for Autologous Bone Grafts? A Systematic Review of the Literature. Plast. Surg. Int. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Herford, A.S.; Lu, M.; Buxton, A.N.; Kim, J.; Henkin, J.; Boyne, P.J.; Caruso, J.M.; Rungcharassaeng, K.; Hong, J. Recombinant Human Bone Morphogenetic Protein 2 Combined With an Osteoconductive Bulking Agent for Mandibular Continuity Defects in Nonhuman Primates. J. Oral Maxillofac. Surg. 2012, 70, 703–716. [Google Scholar] [CrossRef]
- Jahanbin, A.; Rashed, R.; Alamdari, D.H.; Koohestanian, N.; Ezzati, A.; Kazemian, M.; Saghafi, S.; Raisolsadat, M.A. Success of Maxillary Alveolar Defect Repair in Rats Using Osteoblast-Differentiated Human Deciduous Dental Pulp Stem Cells. J. Oral Maxillofac. Surg. 2016, 74, 829.e1–829.e9. [Google Scholar] [CrossRef]
- Wang, F.; Yu, M.; Yan, X.; Wen, Y.; Zeng, Q.; Yue, W.; Yang, P.; Pei, X. Gingiva-Derived Mesenchymal Stem Cell-Mediated Therapeutic Approach for Bone Tissue Regeneration. Stem Cells Dev. 2011, 20, 2093–2102. [Google Scholar] [CrossRef]
- Probst, F.A.; Fliefel, R.; Burian, E.; Probst, M.; Eddicks, M.; Cornelsen, M.; Riedl, C.; Seitz, H.; Aszódi, A.; Schieker, M.; et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep. 2020, 10, 2062. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.; Sonoyama, W.; Yamaza, T.; Coppe, C.; Kikuiri, T.; Akiyama, K.; Lee, J.; Shi, S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008, 14, 428–434. [Google Scholar] [CrossRef]
- Rezai-Rad, M.; Bova, J.F.; Orooji, M.; Pepping, J.; Qureshi, A.; Del Piero, F.; Hayes, D.; Yao, S. Evaluation of bone regeneration potential of dental follicle stem cells for treatment of craniofacial defects. Cytotherapy 2015, 17, 1572–1581. [Google Scholar] [CrossRef]
- Johnson, Z.M.; Yuan, Y.; Li, X.; Jashashvili, T.; Jamieson, M.; Urata, M.; Chen, Y.; Chai, Y. Mesenchymal Stem Cells and Three-Dimensional-Osteoconductive Scaffold Regenerate Calvarial Bone in Critical Size Defects in Swine. Stem Cells Transl. Med. 2021, 10, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Khosronejad, A.; Arabion, H.; Iraji, A.; Mokhtarzadegan, M.; Daneshi, S.S.; Asadi-Yousefabad, S.L.; Zare, S.; Nowzari, F.; Abbaspour, S.; Akbarizadeh, F.; et al. Mandibular bone defect healing using polylactic acid–nano-hydroxyapatite–gelatin scaffold loaded with hesperidin and dental pulp stem cells in rat. Tissue Cell 2025, 93, 102700. [Google Scholar] [CrossRef] [PubMed]
- Petridis, X.; Diamanti, E.; Trigas, G.C.; Kalyvas, D.; Kitraki, E. Bone regeneration in critical-size calvarial defects using human dental pulp cells in an extracellular matrix-based scaffold. J. Cranio-Maxillofac. Surg. 2015, 43, 483–490. [Google Scholar] [CrossRef]
- Dehghanian, F.; Soltani, Z.; Farsinejad, A.; Khaksari, M.; Jafari, E.; Darakhshani, A.; Sabet, N.; Bashiri, H. The Effect of Oral Mucosal Mesenchymal Stem Cells on Pathological and Long-Term Outcomes in Experimental Traumatic Brain Injury. BioMed Res. Int. 2022, 2022, 4065118. [Google Scholar] [CrossRef]
- Alge, D.L.; Zhou, D.; Adams, L.L.; Wyss, B.K.; Shadday, M.D.; Woods, E.J.; Gabriel Chu, T.M.; Goebel, W.S. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J. Tissue Eng. Regen. Med. 2010, 4, 73–81. [Google Scholar] [CrossRef]
- Xiao, C.; Lu, D.; Chen, J.; Chen, X.; Lin, H.; Huang, M.; Cheng, S.; Wang, Y.; Liu, Q.; Zheng, H. Human Olfactory Mesenchymal Stem Cells Are a Novel Candidate for Neurological Autoimmune Disease. Front. Pharmacol. 2021, 12, 770884. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Xu, X.; Chen, C.; Akiyama, K.; Snead, M.L.; Shi, S. Dental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regeneration. Acta Biomater. 2013, 9, 9343–9350. [Google Scholar] [CrossRef]
- Mata, M.; Milian, L.; Oliver, M.; Zurriaga, J.; Sancho-Tello, M.; de Llano, J.J.M.; Carda, C. In Vivo Articular Cartilage Regeneration Using Human Dental Pulp Stem Cells Cultured in an Alginate Scaffold: A Preliminary Study. Stem Cells Int. 2017, 2017, 8309256. [Google Scholar] [CrossRef]
- Zhu, F.; Meng, Q.; Yu, Y.; Shao, L.; Shen, Z. Adult Cardiomyocyte Proliferation: A New Insight for Myocardial Infarction Therapy. J. Cardiovasc. Transl. Res. 2021, 14, 457–466. [Google Scholar] [CrossRef]
- Cochain, C.; Channon, K.M.; Silvestre, J.S. Angiogenesis in the Infarcted Myocardium. Antioxid. Redox Signal. 2013, 18, 1100–1113. [Google Scholar] [CrossRef]
