Spectral Analysis of Extrahepatic Bile Ducts During Normothermic Liver Machine Perfusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Procedure
2.3. Normothermic Liver Machine Perfusion—Centre-Specific Protocol
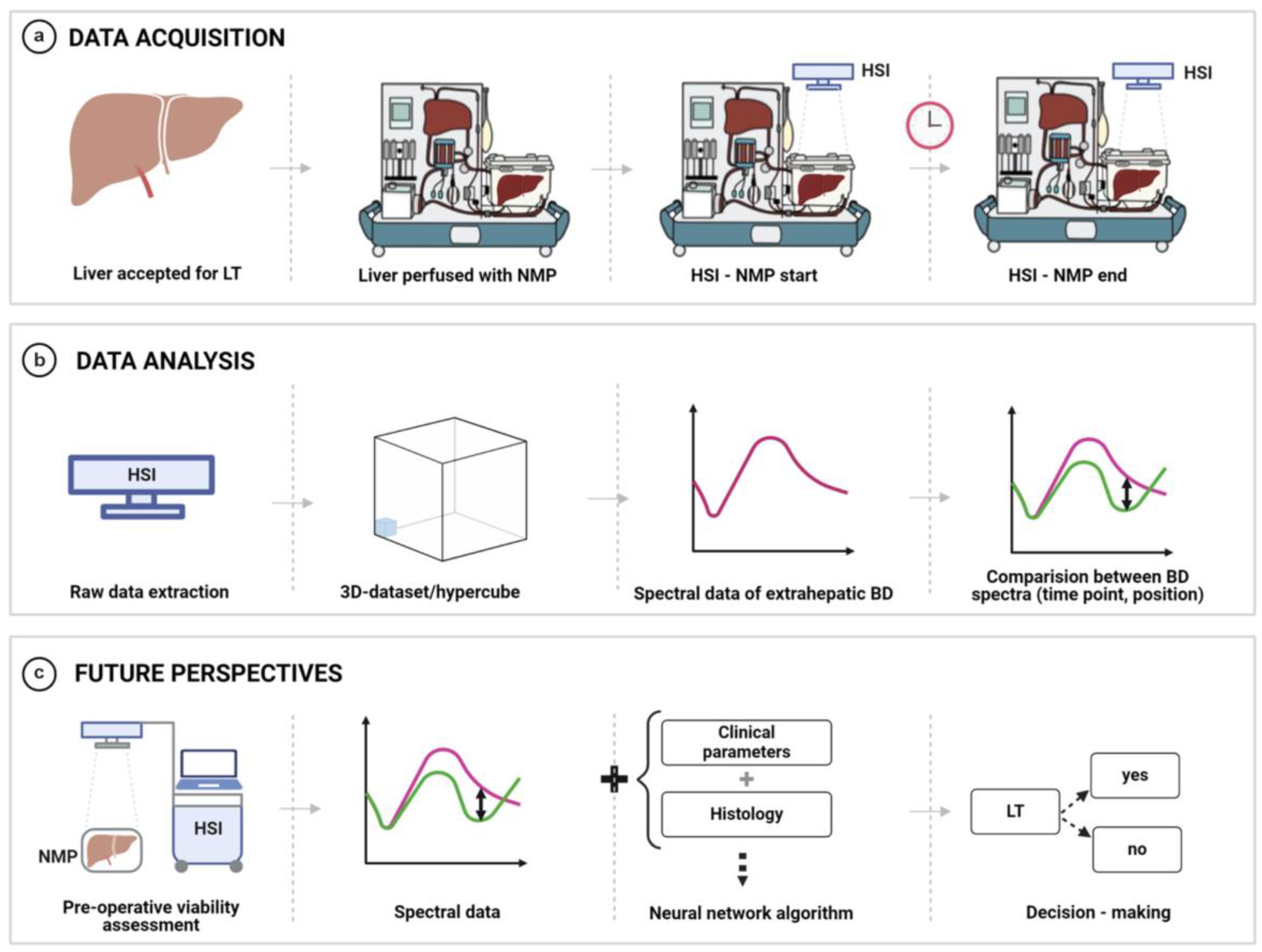
2.4. Hyperspectral Imaging of Extrahepatic Bile Ducts During Normothermic Liver Machine Perfusion
2.5. Definitions
2.5.1. Graft Loss and Graft Dysfunction
2.5.2. Rejections
2.5.3. Infectious Complications and Sepsis
2.5.4. Balance of Risk (BAR) Score
2.5.5. Classification and Quantification of Complications
2.6. Spectral Analysis and Data Processing
2.7. Statistical Analysis
3. Results
3.1. Recipient, Donor, and Preservation Characteristics
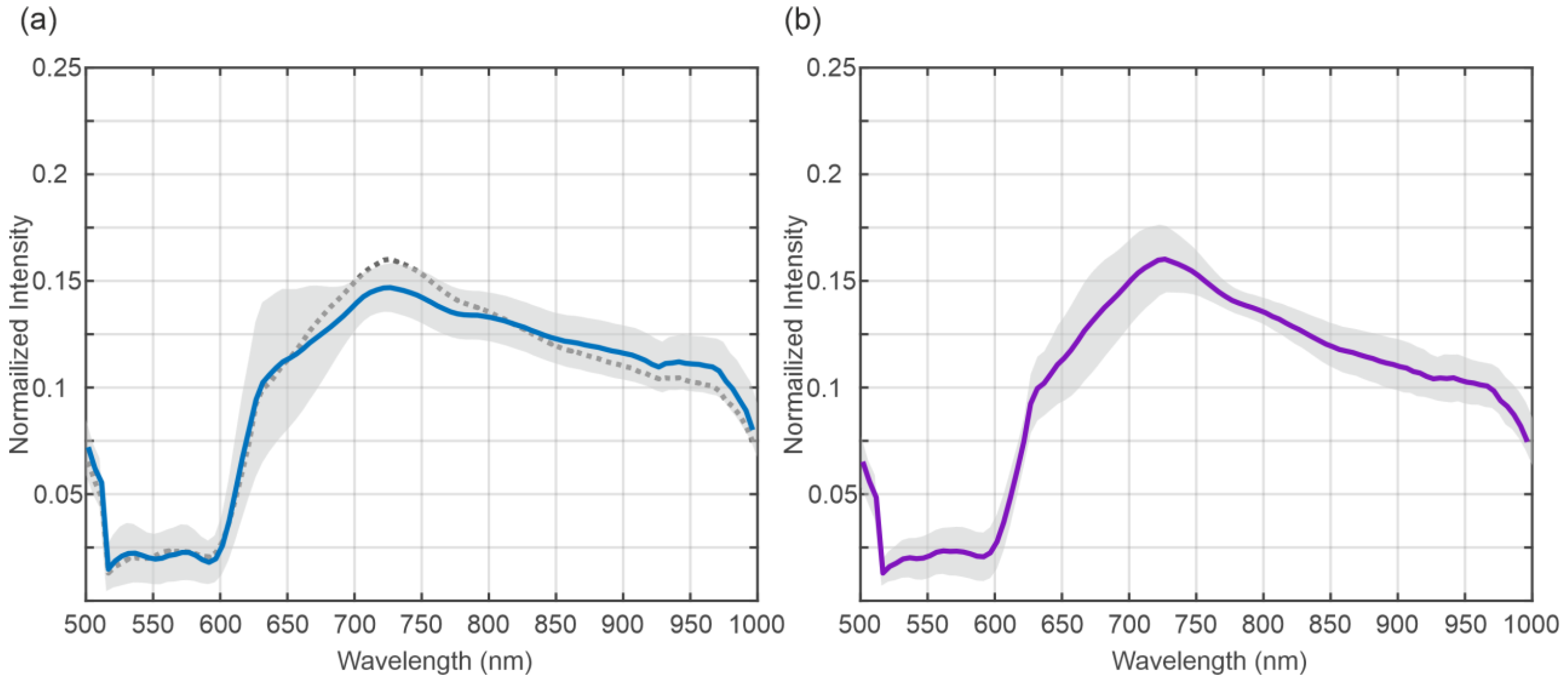
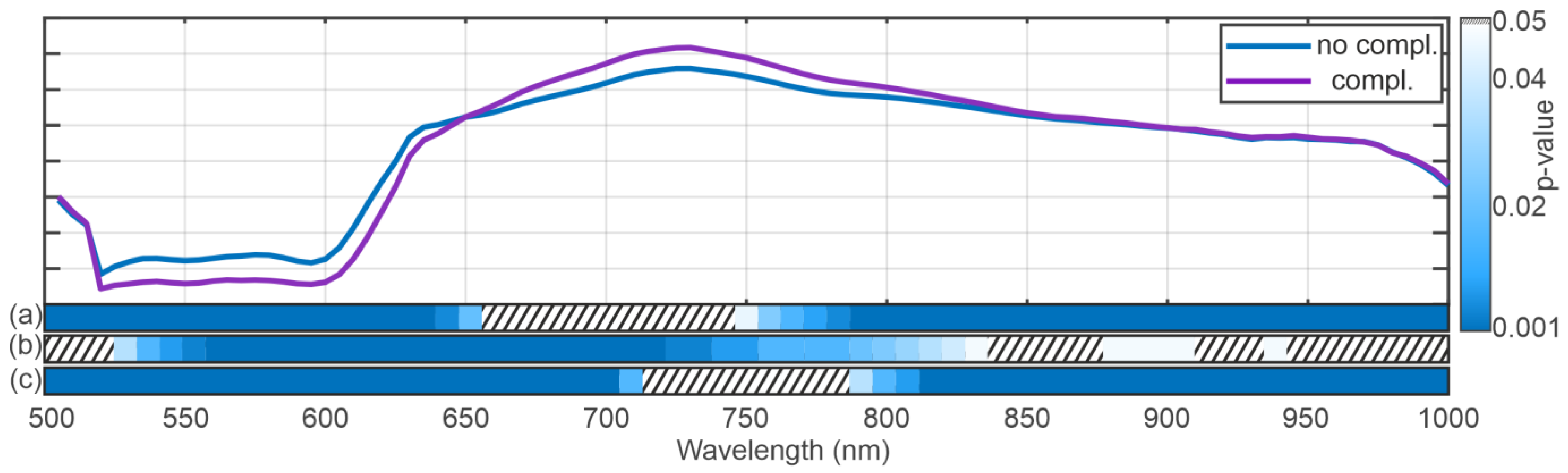
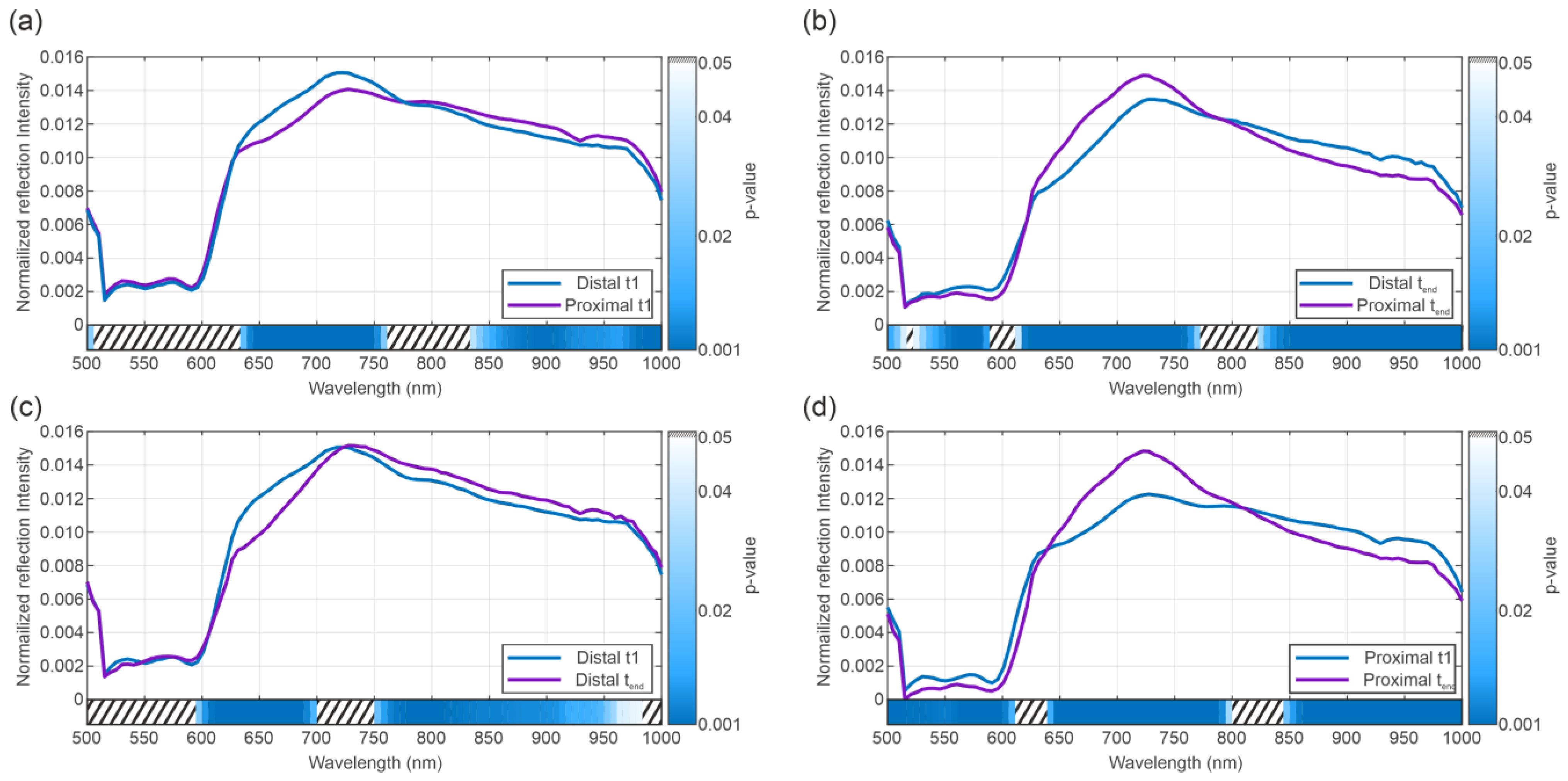
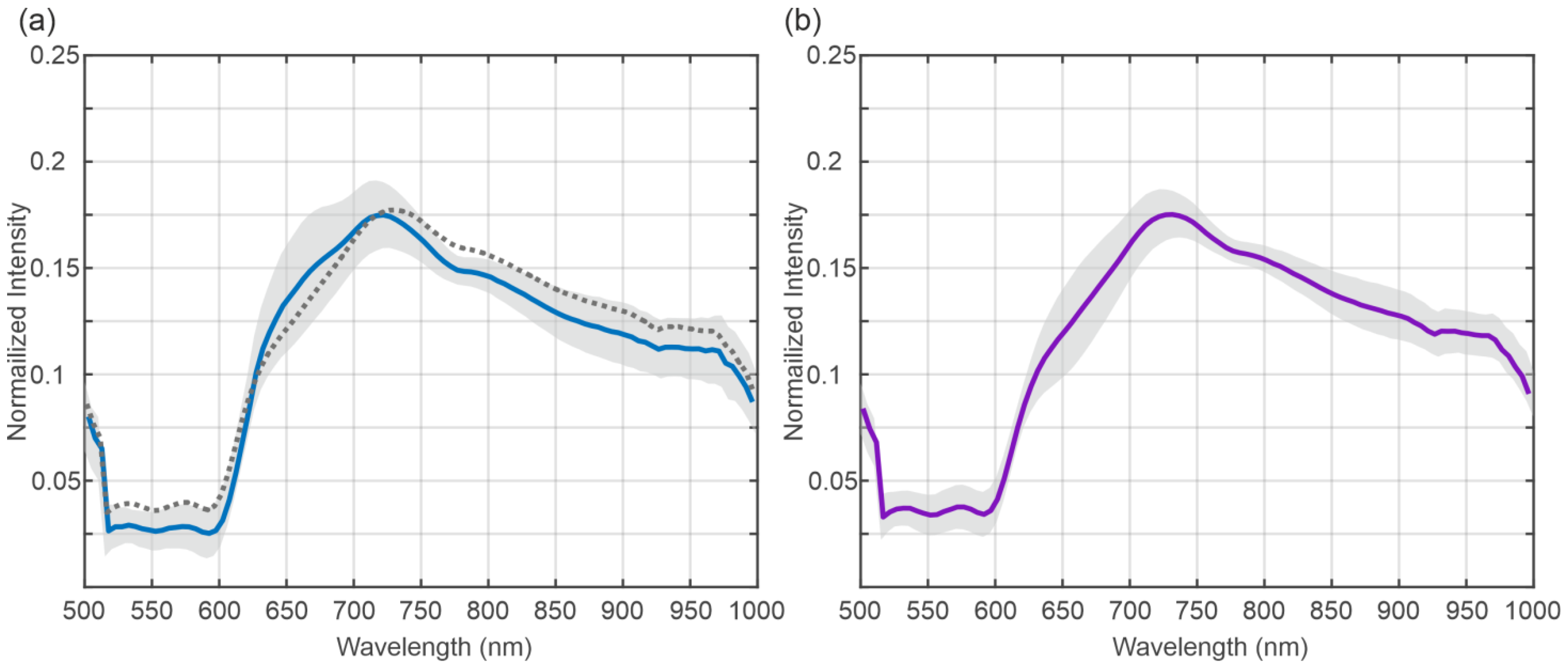
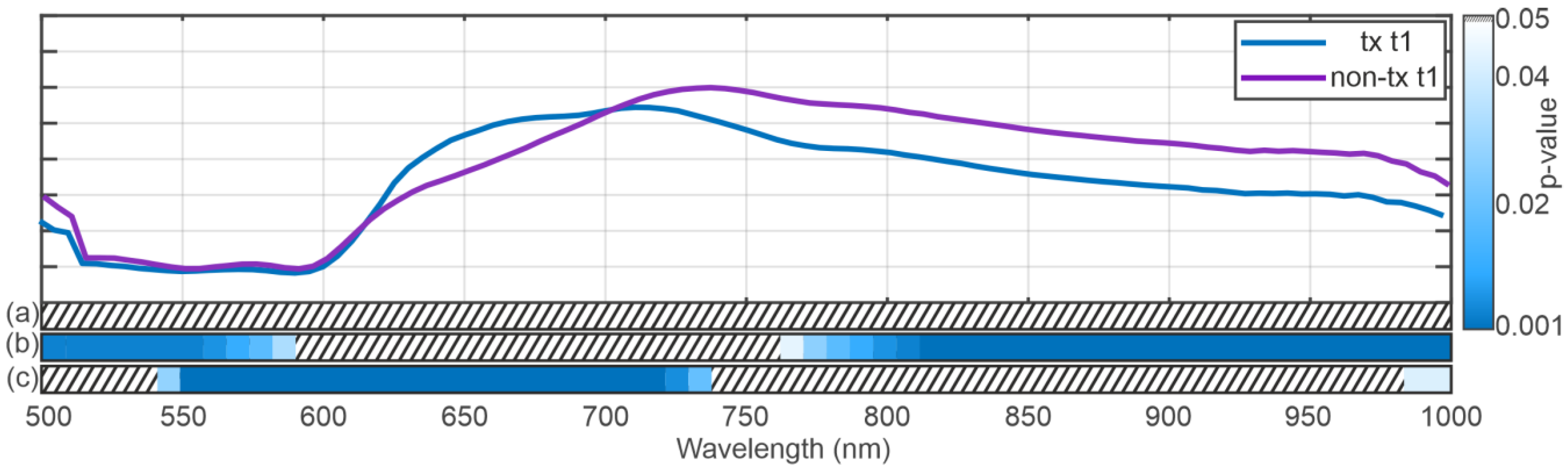
3.2. Spectral Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AS | Anastomotic stricture |
| AST | Aspartate aminotransferase |
| BAR | Balance of risk score |
| BD | Bile duct |
| BC | Biliary complications |
| CIT | Cold ischemia time |
| DCD | Donation after cardiac death |
| DBD | Donation after brain death |
| EAD | Early allograft dysfunction |
| ECD | Extended criteria donors |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| HSI | Hyperspectral imaging |
| HTK | Histidine-tryptophan-ketoglutarate |
| ICU | Intensive care unit |
| IGL-1 | Institut Georges Lopez |
| IQR | Interquartile range |
| IRI | Ischemia–reperfusion injury |
| LT | Liver transplantation |
| MELD | Model for end-stage liver disease |
| MRCP | Magnetic resonance cholangiopancreatography |
| NAS | Non-anastomotic stricture |
| NLMP | Normothermic liver machine perfusion |
| PNF | Primary non-function |
| ROI | Region of interest |
References
- Kochhar, G.; Parungao, J.M.; Hanouneh, I.A.; Parsi, M.A. Biliary complications following liver transplantation. World J. Gastroenterol. 2013, 19, 2841–2846. [Google Scholar] [CrossRef]
