Immunomodulatory and Regenerative Functions of MSC-Derived Exosomes in Bone Repair
Abstract
1. Introduction
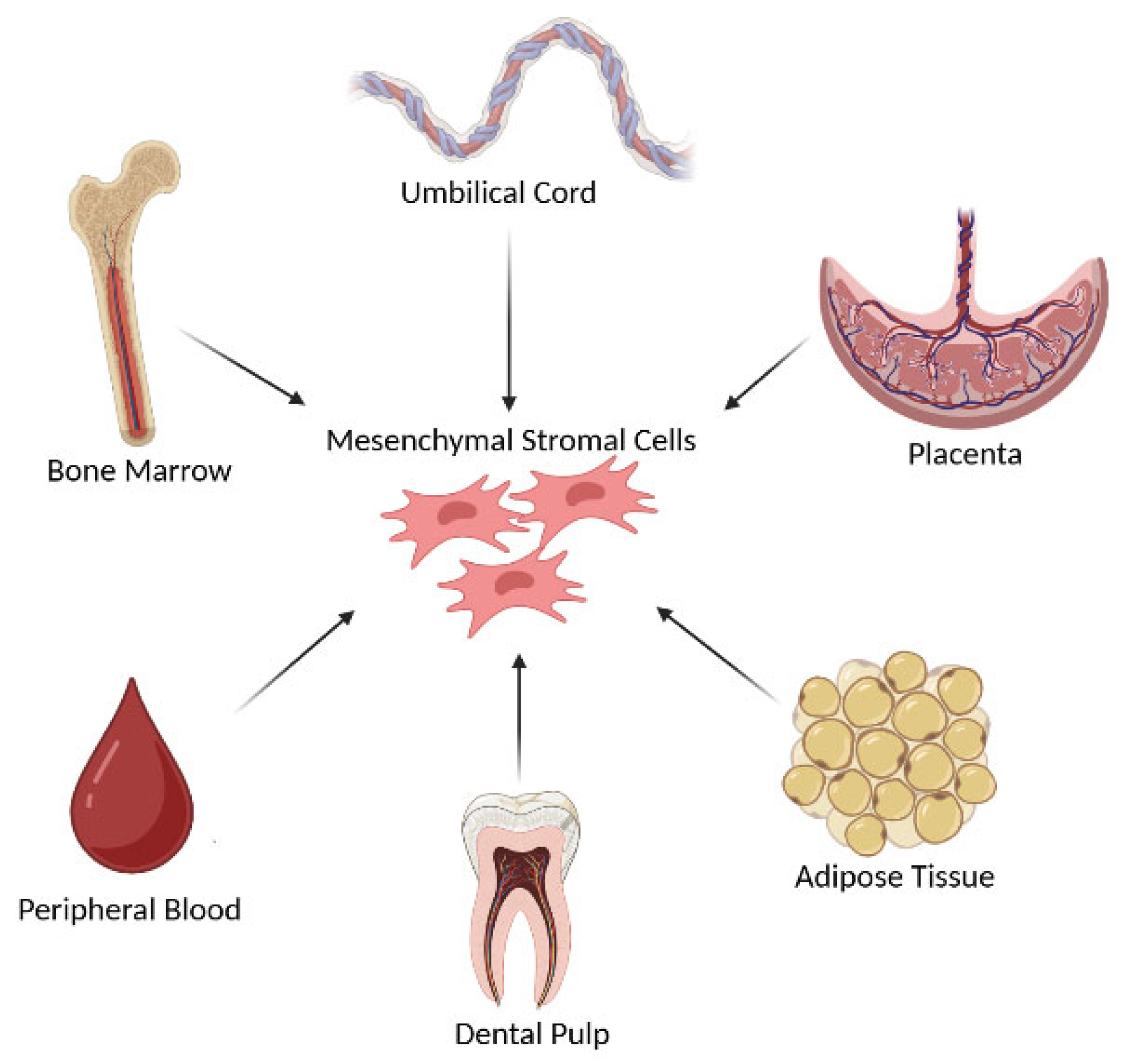
2. Mesenchymal Stromal Cells and Exosomes: An Overview
3. Exosomes from iPSC-Derived MSCs (iMSCs)
4. MSC-Derived Exosomes in Bone Regeneration
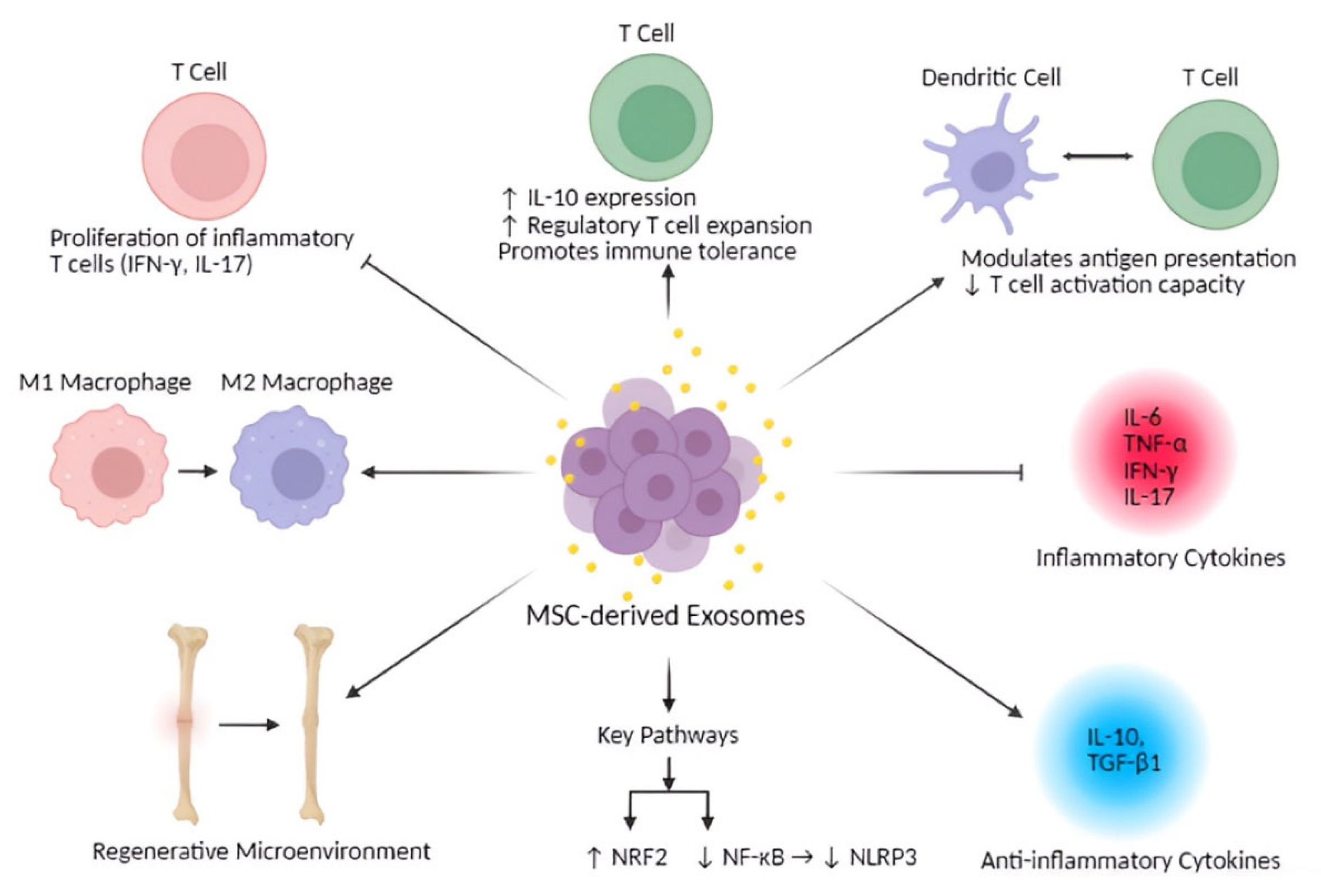
5. Immunomodulatory Properties of MSC-Derived Exosomes
6. Non-Immunomodulatory Properties of MSC-Derived Exosomes
7. Evidence of MSC-Derived Exosomes Promoting Osteocyte Differentiation
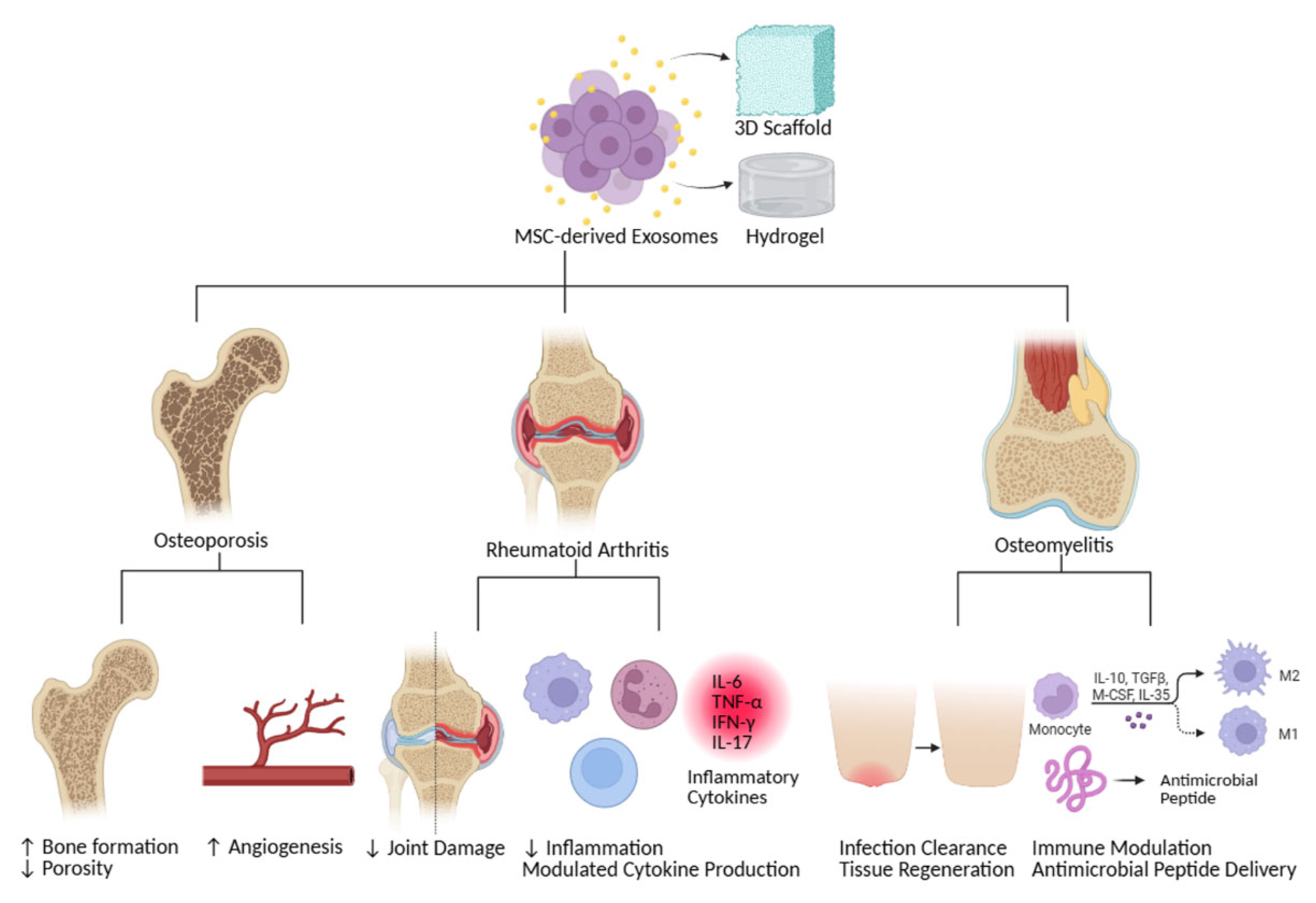
8. Therapeutic Potential and Clinical Implications
9. Challenges and Future Directions
- (1)
- Dissecting the molecular pathways through which MSC exosomes influence osteocyte differentiation and function.
- (2)
- Establishing standardized, scalable protocols for exosome production, purification, and characterization.
- (3)
- Innovating delivery systems—such as engineered scaffolds and targeting ligands—to maximize therapeutic precision.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Helder, M.N.; Bravenboer, N.; Wu, G.; Jin, J.; Ten Bruggenkate, C.M.; Klein-Nulend, J.; Schulten, E. Is There a Governing Role of Osteocytes in Bone Tissue Regeneration? Curr. Osteoporos. Rep. 2020, 18, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Tresguerres, F.G.F.; Torres, J.; López-Quiles, J.; Hernández, G.; Vega, J.A.; Tresguerres, I.F. The osteocyte: A multifunctional cell within the bone. Ann. Anat. 2020, 227, 151422. [Google Scholar] [CrossRef]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Noguchi, T.; Mizoguchi, I. The osteocyte and its osteoclastogenic potential. Front. Endocrinol. 2023, 14, 1121727. [Google Scholar] [CrossRef]
- Du, J.H.; Lin, S.X.; Wu, X.L.; Yang, S.M.; Cao, L.Y.; Zheng, A.; Wu, J.N.; Jiang, X.Q. The Function of Wnt Ligands on Osteocyte and Bone Remodeling. J. Dent. Res. 2019, 98, 930–938. [Google Scholar] [CrossRef]
- Niedźwiedzki, T.; Filipowska, J. Bone remodeling in the context of cellular and systemic regulation: The role of osteocytes and the nervous system. J. Mol. Endocrinol. 2015, 55, R23–R36. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, M.H.; Kink, J.A.; Hematti, P.; Capitini, C.M. Mesenchymal Stromal Cells and Exosomes: Progress and Challenges. Front. Cell Dev. Biol. 2020, 8, 665. [Google Scholar] [CrossRef]
- Akbar, N.; Razzaq, S.S.; Salim, A.; Haneef, K. Mesenchymal Stem Cell-Derived Exosomes and Their MicroRNAs in Heart Repair and Regeneration. J. Cardiovasc. Transl. Res. 2024, 17, 505–522. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Rahman, H.S.; Markov, A.; Endjun, J.J.; Zekiy, A.O.; Chartrand, M.S.; Beheshtkhoo, N.; Kouhbanani, M.A.J.; Marofi, F.; Nikoo, M.; et al. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res. Ther. 2021, 12, 297. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Gao, B.; Zhang, L. Exosome mediated biological functions within skeletal microenvironment. Front. Bioeng. Biotechnol. 2022, 10, 953916. [Google Scholar] [CrossRef]
- Torrecillas-Baena, B.; Pulido-Escribano, V.; Dorado, G.; Gálvez-Moreno, M.; Camacho-Cardenosa, M.; Casado-Díaz, A. Clinical Potential of Mesenchymal Stem Cell-Derived Exosomes in Bone Regeneration. J. Clin. Med. 2023, 12, 4385. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, R.; Katagiri, W.; Endo, S.; Kobayashi, T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS ONE 2019, 14, e0225472. [Google Scholar] [CrossRef] [PubMed]
- Baharlooi, H.; Salehi, Z.; Minbashi Moeini, M.; Rezaei, N.; Azimi, M. Immunomodulatory Potential of Human Mesenchymal Stem Cells and their Exosomes on Multiple Sclerosis. Adv. Pharm. Bull. 2022, 12, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Wang, H.; Dong, J.; Wu, Y.; Zhang, H.; Fu, L.; Zhang, J. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate neuroinflammation and oxidative stress through the NRF2/NF-κB/NLRP3 pathway. CNS Neurosci. Ther. 2024, 30, e14454. [Google Scholar] [CrossRef]
- He, J.G.; Wu, X.X.; Li, S.; Yan, D.; Xiao, G.P.; Mao, F.G. Exosomes derived from microRNA-540-3p overexpressing mesenchymal stem cells promote immune tolerance via the CD74/nuclear factor-kappaB pathway in cardiac allograft. World J. Stem Cells 2024, 16, 1022–1046. [Google Scholar] [CrossRef]
- Guo, G.; Tan, Z.; Liu, Y.; Shi, F.; She, J. The therapeutic potential of stem cell-derived exosomes in the ulcerative colitis and colorectal cancer. Stem Cell Res. Ther. 2022, 13, 138. [Google Scholar] [CrossRef]
- Liu, W.; Huang, J.; Chen, F.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Li, X.; Li, X.; Yang, L. MSC-derived small extracellular vesicles overexpressing miR-20a promoted the osteointegration of porous titanium alloy by enhancing osteogenesis via targeting BAMBI. Stem Cell Res. Ther. 2021, 12, 348. [Google Scholar] [CrossRef]
- Akhlaghpasand, M.; Tavanaei, R.; Hosseinpoor, M.; Yazdani, K.O.; Soleimani, A.; Zoshk, M.Y.; Soleimani, M.; Chamanara, M.; Ghorbani, M.; Deylami, M.; et al. Safety and potential effects of intrathecal injection of allogeneic human umbilical cord mesenchymal stem cell-derived exosomes in complete subacute spinal cord injury: A first-in-human, single-arm, open-label, phase I clinical trial. Stem Cell Res. Ther. 2024, 15, 264. [Google Scholar] [CrossRef]
- Ponzetti, M.; Rucci, N. Updates on Osteoimmunology: What’s New on the Cross-Talk Between Bone and Immune System. Front. Endocrinol. 2019, 10, 236. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, L. Osteoimmunology: The Crosstalk between T Cells, B Cells, and Osteoclasts in Rheumatoid Arthritis. Int. J. Mol. Sci. 2024, 25, 2688. [Google Scholar] [CrossRef]
- Lin, T.; Pajarinen, J.; Nabeshima, A.; Lu, L.; Nathan, K.; Jämsen, E.; Yao, Z.; Goodman, S.B. Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodulation and osteogenesis. Stem Cell Res. Ther. 2017, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Shin, M.K.; Jang, B.Y.; Lee, S.H.; Kim, M.; Sung, J.S. Immunomodulatory Effect and Bone Homeostasis Regulation in Osteoblasts Differentiated from hADMSCs via the PD-1/PD-L1 Axis. Cells 2022, 11, 3152. [Google Scholar] [CrossRef] [PubMed]