- Serbo, J.V.; Gerecht, S. Vascular tissue engineering: Biodegradable scaffold platforms to promote angiogenesis. Stem Cell Res. Ther. 2013, 4, 8. [Google Scholar] [CrossRef]
- Gandia, C.; Armiñan, A.; García-Verdugo, J.M.; Lledó, E.; Ruiz, A.; Miñana, M.D.; Sanchez-Torrijos, J.; Payá, R.; Mirabet, V.; Carbonell-Uberos, F.; et al. Human Dental Pulp Stem Cells Improve Left Ventricular Function, Induce Angiogenesis, and Reduce Infarct Size in Rats with Acute Myocardial Infarction. Stem Cells 2008, 26, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Zhou, J.; Solomon, C.; Zheng, Y.; Suzuki, T.; Chen, M.; Song, S.; Jiang, N.; Cho, S.; Mao, J.J. Effects of Growth Factors on Dental Stem/Progenitor Cells. Dent. Clin. N. Am. 2012, 56, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cho, H.J.; Han, Y.; Lee, S.H. Mid- to long-term efficacy and safety of stem cell therapy for acute myocardial infarction: A systematic review and meta-analysis. Stem Cell Res. Ther. 2024, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Castellani, M.; Piccaluga, E.; Pusineri, E.; Palatresi, S.; Longari, V.; Canzi, C.; Sacchi, E.; Rossi, E.; Rech, R.; et al. Myocardial blood flow and infarct size after CD133+ cell injection in large myocardial infarction with good recanalization and poor reperfusion: Results from a randomized controlled trial. J. Cardiovasc. Med. 2011, 12, 239–248. [Google Scholar] [CrossRef]
- Mansour, S.; Roy, D.C.; Bouchard, V.; Stevens, L.M.; Gobeil, F.; Rivard, A.; Leclerc, G.; Reeves, F.; Noiseux, N. One-Year Safety Analysis of the COMPARE-AMI Trial: Comparison of Intracoronary Injection of CD133+ Bone Marrow Stem Cells to Placebo in Patients after Acute Myocardial Infarction and Left Ventricular Dysfunction. Bone Marrow Res. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Linard, C.; Brachet, M.; L’homme, B.; Strup-Perrot, C.; Busson, E.; Bonneau, M.; Lataillade, J.J.; Bey, E.; Benderitter, M. Long-term effectiveness of local BM-MSCs for skeletal muscle regeneration: A proof of concept obtained on a pig model of severe radiation burn. Stem Cell Res. Ther. 2018, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Owston, H.; Giannoudis, P.V.; Jones, E. Do skeletal muscle MSCs in humans contribute to bone repair? A systematic review. Injury 2016, 47, S3–S15. [Google Scholar] [CrossRef]
- Sandonà, M.; Di Pietro, L.; Esposito, F.; Ventura, A.; Silini, A.R.; Parolini, O.; Saccone, V. Mesenchymal Stromal Cells and Their Secretome: New Therapeutic Perspectives for Skeletal Muscle Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 652970. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef]
- Zhang, M.H.; Yu, L.M.; Zhang, W.H.; Deng, J.J.; Sun, B.J.; Chen, M.H.; Huang, W.; Li, J.; He, H.; Han, X.X.; et al. Noggin Combined With Human Dental Pulp Stem Cells to Promote Skeletal Muscle Regeneration. Stem Cells Int. 2024, 2024, 2812390. [Google Scholar] [CrossRef]
- Martínez-Sarrà, E.; Montori, S.; Gil-Recio, C.; Núñez-Toldrà, R.; Costamagna, D.; Rotini, A.; Atari, M.; Luttun, A.; Sampaolesi, M. Human dental pulp pluripotent-like stem cells promote wound healing and muscle regeneration. Stem Cell Res. Ther. 2017, 8, 175. [Google Scholar] [CrossRef]
- Nosrat, I.V.; Widenfalk, J.; Olson, L.; Nosrat, C.A. Dental Pulp Cells Produce Neurotrophic Factors, Interact with Trigeminal Neurons in Vitro, and Rescue Motoneurons after Spinal Cord Injury. Dev. Biol. 2001, 238, 120–132. [Google Scholar] [CrossRef]
- Gonmanee, T.; Thonabulsombat, C.; Vongsavan, K.; Sritanaudomchai, H. Differentiation of stem cells from human deciduous and permanent teeth into spiral ganglion neuron-like cells. Arch. Oral Biol. 2018, 88, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Gervois, P.; Struys, T.; Hilkens, P.; Bronckaers, A.; Ratajczak, J.; Politis, C.; Brône, B.; Lambrichts, I.; Martens, W. Neurogenic Maturation of Human Dental Pulp Stem Cells Following Neurosphere Generation Induces Morphological and Electrophysiological Characteristics of Functional Neurons. Stem Cells Dev. 2015, 24, 296–311. [Google Scholar] [CrossRef]
- Pisciotta, A.; Bertoni, L.; Riccio, M.; Mapelli, J.; Bigiani, A.; La Noce, M.; Orciani, M.; De Pol, A.; Carnevale, G. Use of a 3D Floating Sphere Culture System to Maintain the Neural Crest-Related Properties of Human Dental Pulp Stem Cells. Front. Physiol. 2018, 9, 547. [Google Scholar] [CrossRef]