- Manay, P.; Seth, A.; Jackson, K.; Lentine, K.L.; Schnitzler, M.A.; Xiao, H.; Segev, D.L.; Axelrod, D.A. Biliary Complications After Liver Transplantation in the United States: Changing Trends and Economic Implications. Transplantation 2023, 107, e127–e138. [Google Scholar] [CrossRef] [PubMed]
- Cardini, B.; Oberhuber, R.; Fodor, M.; Hautz, T.; Margreiter, C.; Resch, T.; Scheidl, S.; Maglione, M.; Bösmüller, C.; Mair, H.; et al. Clinical Implementation of Prolonged Liver Preservation and Monitoring Through Normothermic Machine Perfusion in Liver Transplantation. Transplantation 2020, 104, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Margreiter, C.; Maglione, M.; Irsara, C.; Griesmacher, A.; Raynaud, M.; Breitkopf, R.; Troppmair, J.; Öfner, D.; Cardini, B.; Schneeberger, S. Perfusate Enzymes and Platelets Indicate Early Allograft Dysfunction after Transplantation of Normothermically Preserved Livers. Transplantation 2022, 106, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Fodor, M.; Zoller, H.; Oberhuber, R.; Sucher, R.; Seehofer, D.; Cillo, U.; Line, P.D.; Tilg, H.; Schneeberger, S. The Need to Update Endpoints and Outcome Analysis in the Rapidly Changing Field of Liver Transplantation. Transplantation 2022, 106, 938–949. [Google Scholar] [CrossRef]
- Fodor, M.; Cardini, B.; Peter, W.; Weissenbacher, A.; Oberhuber, R.; Hautz, T.; Otarashvili, G.; Margreiter, C.; Maglione, M.; Resch, T.; et al. Static cold storage compared with normothermic machine perfusion of the liver and effect on ischaemic-type biliary lesions after transplantation: A propensity score-matched study. Br. J. Surg. 2021, 108, 1082–1089. [Google Scholar] [CrossRef]
- Fodor, M.; Woerdehoff, A.; Peter, W.; Esser, H.; Oberhuber, R.; Margreiter, C.; Maglione, M.; Cardini, B.; Resch, T.; Weissenbacher, A.; et al. Reassessment of Relevance and Predictive Value of Parameters Indicating Early Graft Dysfunction in Liver Transplantation: AST Is a Weak, but Bilirubin and INR Strong Predictors of Mortality. Front. Surg. 2021, 8, 693288. [Google Scholar] [CrossRef]