- Rajasingh, S.; Sigamani, V.; Selvam, V.; Gurusamy, N.; Kirankumar, S.; Vasanthan, J.; Rajasingh, J. Comparative analysis of human induced pluripotent stem cell-derived mesenchymal stem cells and umbilical cord mesenchymal stem cells. J. Cell. Mol. Med. 2021, 25, 8904–8919. [Google Scholar] [CrossRef] [PubMed]
- Vasanthan, J.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Kirankumar, S.; Thomas, E.L.; Rajasingh, J. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells 2020, 10, 54. [Google Scholar] [CrossRef]
- Lindner, U.; Kramer, J.; Rohwedel, J.; Schlenke, P. Mesenchymal Stem or Stromal Cells: Toward a Better Understanding of Their Biology? Transfus. Med. Hemother. 2010, 37, 75–83. [Google Scholar] [CrossRef]
- Baghaei, K.; Hashemi, S.M.; Tokhanbigli, S.; Asadi Rad, A.; Assadzadeh-Aghdaei, H.; Sharifian, A.; Zali, M.R. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol. Hepatol. Bed Bench 2017, 10, 208–213. [Google Scholar]
- Nitkin, C.R.; Rajasingh, J.; Pisano, C.; Besner, G.E.; Thébaud, B.; Sampath, V. Stem cell therapy for preventing neonatal diseases in the 21st century: Current understanding and challenges. Pediatr. Res. 2020, 87, 265–276. [Google Scholar] [CrossRef]
- Shah, P.; Aghazadeh, M.; Rajasingh, S.; Dixon, D.; Jain, V.; Rajasingh, J. Stem cells in regenerative dentistry: Current understanding and future directions. J. Oral. Biosci. 2024, 66, 288–299. [Google Scholar] [CrossRef]
- Masuda, K.; Han, X.; Kato, H.; Sato, H.; Zhang, Y.; Sun, X.; Hirofuji, Y.; Yamaza, H.; Yamada, A.; Fukumoto, S. Dental Pulp-Derived Mesenchymal Stem Cells for Modeling Genetic Disorders. Int. J. Mol. Sci. 2021, 22, 2269. [Google Scholar] [CrossRef]
- Maxim, M.A.; Soritau, O.; Baciut, M.; Bran, S.; Baciut, G. The role of dental stem cells in regeneration. Clujul Med. 2015, 88, 479–482. [Google Scholar] [CrossRef]
- Liu, H.; Cao, T. Dental application potential of mesenchymal stromal cells and embryonic stem cells. Chin. J. Dent. Res. 2010, 13, 95–103. [Google Scholar]
- Goriuc, A.; Foia, L.; Cojocaru, K.; Diaconu-Popa, D.; Sandu, D.; Luchian, I. The Role and Involvement of Stem Cells in Periodontology. Biomedicines 2023, 11, 387. [Google Scholar] [CrossRef]
- Roszkowski, S. Therapeutic potential of mesenchymal stem cell-derived exosomes for regenerative medicine applications. Clin. Exp. Med. 2024, 24, 46. [Google Scholar] [CrossRef]
- Mani, S.; Gurusamy, N.; Ulaganathan, T.; Paluck, A.J.; Ramalingam, S.; Rajasingh, J. Therapeutic potentials of stem cell-derived exosomes in cardiovascular diseases. Exp. Biol. Med. 2023, 248, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.J.; Yang, J.J.; Lu, Y.B.; Liu, Z.Y.; Wang, X.X. Mesenchymal stem cell-derived exosomes: Toward cell-free therapeutic strategies in regenerative medicine. World J. Stem Cells 2020, 12, 814–840. [Google Scholar] [CrossRef]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: New molecular targets of diseases. Acta Pharmacol. Sin. 2017, 39, 501–513. [Google Scholar] [CrossRef]
- Sun, S.J.; Wei, R.; Li, F.; Liao, S.Y.; Tse, H.F. Mesenchymal stromal cell-derived exosomes in cardiac regeneration and repair. Stem Cell Rep. 2021, 16, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Kang, I.; Yu, K.R. Therapeutic Features and Updated Clinical Trials of Mesenchymal Stem Cell (MSC)-Derived Exosomes. J. Clin. Med. 2021, 10, 711. [Google Scholar] [CrossRef]
- Vembuli, H.; Rajasingh, S.; Nabholz, P.; Guenther, J.; Morrow, B.R.; Taylor, M.M.; Aghazadeh, M.; Sigamani, V.; Rajasingh, J. Induced mesenchymal stem cells generated from periodontal ligament fibroblast for regenerative therapy. Exp. Biol. Med. 2025, 250, 10342. [Google Scholar] [CrossRef]
- Nakamura, Y.; Niho, S.; Shimizu, Y. Cell-Based Therapy for Fibrosing Interstitial Lung Diseases, Current Status, and Potential Applications of iPSC-Derived Cells. Cells 2024, 13, 893. [Google Scholar] [CrossRef]
- Sheyn, D.; Ben-David, S.; Shapiro, G.; De Mel, S.; Bez, M.; Ornelas, L.; Sahabian, A.; Sareen, D.; Da, X.; Pelled, G.; et al. Human Induced Pluripotent Stem Cells Differentiate Into Functional Mesenchymal Stem Cells and Repair Bone Defects. Stem Cells Transl. Med. 2016, 5, 1447–1460. [Google Scholar] [CrossRef]
- Jungbluth, P.; Spitzhorn, L.S.; Grassmann, J.; Tanner, S.; Latz, D.; Rahman, M.S.; Bohndorf, M.; Wruck, W.; Sager, M.; Grotheer, V.; et al. Human iPSC-derived iMSCs improve bone regeneration in mini-pigs. Bone Res. 2019, 7, 32. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, J.; Niu, X.; Hu, G.; Guo, S.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 2015, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Spitzhorn, L.S.; Megges, M.; Wruck, W.; Rahman, M.S.; Otte, J.; Degistirici, Ö.; Meisel, R.; Sorg, R.V.; Oreffo, R.O.C.; Adjaye, J. Human iPSC-derived MSCs (iMSCs) from aged individuals acquire a rejuvenation signature. Stem Cell Res. Ther. 2019, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Hodgson-Garms, M.; Moore, M.J.; Martino, M.M.; Kelly, K.; Frith, J.E. Proteomic profiling of iPSC and tissue-derived MSC secretomes reveal a global signature of inflammatory licensing. NPJ Regen. Med. 2025, 10, 7. [Google Scholar] [CrossRef]
- Chakraborty, A.; Wang, C.; Hodgson-Garms, M.; Broughton, B.R.S.; Frith, J.E.; Kelly, K.; Samuel, C.S. Induced pluripotent stem cell-derived mesenchymal stem cells reverse bleomycin-induced pulmonary fibrosis and related lung stiffness. Biomed. Pharmacother. 2024, 178, 117259. [Google Scholar] [CrossRef]
- Levy, D.; Abadchi, S.N.; Shababi, N.; Ravari, M.R.; Pirolli, N.H.; Bergeron, C.; Obiorah, A.; Mokhtari-Esbuie, F.; Gheshlaghi, S.; Abraham, J.M.; et al. Induced Pluripotent Stem Cell-Derived Extracellular Vesicles Promote Wound Repair in a Diabetic Mouse Model via an Anti-Inflammatory Immunomodulatory Mechanism. Adv. Healthc. Mater. 2023, 12, e2300879. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, B.; Shang, J.; Wang, Y.; Jia, L.; She, X.; Xu, X.; Zhang, D.; Guo, J.; Zhang, F. Diabetic and nondiabetic BMSC-derived exosomes affect bone regeneration via regulating miR-17-5p/SMAD7 axis. Int. Immunopharmacol. 2023, 125, 111190. [Google Scholar] [CrossRef]