- Ansari, S.; Diniz, I.M.; Chen, C.; Sarrion, P.; Tamayol, A.; Wu, B.M.; Moshaverinia, A. Human Periodontal Ligament- and Gingiva-derived Mesenchymal Stem Cells Promote Nerve Regeneration When Encapsulated in Alginate/Hyaluronic Acid 3D Scaffold. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Abe, S.; Hamada, K.; Miura, M.; Yamaguchi, S. Neural crest stem cell property of apical pulp cells derived from human developing tooth. Cell Biol. Int. 2012, 36, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Sugiyama, M.; Hattori, H.; Wakita, H.; Wakabayashi, T.; Ueda, M. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng. Part A 2013, 19, 24–29. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Sun, Z.; Wang, X.; Yang, H.; Shi, S.; Wang, S. Stem Cells from Human-Exfoliated Deciduous Teeth Can Differentiate into Dopaminergic Neuron-Like Cells. Stem Cells Dev. 2010, 19, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.D.; Kim, K.H.; Lee, Y.M.; Ku, Y.; Seol, Y.J. Dental-derived cells for regenerative medicine: Stem cells, cell reprogramming, and transdifferentiation. J. Periodontal Implant. Sci. 2022, 52, 437. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Shao, J.; Ge, S. Immunomodulatory functions of oral mesenchymal stem cells: Novel force for tissue regeneration and disease therapy. J. Leukoc. Biol. 2021, 110, 539–552. [Google Scholar] [CrossRef]
- Hong, R.; Wang, Z.; Sui, A.; Liu, X.; Fan, C.; Lipkind, S.; Xu, Q. Gingival mesenchymal stem cells attenuate pro-inflammatory macrophages stimulated with oxidized low-density lipoprotein and modulate lipid metabolism. Arch. Oral Biol. 2019, 98, 92–98. [Google Scholar] [CrossRef]
- Tang, L.; Li, N.; Xie, H.; Jin, Y. Characterization of mesenchymal stem cells from human normal and hyperplastic gingiva. J. Cell. Physiol. 2011, 226, 832–842. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, A.L.; Shi, S.; Hill, C.; Wilder-Smith, P.; Krasieva, T.B.; Le, A.D. Three-Dimensional Spheroid Culture of Human Gingiva-Derived Mesenchymal Stem Cells Enhances Mitigation of Chemotherapy-Induced Oral Mucositis. Stem Cells Dev. 2012, 21, 937–947. [Google Scholar] [CrossRef]
- Israel, E.; Reddel, H.K. Severe and Difficult-to-Treat Asthma in Adults. N. Engl. J. Med. 2017, 377, 965–976. [Google Scholar] [CrossRef]
- Wakayama, H.; Hashimoto, N.; Matsushita, Y.; Matsubara, K.; Yamamoto, N.; Hasegawa, Y.; Ueda, M.; Yamamoto, A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy 2015, 17, 1119–1129. [Google Scholar] [CrossRef]
- Komlósi, Z.I.; van de Veen, W.; Kovács, N.; Szűcs, G.; Sokolowska, M.; O’Mahony, L.; Akdis, M.; Akdis, C.A. Cellular and molecular mechanisms of allergic asthma. Mol. Asp. Med. 2022, 85, 100995. [Google Scholar] [CrossRef]
- Genç, D.; Zibandeh, N.; Nain, E.; Gökalp, M.; Özen, A.O.; Göker, M.K.; Akkoç, T. Dental follicle mesenchymal stem cells down-regulate Th2-mediated immune response in asthmatic patients mononuclear cells. Clin. Exp. Allergy 2018, 48, 663–678. [Google Scholar] [CrossRef]
- Genç, D.; Zibandeh, N.; Nain, E.; Arığ, U.; Göker, K.; Aydıner, E.K.; Akkoç, T. IFN-γ stimulation of dental follicle mesenchymal stem cells modulates immune response of CD4+ T lymphocytes in Der p1+ asthmatic patients in vitro. Allergol. Immunopathol. 2019, 47, 467–476. [Google Scholar] [CrossRef]
- Ehrenstein, M.R.; Evans, J.G.; Singh, A.; Moore, S.; Warnes, G.; Isenberg, D.A.; Mauri, C. Compromised Function of Regulatory T Cells in Rheumatoid Arthritis and Reversal by Anti-TNFα Therapy. J. Exp. Med. 2004, 200, 277–285. [Google Scholar] [CrossRef]
- Zibandeh, N. IFN-γ stimulated dental follicle mesenchymal stem cells regulate activated lymphocyte response in rheumatoid arthritis patients in vitro. Turk. J. Med. Sci. 2019, 49, 1779–1788. [Google Scholar] [CrossRef]
- Chandrashekara, S. The treatment strategies of autoimmune disease may need a different approach from conventional protocol: A review. Indian J. Pharmacol. 2012, 44, 665. [Google Scholar] [CrossRef]
- Wnorowski, A.; Yang, H.; Wu, J.C. Progress, obstacles, and limitations in the use of stem cells in organ-on-a-chip models. Adv. Drug Deliv. Rev. 2019, 140, 3–11. [Google Scholar] [CrossRef]
- Igarashi, A.; Segoshi, K.; Sakai, Y.; Pan, H.; Kanawa, M.; Higashi, Y.; Sugiyama, M.; Nakamura, K.; Kurihara, H.; Yamaguchi, S.; et al. Selection of Common Markers for Bone Marrow Stromal Cells from Various Bones Using Real-Time RT-PCR: Effects of Passage Number and Donor Age. Tissue Eng. 2007, 13, 2405–2417. [Google Scholar] [CrossRef]