- Cardini, B.; Fodor, M. Live Confocal Imaging as a Novel Tool to Assess Liver Quality: Insights From a Murine Model. Transplantation 2020, 104, 2528–2537. [Google Scholar] [CrossRef]
- Neuberger, J.; Callaghan, C. Organ utilization—The next hurdle in transplantation? Transpl. Int. 2020, 33, 1597–1609. [Google Scholar] [CrossRef]
- Ivanics, T.; Abreu, P.; De Martin, E.; Sapisochin, G. Changing Trends in Liver Transplantation: Challenges and Solutions. Transplantation 2021, 105, 743–756. [Google Scholar] [CrossRef]
- Ivanics, T.; Shwaartz, C.; Claasen, M.P.A.W.; Patel, M.S.; Yoon, P.; Raschzok, N.; Wallace, D.; Muaddi, H.; Murillo Perez, C.F.; Hansen, B.E.; et al. Trends in indications and outcomes of liver transplantation in Canada: A multicenter retrospective study. Transpl. Int. 2021, 34, 1444–1454. [Google Scholar] [CrossRef]
- Gilbo, N.; Neil, D.; Brais, R.; Fieuws, S.; Lo Faro, L.; Friend, P.; Ploeg, R.; Monbaliu, D. The Effect of Continuous Liver Normothermic Machine Perfusion on the Severity of Histological Bile Duct Injury. Transpl. Int. 2023, 36, 11645. [Google Scholar] [CrossRef]
- Fodor, M.; Zelger, P.; Pallua, J.D.; Huck, C.W.; Hofmann, J.; Otarashvili, G.; Pühringer, M.; Zelger, B.; Hermann, M.; Resch, T.; et al. Prediction of Biliary Complications After Human Liver Transplantation Using Hyperspectral Imaging and Convolutional Neural Networks: A Proof-of-concept Study. Transplantation 2023, 108, 506–515. [Google Scholar] [CrossRef]
- Karangwa, S.A.; Dutkowski, P.; Fontes, P.; Friend, P.J.; Guarrera, J.V.; Markmann, J.F.; Mergental, H.; Minor, T.; Quintini, C.; Selzner, M.; et al. Machine Perfusion of Donor Livers for Transplantation: A Proposal for Standardized Nomenclature and Reporting Guidelines. Am. J. Transplant. 2016, 16, 2932–2942. [Google Scholar] [CrossRef]