- Wei, F.; Li, Z.; Crawford, R.; Xiao, Y.; Zhou, Y. Immunoregulatory role of exosomes derived from differentiating mesenchymal stromal cells on inflammation and osteogenesis. J. Tissue Eng. Regen. Med. 2019, 13, 1978–1991. [Google Scholar] [CrossRef]
- Wang, D.; Cao, H.; Hua, W.; Gao, L.; Yuan, Y.; Zhou, X.; Zeng, Z. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Bone Defect Repair. Membranes 2022, 12, 716. [Google Scholar] [CrossRef]
- Huang, D.; Shen, H.; Xie, F.; Hu, D.; Jin, Q.; Hu, Y.; Zhong, T. Role of mesenchymal stem cell-derived exosomes in the regeneration of different tissues. J. Biol. Eng. 2024, 18, 36. [Google Scholar] [CrossRef]
- Ren, S.; Lin, Y.; Liu, W.; Yang, L.; Zhao, M. MSC-Exos: Important active factor of bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1136453. [Google Scholar] [CrossRef] [PubMed]
- Huo, K.L.; Yang, T.Y.; Zhang, W.W.; Shao, J. Mesenchymal stem/stromal cells-derived exosomes for osteoporosis treatment. World J. Stem Cells 2023, 15, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, J.; Xiao, B.; Li, C. BMSC-derived exosomes promote osteoporosis alleviation via M2 macrophage polarization. Mol. Med. 2024, 30, 220. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Sun, R.; Wu, C.; Wang, L.; Zhang, C. Exosome: A Novel Approach to Stimulate Bone Regeneration through Regulation of Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2016, 17, 712. [Google Scholar] [CrossRef]
- Harrell, C.R.; Djonov, V.; Volarevic, A.; Arsenijevic, A.; Volarevic, V. Molecular Mechanisms Responsible for the Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Lung Fibrosis. Int. J. Mol. Sci. 2024, 25, 4378. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Fu, X.; Han, W. Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci. China Life Sci. 2016, 59, 1305–1312. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef]
- Wang, X.; Gong, W.; Li, R.; Li, L.; Wang, J. Preparation of genetically or chemically engineered exosomes and their therapeutic effects in bone regeneration and anti-inflammation. Front. Bioeng. Biotechnol. 2024, 12, 1329388. [Google Scholar] [CrossRef]
- Hong, H.; Lin, C.; Fang, M.; Liu, J.; Hsu, H.C.; Chang, C.J.; Wang, H. Proteomic analysis of exosomal proteins associated with bone healing speed in a rat tibial fracture model. Biomed. Chromatogr. 2024, 38, e5846. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, Y.; Zhang, Q. Emerging Role of Extracellular Vesicles in Bone Remodeling. J. Dent. Res. 2018, 97, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Y.; Feng, T.; Li, R.; Wang, Z.; Zhang, L.; Yin, P.; Tang, P. Bone Engineering Scaffolds With Exosomes: A Promising Strategy for Bone Defects Repair. Front. Bioeng. Biotechnol. 2022, 10, 920378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, Y.; Luo, D.; Li, P.; Chen, Y.; Fu, X.; Yue, Y.; Hou, R.; Liu, J.; Wang, X. Advantages and disadvantages of various hydrogel scaffold types: A research to improve the clinical conversion rate of loaded MSCs-Exos hydrogel scaffolds. Biomed. Pharmacother. 2024, 179, 117386. [Google Scholar] [CrossRef]
- Silva, A.M.; Teixeira, J.H.; Almeida, M.I.; Gonçalves, R.M.; Barbosa, M.A.; Santos, S.G. Extracellular Vesicles: Immunomodulatory messengers in the context of tissue repair/regeneration. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 98, 86–95. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Long, R.; Wang, S. Exosomes from preconditioned mesenchymal stem cells: Tissue repair and regeneration. Regen. Ther. 2024, 25, 355–366. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Rong, Y.; Qian, D.; Chen, J.; Zhou, Z.; Luo, Y.; Jiang, D.; Cheng, L.; Zhao, S.; et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020, 103, 196–212. [Google Scholar] [CrossRef]
- Wang, C.; Shou, Z.; Xu, C.; Huo, K.; Liu, W.; Liu, H.; Zan, X.; Wang, Q.; Li, L. Enhancing the Implant Osteointegration via Supramolecular Co-Assembly Coating with Early Immunomodulation and Cell Colonization. Adv. Sci. 2025, 12, e2410595. [Google Scholar] [CrossRef]
- Chu, C.H.; Lee, R.P.; Wu, W.T.; Chen, I.H.; Yeh, K.T.; Wang, C.C. Advancing Osteoarthritis Treatment: The Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes and Biomaterial Integration. Biomedicines 2024, 12, 2478. [Google Scholar] [CrossRef]
- Zhu, M.; Li, S.; Li, S.; Wang, H.; Xu, J.; Wang, Y.; Liang, G. Strategies for Engineering Exosomes and Their Applications in Drug Delivery. J. Biomed. Nanotechnol. 2021, 17, 2271–2297. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Amiri, A.; Bagherifar, R.; Ansari Dezfouli, E.; Kiaie, S.H.; Jafari, R.; Ramezani, R. Exosomes as bio-inspired nanocarriers for RNA delivery: Preparation and applications. J. Transl. Med. 2022, 20, 125. [Google Scholar] [CrossRef]
- Kang, M.; Huang, C.C.; Gajendrareddy, P.; Lu, Y.; Shirazi, S.; Ravindran, S.; Cooper, L.F. Extracellular Vesicles From TNFα Preconditioned MSCs: Effects on Immunomodulation and Bone Regeneration. Front. Immunol. 2022, 13, 878194. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Kushioka, J.; Chow, S.K.; Toya, M.; Tsubosaka, M.; Shen, H.; Gao, Q.; Li, X.; Zhang, N.; Goodman, S.B. Bone regeneration in inflammation with aging and cell-based immunomodulatory therapy. Inflamm. Regen. 2023, 43, 29. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Huang, Y.; Han, J.; Yu, L.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F.; et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016, 64, 831–840. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, F.; Qian, H.; Xu, W.; Jiang, J. Preconditioning and Engineering Strategies for Improving the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cell-Free Therapy. Stem Cells Int. 2022, 2022, 1779346. [Google Scholar] [CrossRef] [PubMed]