- Borstlap, W.A.; Heidbuchel, K.L.; Freihofer, H.P.M.; Kuijpers-Jagtman, A.M. Early secondary bone grafting of alveolar cleft defects. J. Cranio-Maxillofac. Surg. 1990, 18, 201–205. [Google Scholar] [CrossRef]
- Koole, R.; Bosker, H.; Van Der Dussen, F.N. Late secondary autogenous bone grafting in cleft patients comparing mandibular (ectomesenchymal) and iliac crest (mesenchymal) grafts. J. Cranio-Maxillofac. Surg. 1989, 17, 28–30. [Google Scholar] [CrossRef]
- Chai, Y.; Jiang, X.; Ito, Y.; Bringas, P.; Han, J.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 2000, 127, 1671–1679. [Google Scholar] [CrossRef]
- Mackie, E.; Ahmed, Y.; Tatarczuch, L.; Chen, K.S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Dörfer, C.E. Gingival Mesenchymal Stem/Progenitor Cells: A Unique Tissue Engineering Gem. Stem Cells Int. 2016, 2016, 7154327. [Google Scholar] [CrossRef]
- Kim, M.K.; Paek, K.; Woo, S.M.; Kim, J.A. Bone-on-a-Chip: Biomimetic Models Based on Microfluidic Technologies for Biomedical Applications. Biomater. Sci. Eng. 2023, 9, 3058–3073. [Google Scholar] [CrossRef]
- Gao, J.; Li, L. Enhancement of neural regeneration as a therapeutic strategy for Alzheimer’s disease (Review). Exp. Ther. Med. 2023, 26, 444. [Google Scholar] [CrossRef]
- Ryu, N.E.; Lee, S.H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef]
- Hsu, S.h.; Huang, G.S.; Feng, F. Isolation of the multipotent MSC subpopulation from human gingival fibroblasts by culturing on chitosan membranes. Biomaterials 2012, 33, 2642–2655. [Google Scholar] [CrossRef]
- Hsu, S.h.; Huang, G.S.; Lin, S.Y.F.; Feng, F.; Ho, T.T.; Liao, Y.C. Enhanced Chondrogenic Differentiation Potential of Human Gingival Fibroblasts by Spheroid Formation on Chitosan Membranes. Tissue Eng. Part A 2012, 18, 67–79. [Google Scholar] [CrossRef]
- Khojasteh, A.; Hosseinpour, S.; Rad, M.R.; Alikhasi, M. Buccal Fat Pad–Derived Stem Cells in Three-Dimensional Rehabilitation of Large Alveolar Defects: A Report of Two Cases. J. Oral Implantol. 2019, 45, 45–54. [Google Scholar] [CrossRef]
- Camacho-Alonso, F.; Tudela-Mulero, M.R.; Navarro, J.A.; Buendía, A.J.; Mercado-Díaz, A.M. Use of buccal fat pad-derived stem cells cultured on bioceramics for repair of critical-sized mandibular defects in healthy and osteoporotic rats. Clin. Oral Investig. 2022, 26, 5389–5408. [Google Scholar] [CrossRef]
- University of Turin, Italy. Periodontal Regeneration Using Dental Pulp Stem Cells (DPSCs). ClinicalTrials.gov. 2017. Available online: https://clinicaltrials.gov/study/NCT03386877 (accessed on 22 August 2025).
- JCR Pharmaceuticals Co., Ltd., Japan. A Randomized Placebo-controlled Multicenter Trial to Evaluate the Efficacy and Safety of JTR-161, Allogeneic Human Dental Pulp Stem Cell, in Patients With Acute Ischemic stRoke (J-REPAIR) (J-REPAIR). ClinicalTrials.gov. 2022. Available online: https://clinicaltrials.gov/study/NCT04608838 (accessed on 30 June 2025).
- Central Hospital, Nancy, France. Dental Stem Cells and Bone Tissue Engineering (CELSORDINO) (CELSORDINO). ClinicalTrials.gov. 2017. Available online: https://clinicaltrials.gov/study/NCT03194451 (accessed on 22 August 2025).
- Translational Research Center for Medical Innovation, Kobe, Hyogo, Japan. Clinical Trials of Regeneration for Periodontal Tissue. ClinicalTrials.gov. 2009. Available online: https://clinicaltrials.gov/study/NCT00221130 (accessed on 22 August 2025).
- Air Force Military Medical University, China. Periodontal Tissue Regeneration Using Autologous Periodontal Ligament Stem Cells (PDLSC). ClinicalTrials.gov. 2011. Available online: https://clinicaltrials.gov/study/NCT01357785 (accessed on 22 August 2025).
- Air Force Military Medical University, China. Periodontal Ligament Stem Cell Implantation in the Treatment of Periodontitis. ClinicalTrials.gov. 2010. Available online: https://clinicaltrials.gov/study/NCT01082822 (accessed on 22 August 2025).
- Baskent University. Effect of Dental Pulp Stem Cells and L-PRF After Impacted Third Molar Extraction. ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/study/NCT04641533 (accessed on 22 August 2025).
- CAR-T (Shanghai) Biotechnology Co., Ltd. Stem Cells From Human Exfoliated Teeth in Treatment of Type 2 Diabetes. ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/study/NCT03658655 (accessed on 22 August 2025).