- Kulcke, A. PRINCIPLES AND CONTEXTS OF TISSUE OXYGENATION IMAGING (HSI TECHNOLOGY) AND TRANSCUTANEOUS OXYGEN MEASUREMENT IN TISSUE (TcPO2). 2015. Available online: https://www.researchgate.net/publication/331430995_PRINCIPLES_AND_CONTEXTS_OF_TISSUE_OXYGENATION_IMAGING_HSI_TECHNOLOGY_AND_TRANSCUTANEOUS_OXYGEN_MEASUREMENT_IN_TISSUE_TcPO2 (accessed on 19 July 2025).
- Fodor, M.; Hofmann, J.; Lanser, L.; Otarashvili, G.; Pühringer, M.; Hautz, T.; Sucher, R.; Schneeberger, S. Hyperspectral Imaging and Machine Perfusion in Solid Organ Transplantation: Clinical Potentials of Combining Two Novel Technologies. J. Clin. Med. 2021, 10, 3838. [Google Scholar] [CrossRef] [PubMed]
- Holmer, A.; Marotz, J.; Wahl, P.; Dau, M.; Kämmerer, P.W. Hyperspectral imaging in perfusion and wound diagnostics—Methods and algorithms for the determination of tissue parameters. Biomed. Tech. 2018, 63, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 10901. [Google Scholar] [CrossRef]
- Moulla, Y.; Buchloh, D.C.; Köhler, H.; Rademacher, S.; Denecke, T.; Meyer, H.J.; Mehdorn, M.; Lange, U.G.; Sucher, R.; Seehofer, D.; et al. Hyperspectral Imaging (HSI)—A New Tool to Estimate the Perfusion of Upper Abdominal Organs during Pancreatoduodenectomy. Cancers 2021, 13, 2846. [Google Scholar] [CrossRef]
- Mühle, R.; Ernst, H.; Sobottka, S.B.; Morgenstern, U. Workflow and hardware for intraoperative hyperspectral data acquisition in neurosurgery. Biomed. Tech. 2020, 66, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Mühle, R.; Markgraf, W.; Hilsmann, A.; Malberg, H.; Eisert, P.; Wisotzky, E.L. Comparison of different spectral cameras for image-guided organ transplantation. J. Biomed. Opt. 2021, 26, 076007. [Google Scholar] [CrossRef] [PubMed]
- Sucher, R.; Scheuermann, U.; Rademacher, S.; Lederer, A.; Sucher, E.; Hau, H.M.; Brandacher, G.; Schneeberger, S.; Gockel, I.; Seehofer, D. Intraoperative reperfusion assessment of human pancreas allografts using hyperspectral imaging (HSI). HepatoBiliary Surg. Nutr. 2021, 11, 67–77. [Google Scholar] [CrossRef]