- Kahrizi, M.S.; Mousavi, E.; Khosravi, A.; Rahnama, S.; Salehi, A.; Nasrabadi, N.; Ebrahimzadeh, F.; Jamali, S. Recent advances in pre-conditioned mesenchymal stem/stromal cell (MSCs) therapy in organ failure; a comprehensive review of preclinical studies. Stem Cell Res. Ther. 2023, 14, 155. [Google Scholar] [CrossRef]
- Li, L.; Jin, S.; Zhang, Y. Ischemic preconditioning potentiates the protective effect of mesenchymal stem cells on endotoxin-induced acute lung injury in mice through secretion of exosome. Int. J. Clin. Exp. Med. 2015, 8, 3825–3832. [Google Scholar] [PubMed]
- Yang, Y.; Lee, E.H.; Yang, Z. Hypoxia-Conditioned Mesenchymal Stem Cells in Tissue Regeneration Application. Tissue Eng. Part. B Rev. 2022, 28, 966–977. [Google Scholar] [CrossRef]
- Zhuo, H.; Chen, Y.; Zhao, G. Advances in application of hypoxia-preconditioned mesenchymal stem cell-derived exosomes. Front. Cell Dev. Biol. 2024, 12, 1446050. [Google Scholar] [CrossRef]
- Pendse, S.; Kale, V.; Vaidya, A. Extracellular Vesicles Isolated from Mesenchymal Stromal Cells Primed with Hypoxia: Novel Strategy in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2021, 16, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Mullen, M.; Williams, K.; LaRocca, T.; Duke, V.; Hambright, W.S.; Ravuri, S.K.; Bahney, C.S.; Ehrhart, N.; Huard, J. Mechanical strain drives exosome production, function, and miRNA cargo in C2C12 muscle progenitor cells. J. Orthop. Res. 2023, 41, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Papoutsakis, E.T. The role of biomechanical stress in extracellular vesicle formation, composition and activity. Biotechnol. Adv. 2023, 66, 108158. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Wang, X.; Thomsen, P. Mesenchymal stem cell–derived small extracellular vesicles and bone regeneration. Basic. Clin. Pharmacol. Toxicol. 2021, 128, 18–36. [Google Scholar] [CrossRef]
- Liang, W.; Han, B.; Hai, Y.; Sun, D.; Yin, P. Mechanism of Action of Mesenchymal Stem Cell-Derived Exosomes in the Intervertebral Disc Degeneration Treatment and Bone Repair and Regeneration. Front. Cell Dev. Biol. 2021, 9, 833840. [Google Scholar] [CrossRef]
- Wa, Q.; Luo, Y.; Tang, Y.; Song, J.; Zhang, P.; Linghu, X.; Lin, S.; Li, G.; Wang, Y.; Wen, Z.; et al. Mesoporous bioactive glass-enhanced MSC-derived exosomes promote bone regeneration and immunomodulation in vitro and in vivo. J. Orthop. Transl. 2024, 49, 264–282. [Google Scholar] [CrossRef]
- Wang, X.; Fu, L.; Sun, R.; Zhang, C.; Zhang, Y. Bone Marrow Mesenchymal Stem Cell-Exosomes (BMSC-ExO) Promote Osteogenic Differentiation In Vitro and Osteogenesis In Vivo by Regulating miR-318/Runt-Related Transcription Factor 2 (RUNX2). J. Biomater. Tissue Eng. 2022, 12, 1266–1271. [Google Scholar] [CrossRef]
- Soriano-Cruz, M.; Vázquez-González, W.G.; Molina-Vargas, P.; Faustino-Trejo, A.; Chávez-Rueda, A.K.; Legorreta-Haquet, M.V.; Aguilar-Ruíz, S.R.; Chávez-Sánchez, L. Exosomes as Regulators of Macrophages in Cardiovascular Diseases. Biomedicines 2024, 12, 2683. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, S.; Guo, W.Z.; Li, X.K. The Unique Immunomodulatory Properties of MSC-Derived Exosomes in Organ Transplantation. Front. Immunol. 2021, 12, 659621. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.M.; Yang, M.F.; Xu, H.M.; Zhu, M.Z.; Zhang, Y.; Yao, J.; Wang, L.S.; Liang, Y.J.; Li, D.F. Mesenchymal Stem Cell-derived Exosomes: Novel Therapeutic Approach for Inflammatory Bowel Diseases. Stem Cells Int. 2023, 2023, 4245704. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.; Jamal, R.; Abu, N. Cancer-Derived Exosomes as Effectors of Key Inflammation-Related Players. Front. Immunol. 2019, 10, 2103. [Google Scholar] [CrossRef]
- Blazquez, R.; Sanchez-Margallo, F.M.; de la Rosa, O.; Dalemans, W.; Alvarez, V.; Tarazona, R.; Casado, J.G. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front. Immunol. 2014, 5, 556. [Google Scholar] [CrossRef]
- Liu, H.; Li, R.; Liu, T.; Yang, L.; Yin, G.; Xie, Q. Immunomodulatory Effects of Mesenchymal Stem Cells and Mesenchymal Stem Cell-Derived Extracellular Vesicles in Rheumatoid Arthritis. Front. Immunol. 2020, 11, 1912. [Google Scholar] [CrossRef]
- Villatoro, A.J.; Alcoholado, C.; Martín-Astorga, M.C.; Fernández, V.; Cifuentes, M.; Becerra, J. Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Vet. Immunol. Immunopathol. 2019, 208, 6–15. [Google Scholar] [CrossRef]
- Agrawal, M.; Rasiah, P.K.; Bajwa, A.; Rajasingh, J.; Gangaraju, R. Mesenchymal Stem Cell Induced Foxp3(+) Tregs Suppress Effector T Cells and Protect against Retinal Ischemic Injury. Cells 2021, 10, 3006. [Google Scholar] [CrossRef]
- Wang, L.T.; Jiang, S.S.; Ting, C.H.; Hsu, P.J.; Chang, C.C.; Sytwu, H.K.; Liu, K.J.; Yen, B.L. Differentiation of Mesenchymal Stem Cells from Human Induced Pluripotent Stem Cells Results in Downregulation of c-Myc and DNA Replication Pathways with Immunomodulation Toward CD4 and CD8 Cells. Stem Cells 2018, 36, 903–914. [Google Scholar] [CrossRef]
- Xie, Q.H.; Zheng, J.Q.; Ding, J.Y.; Wu, Y.F.; Liu, L.; Yu, Z.L.; Chen, G. Exosome-Mediated Immunosuppression in Tumor Microenvironments. Cells 2022, 11, 1946. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, T.; Zhou, M. Immune-Cell-Derived Exosomes for Cancer Therapy. Mol. Pharm. 2022, 19, 3042–3056. [Google Scholar] [CrossRef] [PubMed]
- Kugeratski, F.G.; Kalluri, R. Exosomes as mediators of immune regulation and immunotherapy in cancer. Febs. J. 2021, 288, 10–35. [Google Scholar] [CrossRef]
- Madel, M.B.; Ibáñez, L.; Wakkach, A.; de Vries, T.J.; Teti, A.; Apparailly, F.; Blin-Wakkach, C. Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions. Front. Immunol. 2019, 10, 1408. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Hertel, F.C.; Silva, A.S.D.; Sabino, A.P.; Valente, F.L.; Reis, E.C.C. Preconditioning Methods to Improve Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Bone Regeneration-A Systematic Review. Biology 2022, 11, 733. [Google Scholar] [CrossRef]