- Matsumoto, R.; Yamamoto, T.; Takahashi, Y. Complex Organ Construction from Human Pluripotent Stem Cells for Biological Research and Disease Modeling with New Emerging Techniques. Int. J. Mol. Sci. 2021, 22, 10184. [Google Scholar] [CrossRef]
- Mansoorifar, A.; Gordon, R.; Bergan, R.C.; Bertassoni, L.E. Bone-on-a-Chip: Microfluidic Technologies and Microphysiologic Models of Bone Tissue. Adv. Funct. Mater. 2021, 31, 2006796. [Google Scholar] [CrossRef]
- Baba, S.; Yamada, Y.; Komuro, A.; Yotsui, Y.; Umeda, M.; Shimuzutani, K.; Nakamura, S. Phase I/II Trial of Autologous Bone Marrow Stem Cell Transplantation with a Three-Dimensional Woven-Fabric Scaffold for Periodontitis. Stem Cells Int. 2016, 2016, 6205910. [Google Scholar] [CrossRef]
- Sieber, S.; Wirth, L.; Cavak, N.; Koenigsmark, M.; Marx, U.; Lauster, R.; Rosowski, M. Bone marrow-on-a-chip: Long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J. Tissue Eng. Regen. Med. 2018, 12, 479–489. [Google Scholar] [CrossRef]
- Fageeh, H.N. Preliminary Evaluation of Proliferation, Wound Healing Properties, Osteogenic and Chondrogenic Potential of Dental Pulp Stem Cells Obtained from Healthy and Periodontitis Affected Teeth. Cells 2021, 10, 2118. [Google Scholar] [CrossRef]
- Qu, C.; Brohlin, M.; Kingham, P.J.; Kelk, P. Evaluation of growth, stemness, and angiogenic properties of dental pulp stem cells cultured in cGMP xeno-/serum-free medium. Cell Tissue Res. 2020, 380, 93–105. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Dong, J.; Jiao, M.; Gu, Y.; Chen, L.; Yuan, N.; Wang, J.; Yang, D.; Meng, F. Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics. Open Life Sci. 2025, 20, 20220998. [Google Scholar] [CrossRef]
- Nesic, D.; Schaefer, B.M.; Sun, Y.; Saulacic, N.; Sailer, I. 3D Printing Approach in Dentistry: The Future for Personalized Oral Soft Tissue Regeneration. J. Clin. Med. 2020, 9, 2238. [Google Scholar] [CrossRef]
- Abedin Zadeh, M.; Khoder, M.; Al-Kinani, A.A.; Younes, H.M.; Alany, R.G. Retinal cell regeneration using tissue engineered polymeric scaffolds. Drug Discov. Today 2019, 24, 1669–1678. [Google Scholar] [CrossRef]
- Shindo, S.; Savitri, I.J.; Ishii, T.; Ikeda, A.; Pierrelus, R.; Heidari, A.; Okubo, K.; Nakamura, S.; Kandalam, U.; Rawas-Qalaji, M.; et al. Dual-Function Semaphorin 4D Released by Platelets: Suppression of Osteoblastogenesis and Promotion of Osteoclastogenesis. Int. J. Mol. Sci. 2022, 23, 2938. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wang, S.; Zhao, J.; Xu, L.; Zhu, C.; Zeng, D.; Chen, J.; Zhang, Z.; Kaplan, D.L.; et al. The use of injectable sonication-induced silk hydrogel for VEGF165 and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials 2011, 32, 9415–9424. [Google Scholar] [CrossRef]
- Caballero-Aguilar, L.M.; Duchi, S.; Quigley, A.; Onofrillo, C.; Di Bella, C.; Moulton, S.E. Microencapsulation of growth factors by microfluidic system. MethodsX 2021, 8, 101324. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, R.; Wu, C.; Wang, L.; Zhang, C. Exosome: A Novel Approach to Stimulate Bone Regeneration through Regulation of Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2016, 17, 712. [Google Scholar] [CrossRef]
- Alvites, R.D.; Branquinho, M.V.; Caseiro, A.R.; Amorim, I.; Santos Pedrosa, S.; Rêma, A.; Faria, F.; Porto, B.; Oliveira, C.; Teixeira, P.; et al. Rat Olfactory Mucosa Mesenchymal Stem/Stromal Cells (OM-MSCs): A Characterization Study. Int. J. Cell Biol. 2020, 2020, 2938258. [Google Scholar] [CrossRef]
- Santos Rosalem, G.; Gonzáles Torres, L.A.; De Las Casas, E.B.; Mathias, F.A.S.; Ruiz, J.C.; Carvalho, M.G.R. Microfluidics and organ-on-a-chip technologies: A systematic review of the methods used to mimic bone marrow. PLoS ONE 2020, 15, e0243840. [Google Scholar] [CrossRef]
- Lv, Z.; Cai, X.; Bian, Y.; Wei, Z.; Zhu, W.; Zhao, X.; Weng, X. Advances in Mesenchymal Stem Cell Therapy for Osteoarthritis: From Preclinical and Clinical Perspectives. Bioengineering 2023, 10, 195. [Google Scholar] [CrossRef]
- Schliephake, H.; Weich, H.A.; Dullin, C.; Gruber, R.; Frahse, S. Mandibular bone repair by implantation of rhBMP-2 in a slow release carrier of polylactic acid—An experimental study in rats. Biomaterials 2008, 29, 103–110. [Google Scholar] [CrossRef]
- Fujita, M.; Kinoshita, Y.; Sato, E.; Maeda, H.; Ozono, S.; Negishi, H.; Kawase, T.; Hiraoka, Y.; Takamoto, T.; Tabata, Y.; et al. Proliferation and Differentiation of Rat Bone Marrow Stromal Cells on Poly(glycolic acid)–Collagen Sponge. Tissue Eng. 2005, 11, 1346–1355. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Scaglione, S.; Martinetti, R.; Dolcini, L.; Beltrame, F.; Cancedda, R.; Quarto, R. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials 2006, 27, 3230–3237. [Google Scholar] [CrossRef]