- Sucher, R.; Athanasios, A.; Köhler, H.; Wagner, T.; Brunotte, M.; Lederer, A.; Gockel, I.; Seehofer, D. Hyperspectral Imaging (HSI) in anatomic left liver resection. Int. J. Surg. Case Rep. 2019, 62, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Florian, T. Hyperspectral imaging for monitoring oxygen saturation levels during normothermic kidney perfusion. J. Sens. Sens. Syst. 2016, 5, 313–318. [Google Scholar]
- Sucher, E.; Sucher, R.; Guice, H.; Schneeberger, S.; Brandacher, G.; Gockel, I.; Berg, T.; Seehofer, D. Hyperspectral Evaluation of the Human Liver During Major Resection. Ann. Surg. Open 2022, 3, e169. [Google Scholar] [CrossRef]
- Chang, C.-I. Hyperspectral Imaging: Techniques for Spectral Detection and Classification; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003; Volume 1. [Google Scholar]
- Pardo, A.; Gutierrez-Gutierrez, J.A.; Lihacova, I.; Lopez-Higuera, J.M.; Conde, O.M. On the spectral signature of melanoma: A non-parametric classification framework for cancer detection in hyperspectral imaging of melanocytic lesions. Biomed. Opt. Express 2018, 9, 6283–6301. [Google Scholar] [CrossRef]
- Bauer, S.; Puente León, F. Gewinnung und Verarbeitung hyperspektraler Fluoreszenzbilder zur optischen Mineralklassifikation. TM-Tech. Messen. 2015, 82, 24–33. [Google Scholar] [CrossRef]
- Bauer, S.; Puente León, F. Spectral and geometric aspects of mineral identification by means of hyperspectral fluorescence imaging. TM-Tech. Messen. 2015, 82, 597–605. [Google Scholar] [CrossRef]
- Bioucas-Dias, J.M.; Plaza, A.; Dobigeon, N.; Parente, M.; Du, Q.; Gader, P.; Chanussot, J. Hyperspectral Unmixing Overview: Geometrical, Statistical, and Sparse Regression-Based Approaches. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 354–379. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Chao, K.; Kim, M.S. Machine vision technology for agricultural applications. Comput. Electron. Agric. 2002, 36, 173–191. [Google Scholar] [CrossRef]