- Huber, J.; Griffin, M.F.; Longaker, M.T.; Quarto, N. Exosomes: A Tool for Bone Tissue Engineering. Tissue Eng. Part. B Rev. 2022, 28, 101–113. [Google Scholar] [CrossRef]
- Tian, J.; Zhu, Q.; Zhang, Y.; Bian, Q.; Hong, Y.; Shen, Z.; Xu, H.; Rui, K.; Yin, K.; Wang, S. Olfactory Ecto-Mesenchymal Stem Cell-Derived Exosomes Ameliorate Experimental Colitis via Modulating Th1/Th17 and Treg Cell Responses. Front. Immunol. 2020, 11, 598322. [Google Scholar] [CrossRef] [PubMed]
- Shahir, M.; Mahmoud Hashemi, S.; Asadirad, A.; Varahram, M.; Kazempour-Dizaji, M.; Folkerts, G.; Garssen, J.; Adcock, I.; Mortaz, E. Effect of mesenchymal stem cell-derived exosomes on the induction of mouse tolerogenic dendritic cells. J. Cell Physiol. 2020, 235, 7043–7055. [Google Scholar] [CrossRef]
- Fathollahi, A.; Hashemi, S.M.; Haji Molla Hoseini, M.; Yeganeh, F. In vitro analysis of immunomodulatory effects of mesenchymal stem cell- and tumor cell -derived exosomes on recall antigen-specific responses. Int. Immunopharmacol. 2019, 67, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, M.Y.; Jasim, S.A.; Altalbawy, F.M.A.; Bansal, P.; Kaur, H.; Al-Hamdani, M.M.; Deorari, M.; Abosaoda, M.K.; Hamzah, H.F.; Mohammed, B.A. A comprehensive insight into the immunomodulatory role of MSCs-derived exosomes (MSC-Exos) through modulating pattern-recognition receptors (PRRs). Cell Biochem. Funct. 2024, 42, e4029. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ge, Y.; Zhang, C.; Zhang, L.; Xu, J.; Qi, L.; Li, W. Progress of Mesenchymal Stem Cell-Derived Exosomes in Tissue Repair. Curr. Pharm. Des. 2020, 26, 2022–2037. [Google Scholar] [CrossRef]
- Elahi, F.M.; Farwell, D.G.; Nolta, J.A.; Anderson, J.D. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells 2020, 38, 15–21. [Google Scholar] [CrossRef]
- Roefs, M.T.; Sluijter, J.P.G.; Vader, P. Extracellular Vesicle-Associated Proteins in Tissue Repair. Trends Cell Biol. 2020, 30, 990–1013. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Lan, Q.; Xiao, X.; Bi, X.; Gu, Y.; Ai, Y. Effects of periodontal ligament stem cell-derived exosomes on osteoblastic proliferation, migration, differentiation, apoptosis, and signaling pathways. Oral. Dis. 2024, 30, 710–718. [Google Scholar] [CrossRef]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- Yu, H.; Cheng, J.; Shi, W.; Ren, B.; Zhao, F.; Shi, Y.; Yang, P.; Duan, X.; Zhang, J.; Fu, X.; et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020, 106, 328–341. [Google Scholar] [CrossRef]
- Rong, X.; Liu, J.; Yao, X.; Jiang, T.; Wang, Y.; Xie, F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res. Ther. 2019, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef]
- Narayanan, R.; Huang, C.C.; Ravindran, S. Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 3808674. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Cao, Z.; Xue, J.; Wang, X.; Hu, T.; Han, B.; Guo, Y. Mechanically strained osteocyte-derived exosomes contained miR-3110-5p and miR-3058-3p and promoted osteoblastic differentiation. Biomed. Eng. Online 2024, 23, 44. [Google Scholar] [CrossRef]
- Wang, B.; Lyu, F.J.; Deng, Z.; Zheng, Q.; Ma, Y.; Peng, Y.; Guo, S.; Lei, G.; Lai, Y.; Li, Q. Therapeutic potential of stem cell-derived exosomes for bone tissue regeneration around prostheses. J. Orthop. Transl. 2025, 52, 85–96. [Google Scholar] [CrossRef]
- Wu, F.; Song, C.; Zhen, G.; Jin, Q.; Li, W.; Liang, X.; Xu, W.; Guo, W.; Yang, Y.; Dong, W.; et al. Exosomes derived from BMSCs in osteogenic differentiation promote type H blood vessel angiogenesis through miR-150-5p mediated metabolic reprogramming of endothelial cells. Cell Mol. Life Sci. CMLS 2024, 81, 344. [Google Scholar] [CrossRef]
- Liang, W.; Li, Y.; Ji, Y.; Kang, R.; Zhang, K.; Su, X.; Li, J.; Ji, M.; Wu, T.; Cao, X.; et al. Exosomes derived from bone marrow mesenchymal stem cells induce the proliferation and osteogenic differentiation and regulate the inflammatory state in osteomyelitis in vitro model. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 1695–1705. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Li, J.; Jiao, T.; Yang, L. Enhancing osteoporosis treatment with engineered mesenchymal stem cell-derived extracellular vesicles: Mechanisms and advances. Cell Death Dis. 2024, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Song, Z.J.; Cai, Q.W.; Chen, R.X.; Zou, Y.; Fu, Q.; Ma, Y.Y. Adipose mesenchymal stem cell-derived exosomes ameliorate hypoxia/serum deprivation-induced osteocyte apoptosis and osteocyte-mediated osteoclastogenesis in vitro. Biochem. Biophys. Res. Commun. 2019, 508, 138–144. [Google Scholar] [CrossRef]
- Kuang, M.J.; Huang, Y.; Zhao, X.G.; Zhang, R.; Ma, J.X.; Wang, D.C.; Ma, X.L. Exosomes derived from Wharton’s jelly of human umbilical cord mesenchymal stem cells reduce osteocyte apoptosis in glucocorticoid-induced osteonecrosis of the femoral head in rats via the miR-21-PTEN-AKT signalling pathway. Int. J. Biol. Sci. 2019, 15, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.T.; Li, T.; Hu, Y.H.; Zou, M.; Guo, Q.; Qu, X.W. Exosomes secreted by mice adipose-derived stem cells after low-level laser irradiation treatment reduce apoptosis of osteocyte induced by hypoxia. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5562–5570. [Google Scholar] [PubMed]
- Xu, J.; Wang, Y.; Hsu, C.Y.; Gao, Y.; Meyers, C.A.; Chang, L.; Zhang, L.; Broderick, K.; Ding, C.; Peault, B.; et al. Human perivascular stem cell-derived extracellular vesicles mediate bone repair. eLife 2019, 8, e48191. [Google Scholar] [CrossRef]