- Komlev, V.; Peyrin, F.; Mastrogiacomo, M.; Cedola, A.; Papadimitropoulos, A.; Rustichelli, F.; Cancedda, R. Kinetics of In Vivo Bone Deposition by Bone Marrow Stromal Cells into Porous Calcium Phosphate Scaffolds: An X-Ray Computed Microtomography Study. Tissue Eng. 2006, 12, 3449–3458. [Google Scholar] [CrossRef]
- Cao, L.; Su, H.; Si, M.; Xu, J.; Chang, X.; Lv, J.; Zhai, Y. Tissue Engineering in Stomatology: A Review of Potential Approaches for Oral Disease Treatments. Front. Bioeng. Biotechnol. 2021, 9, 662418. [Google Scholar] [CrossRef]
- Harris, J.P.; Burrell, J.C.; Struzyna, L.A.; Chen, H.I.; Serruya, M.D.; Wolf, J.A.; Duda, J.E.; Cullen, D.K. Emerging regenerative medicine and tissue engineering strategies for Parkinson’s disease. NPJ Park. Dis. 2020, 6, 4. [Google Scholar] [CrossRef]
- Skardal, A.; Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Effect of Chemistry on Osteogenesis and Angiogenesis Towards Bone Tissue Engineering Using 3D Printed Scaffolds. Ann. Biomed. Eng. 2017, 45, 261–272. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Zhao, N.; Zhu, Y.; Qiu, Z.; Li, Q.; Zhou, Y.; Lin, Z.; Li, X.; Zeng, X.; et al. Integrating 3D Printing and Biomimetic Mineralization for Personalized Enhanced Osteogenesis, Angiogenesis, and Osteointegration. Appl. Mater. Interfaces 2018, 10, 42146–42154. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Guo, J.; Yang, L.; Liu, Y.; Sun, Q.; Li, R.; Yu, W. Hybrid composites of mesenchymal stem cell sheets, hydroxyapatite, and platelet-rich fibrin granules for bone regeneration in a rabbit calvarial critical-size defect model. Exp. Ther. Med. 2017, 13, 1891–1899. [Google Scholar] [CrossRef]
- Mankani, M.H.; Kuznetsov, S.A.; Wolfe, R.M.; Marshall, G.W.; Robey, P.G. In Vivo Bone Formation by Human Bone Marrow Stromal Cells: Reconstruction of the Mouse Calvarium and Mandible. Stem Cells 2006, 24, 2140–2149. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Diomede, F.; Gugliandolo, A.; Chiricosta, L.; Bramanti, P.; Merciaro, I.; Orsini, T.; Mazzon, E.; Trubiani, O. 3D Printing PLA/Gingival Stem Cells/ EVs Upregulate miR-2861 and -210 during Osteoangiogenesis Commitment. Int. J. Mol. Sci. 2019, 20, 3256. [Google Scholar] [CrossRef]
- Yuanzheng, C.; Yan, G.; Ting, L.; Yanjie, F.; Peng, W.; Nan, B. Enhancement of the Repair of Dog Alveolar Cleft by an Autologous Iliac Bone, Bone Marrow–Derived Mesenchymal Stem Cell, and Platelet-Rich Fibrin Mixture. Plast. Reconstr. Surg. 2015, 135, 1405–1412. [Google Scholar] [CrossRef]
- Caballero, M.; Morse, J.C.; Halevi, A.E.; Emodi, O.; Pharaon, M.R.; Wood, J.S.; Van Aalst, J.A. Juvenile Swine Surgical Alveolar Cleft Model to Test Novel Autologous Stem Cell Therapies. Tissue Eng. Part Methods 2015, 21, 898–908. [Google Scholar] [CrossRef]
- Nosrati, H.; Nosrati, M. Artificial Intelligence in Regenerative Medicine: Applications and Implications. Biomimetics 2023, 8, 442. [Google Scholar] [CrossRef]
- Capponi, S.; Wang, S. AI in cellular engineering and reprogramming. Biophys. J. 2024, 123, 2658–2670. [Google Scholar] [CrossRef]
- Lee, S.B.; Abdal Dayem, A.; Kmiecik, S.; Lim, K.M.; Seo, D.S.; Kim, H.T.; Kumar Biswas, P.; Do, M.; Kim, D.H.; Cho, S.G. Efficient improvement of the proliferation, differentiation, and anti-arthritic capacity of mesenchymal stem cells by simply culturing on the immobilized FGF2 derived peptide, 44-ERGVVSIKGV-53. J. Adv. Res. 2024, 62, 119–141. [Google Scholar] [CrossRef]
- Ertl, P.; Sticker, D.; Charwat, V.; Kasper, C.; Lepperdinger, G. Lab-on-a-chip technologies for stem cell analysis. Trends Biotechnol. 2014, 32, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, X.; Zeng, R.; Xu, F.; Li, X. Stem Cell Culture and Differentiation in Microfluidic Devices Toward Organ-on-a-Chip. Future Sci. OA 2017, 3, FSO187. [Google Scholar] [CrossRef]
- Paek, K.; Kim, S.; Tak, S.; Kim, M.K.; Park, J.; Chung, S.; Park, T.H.; Kim, J.A. A high-throughput biomimetic bone-on-a-chip platform with artificial intelligence-assisted image analysis for osteoporosis drug testing. Bioeng. Transl. Med. 2023, 8, e10313. [Google Scholar] [CrossRef]
- Zhang, Q.; Austin, R.H. Applications of Microfluidics in Stem Cell Biology. BioNanoScience 2012, 2, 277–286. [Google Scholar] [CrossRef]
- Wu, H.W.; Lin, C.C.; Lee, G.B. Stem cells in microfluidics. Biomicrofluidics 2011, 5, 013401. [Google Scholar] [CrossRef]
- Park, S.H.; Sim, W.Y.; Min, B.H.; Yang, S.S.; Khademhosseini, A.; Kaplan, D.L. Chip-Based Comparison of the Osteogenesis of Human Bone Marrow- and Adipose Tissue-Derived Mesenchymal Stem Cells under Mechanical Stimulation. PLoS ONE 2012, 7, e46689. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Alam, B.; Chopra, K. Applications of Alphafold and Artificial Intelligence in Biology and Medicine. p. 15. Available online: https://www.academia.edu/127996095 (accessed on 9 August 2025).