- Pallua, J.D.; Brunner, A.; Zelger, B.W.H.; Huck, C.; Schirmer, M.; Laimer, J.; Putzer, D.; Thaler, M.; Zelger, B. New perspectives of hyperspectral imaging for clinical research. NIR News 2021, 32, 5–13. [Google Scholar] [CrossRef]
- Turker-Kaya, S.; Huck, C.W. A Review of Mid-Infrared and Near-Infrared Imaging: Principles, Concepts and Applications in Plant Tissue Analysis. Molecules 2017, 22, 168. [Google Scholar] [CrossRef]
- Huck, C.W. Advances of vibrational spectroscopic methods in phytomics and bioanalysis. J. Pharm. Biomed. Anal. 2014, 87, 26–35. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of Micro-Fourier Transform Infrared Spectroscopy (FTIR) in the Geological Sciences—A Review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef]
- Pallua, J.D.; Brunner, A.; Zelger, B.; Stalder, R.; Unterberger, S.H.; Schirmer, M.; Tappert, M.C. Clinical infrared microscopic imaging: An overview. Pathol. Res. Pract. 2018, 214, 1532–1538. [Google Scholar] [CrossRef]
- Petter, C.H.; Heigl, N.; Rainer, M.; Bakry, R.; Pallua, J.; Bonn, G.K.; Huck, C.W. Development and application of Fourier-transform infrared chemical imaging of tumour in human tissue. Curr. Med. Chem. 2009, 16, 318–326. [Google Scholar] [CrossRef]
- Pezzei, C.; Pallua, J.D.; Schaefer, G.; Seifarth, C.; Huck Pezzei, V.; Bittner, L.K.; Klocker, H.; Bartsch, G.; Bonn, G.K.; Huck, C.W. Characterization of normal and malignant prostate tissue by Fourier transform infrared microspectroscopy. Mol. Biosyst. 2010, 6, 2287–2295. [Google Scholar] [CrossRef]
- Pallua, J.D.; Pezzei, C.; Zelger, B.; Schaefer, G.; Bittner, L.K.; Huck Pezzei, V.A.; Schoenbichler, S.A.; Hahn, H.; Kloss Branstaetter, A.; Kloss, F.; et al. Fourier transform infrared imaging analysis in discrimination studies of squamous cell carcinoma. Analyst 2012, 137, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Bec, K.B.; Grabska, J.; Huck, C.W. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar] [CrossRef]