- Zhang, L.; Seitz, L.C.; Abramczyk, A.M.; Liu, L.; Chan, C. cAMP initiates early phase neuron-like morphology changes and late phase neural differentiation in mesenchymal stem cells. Cell Mol. Life Sci. 2011, 68, 863–876. [Google Scholar] [CrossRef]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell Mol. Life Sci. 2009, 66, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Omar, O.; Vazirisani, F.; Thomsen, P.; Ekström, K. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS ONE 2018, 13, e0193059. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Zhu, Y.; Yang, M.; Mao, C. Human Mesenchymal Stem Cell Derived Exosomes Enhance Cell-Free Bone Regeneration by Altering Their miRNAs Profiles. Adv. Sci. 2020, 7, 2001334. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharabi, N.; Mohamed-Ahmed, S.; Shanbhag, S.; Kampleitner, C.; Elnour, R.; Yamada, S.; Rana, N.; Birkeland, E.; Tangl, S.; Gruber, R.; et al. Osteogenic human MSC-derived extracellular vesicles regulate MSC activity and osteogenic differentiation and promote bone regeneration in a rat calvarial defect model. Stem Cell Res. Ther. 2024, 15, 33. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef]
- Zuo, R.; Liu, M.; Wang, Y.; Li, J.; Wang, W.; Wu, J.; Sun, C.; Li, B.; Wang, Z.; Lan, W.; et al. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem Cell Res. Ther. 2019, 10, 30. [Google Scholar] [CrossRef]
- Luo, Z.W.; Li, F.X.; Liu, Y.W.; Rao, S.S.; Yin, H.; Huang, J.; Chen, C.Y.; Hu, Y.; Zhang, Y.; Tan, Y.J.; et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale 2019, 11, 20884–20892. [Google Scholar] [CrossRef]
- Li, Y.; Jin, D.; Xie, W.; Wen, L.; Chen, W.; Xu, J.; Ding, J.; Ren, D.; Xiao, Z. Mesenchymal Stem Cells-Derived Exosomes: A Possible Therapeutic Strategy for Osteoporosis. Curr. Stem Cell Res. Ther. 2018, 13, 362–368. [Google Scholar] [CrossRef]
- Meng, F.; Wang, G.; Zhou, F.; Li, G.; Wang, M.; Zhou, Z.; Han, Y.; Chen, X.; Hu, Y.; Zhang, Y.; et al. Exosomes from young plasma alleviate osteoporosis through miR-217-5p-regulated osteogenesis of bone marrow mesenchymal stem cell. Compos. Part. B Eng. 2024, 276, 111358. [Google Scholar] [CrossRef]
- Wu, Z.; Su, Y.; Li, J.; Liu, X.; Liu, Y.; Zhao, L.; Li, L.; Zhang, L. Induced pluripotent stem cell-derived mesenchymal stem cells: Whether they can become new stars of cell therapy. Stem Cell Res. Ther. 2024, 15, 367. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Lu, J.; Chen, X.; Zheng, T.; He, R.; Ye, C.; Xu, J. Therapeutic potential of mesenchymal stem cell-derived exosomes in skeletal diseases. Front. Mol. Biosci. 2024, 11, 1268019. [Google Scholar] [CrossRef] [PubMed]
- Janockova, J.; Slovinska, L.; Harvanova, D.; Spakova, T.; Rosocha, J. New therapeutic approaches of mesenchymal stem cells-derived exosomes. J. Biomed. Sci. 2021, 28, 39. [Google Scholar] [CrossRef] [PubMed]
- Oyarce, K.; Cepeda, M.Y.; Lagos, R.; Garrido, C.; Vega-Letter, A.M.; Garcia-Robles, M.; Luz-Crawford, P.; Elizondo-Vega, R. Neuroprotective and Neurotoxic Effects of Glial-Derived Exosomes. Front. Cell. Neurosci. 2022, 16, 920686. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Seo, W.; Kim, D.K.; Moon, P.G.; Kim, S.H.; Lee, B.H.; Song, B.J.; Baek, M.C. Exogenous exosomes from mice with acetaminophen-induced liver injury promote toxicity in the recipient hepatocytes and mice. Sci. Rep. 2018, 8, 16070. [Google Scholar] [CrossRef]
- Chang, X.; Yao, J.; He, Q.; Liu, M.; Duan, T.; Wang, K. Exosomes From Women With Preeclampsia Induced Vascular Dysfunction by Delivering sFlt (Soluble Fms-Like Tyrosine Kinase)-1 and sEng (Soluble Endoglin) to Endothelial Cells. Hypertension 2018, 72, 1381–1390. [Google Scholar] [CrossRef]
- Pishavar, E.; Luo, H.; Naserifar, M.; Hashemi, M.; Toosi, S.; Atala, A.; Ramakrishna, S.; Behravan, J. Advanced Hydrogels as Exosome Delivery Systems for Osteogenic Differentiation of MSCs: Application in Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 6203. [Google Scholar] [CrossRef]
- Zhao, C.; Li, J.; Cai, H.; Wu, D.; Tao, S.; Pi, C.; Zhu, L.; Xu, N.; Zhang, T. An injectable hydrogel scaffold with IL-1β-activated MSC-derived exosomes for the treatment of endometritis. Biomater. Sci. 2023, 11, 1422–1436. [Google Scholar] [CrossRef]
- Ghorbani, R.; Abbaszadeh, H.A.; Ramezani, R.; Taghipour, N.; Rahimpour, A.; Hosseinzadeh, S. Encapsulation of AD-MSC- derived extracellular nanovesicles in an electrospun three-layer scaffold: Characterization and controlled release analysisin vitro. Biomed. Mater. 2025, 20, 015038. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Gardin, C.; Zamparini, F.; Ferroni, L.; Esposti, M.D.; Parchi, G.; Ercan, B.; Manzoli, L.; Fava, F.; Fabbri, P.; et al. Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells. Nanomaterials 2020, 10, 432. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, Y.; Huang, M.; Luo, G.; Ma, Y.; Wang, X. Microdroplets Encapsulated with NFATc1-siRNA and Exosomes-Derived from MSCs Onto 3D Porous PLA Scaffold for Regulating Osteoclastogenesis and Promoting Osteogenesis. Int. J. Nanomed. 2024, 19, 3423–3440. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Wang, X.; Liu, H.; Lu, Y.; Bian, J.; Sun, J.; Huang, D. Zein Increases the Cytoaffinity and Biodegradability of Scaffolds 3D-Printed with Zein and Poly(ε-caprolactone) Composite Ink. ACS Appl. Mater. Interfaces 2018, 10, 18551–18559. [Google Scholar] [CrossRef]
- Rahmati, S.; Khazaei, M.; Nadi, A.; Alizadeh, M.; Rezakhani, L. Exosome-loaded scaffolds for regenerative medicine in hard tissues. Tissue Cell 2023, 82, 102102. [Google Scholar] [CrossRef]
- Chachques, J.C.; Gardin, C.; Lila, N.; Ferroni, L.; Migonney, V.; Falentin-Daudre, C.; Zanotti, F.; Trentini, M.; Brunello, G.; Rocca, T.; et al. Elastomeric Cardiowrap Scaffolds Functionalized with Mesenchymal Stem Cells-Derived Exosomes Induce a Positive Modulation in the Inflammatory and Wound Healing Response of Mesenchymal Stem Cell and Macrophage. Biomedicines 2021, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Gloria, A.; Cometa, S.; Coelho, J.F.J.; Domingos, M. Effect of in vitro enzymatic degradation on 3D printed poly(ε-caprolactone) scaffolds: Morphological, chemical and mechanical properties. J. Appl. Biomater. Funct. Mater. 2017, 15, e185–e195. [Google Scholar] [CrossRef]