- Lee, S.; Kim, Y.G.; Jung, H.I.; Lim, J.S.; Nam, K.C.; Choi, H.S.; Kwak, B.S. Bone-on-a-chip simulating bone metastasis in osteoporosis. Biofabrication 2024, 16, 045025. [Google Scholar] [CrossRef]
- Zaman, Z. Exploring Bone Cell Research Using Bone-on-a-Chip Models and Microfluidics: A Literature Review. Undergrad. Res. Nat. Clin. Sci. Technol. (Urncst) J. 2023, 7, 1–7. [Google Scholar] [CrossRef]
- Yang, J.; Duan, P.; Liu, Q.; Yu, H.; Fang, F.; Liu, X. Microfluidic bone chip to study osteogenesis of porous substrate topographies in normal and osteoporotic microenvironments. Eur. Cells Mater. 2024, 47, 238–252. [Google Scholar] [CrossRef]
- Galván-Chacón, V.P.; Zampouka, A.; Hesse, B.; Bohner, M.; Habibovic, P.; Barata, D. Bone-on-a-Chip: A Microscale 3D Biomimetic Model to Study Bone Regeneration. Adv. Eng. Mater. 2022, 24, 2101467. [Google Scholar] [CrossRef]
- Aleman, J.; George, S.K.; Herberg, S.; Devarasetty, M.; Porada, C.D.; Skardal, A.; Almeida-Porada, G. Deconstructed Microfluidic Bone Marrow On-A-Chip to Study Normal and Malignant Hemopoietic Cell–Niche Interactions. Small 2019, 15, 1902971. [Google Scholar] [CrossRef]
- Kim, S.; Rajendran, A.K.; Amirthalingam, S.; Kim, J.H.; So, K.H.; Hwang, N.S. Recent technological advances in lab-on-a-chip for bone remodeling. Biosens. Bioelectron. X 2023, 14, 100360. [Google Scholar] [CrossRef]
- Hao, S.; Ha, L.; Cheng, G.; Wan, Y.; Xia, Y.; Sosnoski, D.M.; Mastro, A.M.; Zheng, S. A Spontaneous 3D Bone-On-a-Chip for Bone Metastasis Study of Breast Cancer Cells. Small 2018, 14, 1702787. [Google Scholar] [CrossRef]
- Van Noort, D.; Ong, S.M.; Zhang, C.; Zhang, S.; Arooz, T.; Yu, H. Stem cells in microfluidics. Biotechnol. Prog. 2009, 25, 52–60. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; Lai, C.; Li, L.; Wu, H.; Wang, Y.; Shi, X. Fabrication of a hydroxyapatite-PDMS microfluidic chip for bone-related cell culture and drug screening. Bioact. Mater. 2021, 6, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Y.; Yang, C.; Shao, C.; Shi, K.; Shang, L.; Ye, F.; Zhao, Y. Microfluidic 3D Printing Responsive Scaffolds with Biomimetic Enrichment Channels for Bone Regeneration. Adv. Funct. Mater. 2021, 31, 2105190. [Google Scholar] [CrossRef]
- Costa, L.A.; Eiro, N.; Vaca, A.; Vizoso, F.J. Towards a New Concept of Regenerative Endodontics Based on Mesenchymal Stem Cell-Derived Secretomes Products. Bioengineering 2022, 10, 4. [Google Scholar] [CrossRef] [PubMed]
| Cell Type | Markers and Proliferation * | Primary Differentiation Potential | Accessibility | Immunomodulatory Effects |
|---|---|---|---|---|
| Dental Pulp Stem Cells (DPSCs) | Standard MSC markers. High; faster rate than BMSCs [23]. | Readily accessible from the neurovascular bundle of healthy, inflamed, or wisdom tooth pulp. Can be recovered from cryopreservation [12,24,27]. | Immunologically privileged. Higher capacity than BMSCs; suppresses T-cell alloreactivity. Exosomes enhance secretion of anti-inflammatory IL-10 and TGF- [28,29]. | |
| Stem cells from exfoliated deciduous teeth (SHED) | Express neural markers (nestin, III-tubulin, GFAP, NFM) in addition to standard MSC markers [30]. High proliferation rates, significantly greater than DPSCs [31]. |
| Obtained from naturally exfoliated deciduous (“baby”) teeth; considered disposable tissue with limited ethical concerns and no donor site morbidity [34,35]. | Significant potential; adjusts CD4+ T-cell responses [36]. Neuroprotective effects. Conditioned medium (CM) and exosomes show potent anti-apoptotic and anti-inflammatory effects [37]. |
| Periodontal Ligament Stem Cells (PDLSCs) | Standard MSC markers. High expansion capability; higher proliferation rate than DPSCs [38]. | Easily accessible from periodontal ligament of impacted third molars. Can be isolated from cryopreserved ligaments [22,24]. | Properties similar to BMSCs; can suppress immune/inflammatory reactions [40]. Exosomes induce osteogenic differentiation and suppress macrophage inflammasome activation [41]. | |
| Gingival Mesenchymal Stem Cells (GMSCs) | Standard MSC markers. Homogenous population that proliferates faster than BMSCs [12]. | Easily accessible from gingival tissue (lamina propria) with minimal morbidity, rapid healing, and no scarring [12]. | Potent immunomodulatory and anti-inflammatory functions, maintaining potential even in inflammatory environments [42]. Suppresses M1 macrophages while boosting M2. Promotes Regulatory T-cell (Treg) and suppresses lymphocyte proliferation [42,44]. | |
| Dental Follicle Stem Cells (DFSCs) | Express high levels of osteogenic markers (RUNX2, ALP) in addition to standard MSC markers [45]. | Easily isolated from the dental follicle of developing teeth (e.g., wisdom teeth). Highly plastic if sourced early [48,50]. | Superior immune-suppressive effect compared to other dental MSCs [51]. Suppresses lymphocyte proliferation and increases Treg ratio, particularly in autoimmune contexts (e.g., RA) [51,52]. | |