- Huck, C.W.; Ozaki, Y.; Huck-Pezzei, V.A. Critical Review Upon the Role and Potential of Fluorescence and Near-Infrared Imaging and Absorption Spectroscopy in Cancer Related Cells, Serum, Saliva, Urine and Tissue Analysis. Curr. Med. Chem. 2016, 23, 3052–3077. [Google Scholar] [CrossRef]
- Baltussen, E.J.M.; Kok, E.N.D.; Brouwer de Koning, S.G.; Sanders, J.; Aalbers, A.G.J.; Kok, N.F.M.; Beets, G.L.; Flohil, C.C.; Bruin, S.C.; Kuhlmann, K.F.D.; et al. Hyperspectral imaging for tissue classification, a way toward smart laparoscopic colorectal surgery. J. Biomed. Opt. 2019, 24, 016002. [Google Scholar] [CrossRef]
- Kho, E.; de Boer, L.L.; Van de Vijver, K.K.; van Duijnhoven, F.; Vrancken Peeters, M.T.F.D.; Sterenborg, H.J.C.M.; Ruers, T.J.M. Hyperspectral Imaging for Resection Margin Assessment during Cancer Surgery. Clin. Cancer Res. 2019, 25, 3572–3580. [Google Scholar] [CrossRef]
- Tsai, T.J.; Mukundan, A.; Chi, Y.S.; Tsao, Y.M.; Wang, Y.K.; Chen, T.H.; Wu, I.C.; Huang, C.W.; Wang, H.C. Intelligent Identification of Early Esophageal Cancer by Band-Selective Hyperspectral Imaging. Cancers 2022, 14, 4292. [Google Scholar] [CrossRef]
- Wagner, T.; Katou, S.; Wahl, P.; Vogt, F.; Kneifel, F.; Morgul, H.; Vogel, T.; Houben, P.; Becker, F.; Struecker, B.; et al. Hyperspectral imaging for quantitative assessment of hepatic steatosis in human liver allografts. Clin. Transplant. 2022, 36, e14736. [Google Scholar] [CrossRef]
- Chen, H.M.; Shih, Y.H.; Wang, H.C.; Sun, Y.H.; Wang, R.C.; Teng, C.J. Detection of DLBCL by pixel purity index and iterative linearly constrained minimum variance into hyperspectral imaging analysis. J. Biophotonics 2022, 15, e202200143. [Google Scholar] [CrossRef]