- Lian, M.; Qiao, Z.; Qiao, S.; Zhang, X.; Lin, J.; Xu, R.; Zhu, N.; Tang, T.; Huang, Z.; Jiang, W.; et al. Nerve Growth Factor-Preconditioned Mesenchymal Stem Cell-Derived Exosome-Functionalized 3D-Printed Hierarchical Porous Scaffolds with Neuro-Promotive Properties for Enhancing Innervated Bone Regeneration. ACS Nano 2024, 18, 7504–7520. [Google Scholar] [CrossRef]
- Bi, Y.; Qiao, X.; Liu, Q.; Song, S.; Zhu, K.; Qiu, X.; Zhang, X.; Jia, C.; Wang, H.; Yang, Z.; et al. Systemic proteomics and miRNA profile analysis of exosomes derived from human pluripotent stem cells. Stem Cell Res. Ther. 2022, 13, 449. [Google Scholar] [CrossRef]
- González-Cubero, E.; González-Fernández, M.L.; Gutiérrez-Velasco, L.; Navarro-Ramírez, E.; Villar-Suárez, V. Isolation and characterization of exosomes from adipose tissue-derived mesenchymal stem cells. J. Anat. 2021, 238, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Currey, J.D.; Dean, M.N.; Shahar, R. Revisiting the links between bone remodelling and osteocytes: Insights from across phyla. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1702–1719. [Google Scholar] [CrossRef]
- Aguayo-Mazzucato, C. Functional changes in beta cells during ageing and senescence. Diabetologia 2020, 63, 2022–2029. [Google Scholar] [CrossRef]
- Smirnova, A.; Yatsenko, E.; Baranovskii, D.; Klabukov, I. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: The risk of senescent drift induction in secretome-based therapeutics. Mil. Med. Res. 2023, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Altıntaş, Ö.; Saylan, Y. Exploring the Versatility of Exosomes: A Review on Isolation, Characterization, Detection Methods, and Diverse Applications. Anal. Chem. 2023, 95, 16029–16048. [Google Scholar] [CrossRef] [PubMed]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Tan, S.H.S.; Wong, J.R.Y.; Sim, S.J.Y.; Tjio, C.K.E.; Wong, K.L.; Chew, J.R.J.; Hui, J.H.P.; Toh, W.S. Mesenchymal stem cell exosomes in bone regenerative strategies-a systematic review of preclinical studies. Mater. Today Bio 2020, 7, 100067. [Google Scholar] [CrossRef]
- Ma, C.Y.; Zhai, Y.; Li, C.T.; Liu, J.; Xu, X.; Chen, H.; Tse, H.F.; Lian, Q. Translating mesenchymal stem cell and their exosome research into GMP compliant advanced therapy products: Promises, problems and prospects. Med. Res. Rev. 2024, 44, 919–938. [Google Scholar] [CrossRef]
| Effect | Specifications | Citation | |
|---|---|---|---|
| Promotion of Osteogenesis | Enhance osteoblast proliferation and differentiation | Deliver specific microRNAs (miRNAs) that promote osteogenic markers and inhibit negative regulators of bone formation. For instance, miR-21 enhances osteoblast proliferation and differentiation by targeting the TGF-β signaling pathway. miR-29a promotes osteoblast differentiation and extracellular matrix mineralization. | [49] |
| [50] | |||
| [51] | |||
| Influence osteoblast maturation into osteocytes | Exosomes deliver specific microRNAs (miRNAs) that influence the maturation of osteoblasts into osteocytes. | [49] | |
| [50] | |||
| Stimulation of Angiogenesis | Promote formation of new blood vessels | Crucial for supplying nutrients and oxygen to regenerate bone tissue and develop osteocytes within that tissue. | [52] |
| In Vivo Bone Regeneration | Accelerate bone healing, increase bone volume, and improve mechanical strength | Animal models of bone defects have demonstrated this in response to local or systemic administration. Crucial for restoring structural integrity and long-term functionality of bone tissue | [18] |
| [54] | |||
| Modulation of Inflammatory Response | Create a favorable environment for tissue regeneration | MSC-derived exosomes can modulate the inflammatory response in the bone defect site | [55] |
| Bioactive Cargo | Function | Target Factors | Signaling Pathway | MSC Type | Citation |
|---|---|---|---|---|---|
| miR-21 | Enhances osteoblast proliferation and differentiation | TGF-β modulators | TGF-β/Smad | BMSC | [51,57,58] |
| miR-29a | Promotes osteoblast differentiation and ECM mineralization | RUNX2, COL1A1 | Wnt/β-catenin | BMSC | [51] |
| miR-20a | Enhances osteogenic differentiation | BAMBI | TGF-β/Smad | BMSC-derived sEVs | [18] |
| miR-540-3p | Enhances immune tolerance and reduces graft rejection | CD74 | NF-κB pathway | miR-540-3p-overexpressing MSCs | [16] |
| miR-16-5p | Suppresses inflammation and tumor progression | Cyclins, BCL2 | Apoptosis, NF-κB | MSC-derived exosomes | [17] |
| miR-146a | Suppresses inflammation, promotes osteogenic survival | TRAF6, IRAK1 | NF-κB inhibition | MSC-derived exosomes | [57,58] |
| miR-181 | Regulates inflammation and supports osteogenesis | Notch regulators | NF-κB, Notch | MSC-derived exosomes | [57,58] |
| Pro-inflammatory cytokines (IL-1β, IFN-γ) | Inhibit osteogenesis and increase bone resorption | Osteoblasts, immune cells | NF-κB, MAPK | Present in some MSC-derived exosomes (low levels) | [16] |
| Anti-inflammatory cytokines (IL-10, TGF-β1) | Promote osteogenesis and suppress inflammation | Immune cells, osteoblasts | TGF-β/Smad, NRF2 | AD-MSC, UC-MSC, iMSC | [16] |
| VEGF | Promotes angiogenesis and bone vascularization | VEGFR | PI3K/Akt | UC-MSC, iMSC | [59] |
| HGF | Enhances angiogenesis and tissue repair | c-Met | MAPK/ERK | MSC-derived exosomes | [59] |
| BMPs | Induce osteogenesis | BMP receptors | BMP/Smad pathway | iMSC, BM-MSC | [43] |
| Preconditioning Method | Specific Strategy/Stimulus | Enhanced Effects | Citation |
|---|---|---|---|
| Inflammatory Cytokine Stimulation | TNFα Preconditioning | Suppresses IL-1β and iNOS and increases Arg1 and CD206 in macrophages; enhances bone formation | [75] |
| Pro-inflammatory Cytokines (TNFα, IL17A) | Mimics inflammatory conditions to boost immunosuppressive exosomal content | [76,77] | |
| IL-1β and TNF-α Exposure | Reduces IL-1β and TNF-α and increases IL-10 and other anti-inflammatory cytokines | [78] | |
| Hypoxia Preconditioning | Low Oxygen Conditions | Enhances exosome yield, angiogenesis, and anti-inflammatory potential | [79,80,81] |
| Ischemic Preconditioning | Reduces TNFα and neutrophils, increases IL-10, and improves recovery in lung injury models | [82] | |