| Alveolar Bone-Derived Mesenchymal Stem Cells (ABMSCs) | Implied standard MSC markers (as a BMSCs source). | Sourced from alveolar bone marrow, often via minimally invasive aspirates during dental surgeries [53]. | observed to be similar to those of standard BMSCs [54]. | |
| Buccal Fat Pad-Derived Stem Cells (BFPDs) | Implied standard MSC markers (as an adipose-derived source) [5,55]. |
| Available from buccal fat pad; facilitated preparation and lower morbidity than BMSCs harvest [17,56]. | Not detailed, but shares similarities with immunomodulatory Adipose tissue-Derived Stem Cells (ADSCs) [56]. |
| Salivary Gland Stem Cells (SGSCs) | Markers for source cells (e.g., EpCAM+, CD24+/CD29+), not traditional MSCs. Exhibit markers of both embryonic and adult stem cells [11,57]. Can proliferate and form organoids. | Can be induced to differentiate into chondrogenic, osteogenic, and adipogenic cells [58]. | Isolated from salivary glands; focus is often on endogenous cellular plasticity rather than routine harvesting [57,59]. | Indirectly contributes through secretion of paracrine factors (cytokines, growth factors, EVs) [60]. |
| Stem Cells from Apical Papilla (SCAPs) | Standard MSC markers. Highly proliferative, with greater capacity than DPSCs [61]. | Isolated from the apical papilla of incompletely developed teeth. Easy to access [61]. | Reported to have immunomodulatory characteristics similar to BMSCs [11,66]. |
| Mediator | Role in Angiogenic-Osteogenic Coupling |
|---|---|
| Fibroblast growth factor (FGF) | Particularly FGF-2, these are potent mitogens for mesenchymal cells and osteoblasts and are strongly angiogenic [113,114,115]. |
| Platelet-derived growth factors (PDGF) | Released from platelets during clotting, PDGF is chemotactic for osteoblasts and promotes angiogenesis, partly by upregulating VEGF [116,117,118,119]. |
| Bone morphogenetic proteins (BMPs) | Powerful osteoinductive factors (e.g., BMP-2, BMP-7) that stimulate osteoprogenitor differentiation. Their angiogenic effects are primarily indirect, mediated through the upregulation of VEGF [107,120,121]. |
| Placental Growth Factor (PlGF) | A VEGF homolog that stimulates MSC proliferation and differentiation toward osteoblastic and osteoclastic lineages [99,122,123]. |
| Other Factors | Molecules such as Insulin-like Growth Factor (IGF), Erythropoietin (EPO), and Angiopoietins (Ang-1, Ang-2) contribute to neovascularization and vessel maturation [99,110,116,124,125,126,127]. |
| Metallic Ions & Exosomes | Emerging evidence shows that metallic ions (e.g., Co, Mg, Zn) can promote angiogenesis by upregulating HIF-1 and VEGF. Similarly, MSC-derived exosomes promote both angiogenesis and osteogenesis by activating pathways such as HIF-1/VEGF and BMP-2/Smad1/Runx2 [100,128,129,130,131,132]. |
| Parameter | Mandiblar Defect Study [87,143,144] | Maxillary Alveolar Defect Study [18,142,145] | Calvarial Defect Study [71,146,147] |
|---|---|---|---|
| Stem cell sources | |||
| Scafold | |||
| Animal | |||
| Conclusion |
| Registration ID | Status (Phase) | Condition Investigated | Cell Type Used |
|---|---|---|---|
| NCT03386877 [204] | Completed (2017) | Periodontal Intrabony Defects | Autologous DPSCs |
| NCT03194451 [206] | Completed (2019) | Bone Tissue Engineering | Autologous DPSCs |
| NCT00221130 [207] | Completed (2005) | Adult Periodontitis | Autologous MSCs/Osteoblasts |
| NCT01357785 [208] | Completed (2014) | Periodontal Intrabony Defects | Autologous DPSCs |
| NCT01082822 [209] | Completed (2012) | Chronic Periodontitis | Autologous DPSCs |
| NCT04641533 [210] | Completed (2020) | Third Molar Extraction Sockets | Autologous DPSCs |
| NCT04608838 [205] | Completed (2021) | Acute Ischemic Stroke | Allogeneic DPSCs (JTR-161) |
| NCT03658655 [211] | Completed (2019) | Type 2 Diabetes | SHED |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaddaram, B.C.; Shakya, A.K.; Zadeh, B.R.; Lopez, D.M.; Wagner, J.; Parco, T.; Kandalam, U. The Therapeutic Scope of Orofacial Mesenchymal Stem Cells. Bioengineering 2025, 12, 970. https://doi.org/10.3390/bioengineering12090970
Vaddaram BC, Shakya AK, Zadeh BR, Lopez DM, Wagner J, Parco T, Kandalam U. The Therapeutic Scope of Orofacial Mesenchymal Stem Cells. Bioengineering. 2025; 12(9):970. https://doi.org/10.3390/bioengineering12090970
Chicago/Turabian StyleVaddaram, Bharath Chandra, Akhilesh Kumar Shakya, Brandon R. Zadeh, Diariza M. Lopez, Jon Wagner, Todd Parco, and Umadevi Kandalam. 2025. "The Therapeutic Scope of Orofacial Mesenchymal Stem Cells" Bioengineering 12, no. 9: 970. https://doi.org/10.3390/bioengineering12090970
APA StyleVaddaram, B. C., Shakya, A. K., Zadeh, B. R., Lopez, D. M., Wagner, J., Parco, T., & Kandalam, U. (2025). The Therapeutic Scope of Orofacial Mesenchymal Stem Cells. Bioengineering, 12(9), 970. https://doi.org/10.3390/bioengineering12090970