| Secretome Modulation | Boosts MSC survival, migration, and regenerative paracrine activity | [83,84,85] | |
| Mechanical Stress | Mechanical Strain (Exercise Mimetic) | Increases exosome production and enhances myogenic differentiation and cell proliferation | [86] |
| Mechanical Stress-Induced Cargo Modulation | Alters exosome miRNA cargo and modulates BMP signaling | [86,87] |
| Immune Cell Type | Experimental Model | Observed Immunomodulatory Effects | Citation |
|---|---|---|---|
| Macrophages | In Vitro | Induce either pro-inflammatory or anti-inflammatory phenotypes depending on the microenvironment | [93] |
| In Vivo | Promote the M2 macrophage phenotype in neuroinflammatory models | [15] | |
| Macrophages (DPSC-Exo specific) | In Vivo | Facilitate the conversion of macrophages from a pro-inflammatory to an anti-inflammatory phenotype, thereby suppressing periodontal inflammation | [29] |
| T cells | In Vitro | Suppress proliferation of inflammatory T cells (producing interferon-γ and IL-17) | [14] |
| T cells | In Vitro | Enhance IL-10 expression | [94] |
| T cells | In Vivo | Encourage regulatory T cell expansion and immune tolerance | [95] |
| Dendritic cells | In Vitro | Alter antigen presentation and T cell activation capacity | [96] |
| Dendritic cells | In Vitro | miR-540-3p modulates dendritic cells via the CD74/NF-κB pathway | [16] |
| Disease/Experimental Model | Exosome Origin | Key Findings | Citation |
|---|---|---|---|
| In Vitro Studies | |||
| Hypoxia/serum-deprived osteocytes | Adipose MSCs (ADSCs) | Exosomes reduce osteocyte apoptosis | [128] |
| Glucocorticoid-induced osteonecrosis model | Wharton’s jelly MSCs (WJ-MSCs) | Exosomes ameliorate osteocyte apoptosis | [129] |
| Hypoxia-induced apoptosis | Mouse-adipose-derived MSCs (ADSCs), post-laser | Exosomes reduce osteocyte apoptosis under stress | [130] |
| Mechanical stimulation | Osteocyte-derived EVs | miR-3110-5p and miR-3058-3p promote osteoblast differentiation | [123] |
| Osteoblast precursor culture | Bone marrow MSCs (BMSCs) | Increased ALP and RUNX2 expression, promoting osteogenesis | [92] |
| In Vivo Studies | |||
| Ovariectomy-induced osteoporosis | hiPSC-MSCs | Promote bone regeneration and angiogenesis | [137] |
| Glucocorticoid-induced osteonecrosis | MSCs | Prevent disease progression and support osteocyte survival | [129] |
| Femur fracture and OVX model | BM-MSCs with aptamer-exosomes | Improve bone mass and target bone delivery | [139] |
| Bone defect model in osteoporotic rats | UC-MSCs/plasma exosomes | Enhance bone formation and implant integration | [18,141] |
| Scaffold Type | Material Composition | Biodegradability | Mechanical Strength | Surface Characteristics | Citation |
|---|---|---|---|---|---|
| Hydrogels | Naturally derived or synthetic polymers | Enable sustained release of exosomes | Allow for controlled and prolonged release of biomolecules | Water-swollen networks | [148,149,150] |
| Mineral-Doped Poly(L-lactide) Acid Porous Scaffolds | Polylactic acid (PLA), calcium silicates (CaSi), dicalcium phosphate dihydrate (DCPD) | Porosity decreases after 28 days in simulated body fluid; bioresorbable | Pores range from 10 to 30 µm in diameter | Circular and elliptic pores; dynamic surface that creates a bone-forming microenvironment; exosomes are easily entrapped on the surface | [151] |
| 3D-Printed Composites | Poly(l-lactide) (PLA) | Often biodegradable, with properties that can be tuned; zein-containing scaffolds show dose-responsive improvement in degradation rate | Mechanical strength can be enhanced by incorporating materials like multi-walled carbon nanotubes (MWCNTs) in poly(L-lactide); addition of pristine graphene improves mechanical performance; Young’s modulus and yield stress can be enhanced | Can have specific architectures and porous structures; can be coated with materials like poly(dopamine) and fibrin gel for bioactivity; can exhibit better cell affinity with components like zein | [49,152,153] |
| Acellular Extracellular Matrices (ECMs) | Decellularized tissues, preserving natural tissue architecture | Biodegradable | Provide structural support | Mimic the native extracellular environment, promoting cell adhesion and growth | [154] |
| Hyaluronic Acid (HA) | Natural polysaccharide | Biodegradable | Can be formulated to have various mechanical properties | Highly biocompatible, often used in hydrogels | [148] |
| Polymer-Based Elastomeric Membranes | Polycaprolactone (PCL) | Biodegradable, with degradation rates that can be matched to tissue regeneration; zein can increase biodegradability | Can be designed with favorable mechanical properties; PCL/pristine graphene scaffolds show improved mechanical performance; PCL/zein composite inks can significantly improve Young’s modulus and yield stress | Exosomes are easily entrapped on the surface; electrospun nanofibers offer a high surface-to-volume ratio; hydrophobicity can be reduced by adding pristine graphene | [150,153,155,156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arun, M.; Rajasingh, S.; Madasamy, P.; Rajasingh, J. Immunomodulatory and Regenerative Functions of MSC-Derived Exosomes in Bone Repair. Bioengineering 2025, 12, 844. https://doi.org/10.3390/bioengineering12080844
Arun M, Rajasingh S, Madasamy P, Rajasingh J. Immunomodulatory and Regenerative Functions of MSC-Derived Exosomes in Bone Repair. Bioengineering. 2025; 12(8):844. https://doi.org/10.3390/bioengineering12080844
Chicago/Turabian StyleArun, Manorathna, Sheeja Rajasingh, Parani Madasamy, and Johnson Rajasingh. 2025. "Immunomodulatory and Regenerative Functions of MSC-Derived Exosomes in Bone Repair" Bioengineering 12, no. 8: 844. https://doi.org/10.3390/bioengineering12080844
APA StyleArun, M., Rajasingh, S., Madasamy, P., & Rajasingh, J. (2025). Immunomodulatory and Regenerative Functions of MSC-Derived Exosomes in Bone Repair. Bioengineering, 12(8), 844. https://doi.org/10.3390/bioengineering12080844