- Woess, C.; Unterberger, S.H.; Roider, C.; Ritsch Marte, M.; Pemberger, N.; Cemper Kiesslich, J.; Hatzer Grubwieser, P.; Parson, W.; Pallua, J.D. Assessing various Infrared (IR) microscopic imaging techniques for post-mortem interval evaluation of human skeletal remains. PLoS ONE 2017, 12, e0174552. [Google Scholar] [CrossRef]
- Cucci, C.; Delaney, J.K.; Picollo, M. Reflectance Hyperspectral Imaging for Investigation of Works of Art: Old Master Paintings and Illuminated Manuscripts. Acc. Chem. Res. 2016, 49, 2070–2079. [Google Scholar] [CrossRef]
- Lugli, F.; Sciutto, G.; Oliveri, P.; Malegori, C.; Prati, S.; Gatti, L.; Silvestrini, S.; Romandini, M.; Catelli, E.; Casale, M.; et al. Near-infrared hyperspectral imaging (NIR-HSI) and normalized difference image (NDI) data processing: An advanced method to map collagen in archaeological bones. Talanta 2021, 226, 122126. [Google Scholar] [CrossRef]
- Feng, L.; Wu, B.; Zhu, S.; He, Y.; Zhang, C. Application of Visible/Infrared Spectroscopy and Hyperspectral Imaging With Machine Learning Techniques for Identifying Food Varieties and Geographical Origins. Front. Nutr. 2021, 8, 680357. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Yao, H.; Liu, Y.; Dai, X.; Brown, R.L.; Bhatnagar, D. Recent developments and applications of hyperspectral imaging for rapid detection of mycotoxins and mycotoxigenic fungi in food products. Crit. Rev. Food Sci. Nutr. 2019, 59, 173–180. [Google Scholar] [CrossRef]
- Jiang, H.; Yuan, W.; Ru, Y.; Chen, Q.; Wang, J.; Zhou, H. Feasibility of identifying the authenticity of fresh and cooked mutton kebabs using visible and near-infrared hyperspectral imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 282, 121689. [Google Scholar] [CrossRef]
- Yin, H.; Li, B.; Liu, Y.D.; Zhang, F.; Su, C.T.; Ou-Yang, A.G. Detection of early bruises on loquat using hyperspectral imaging technology coupled with band ratio and improved Otsu method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 283, 121775. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Sun, L.; Bai, H.; Li, X.; Wang, J.; Bai, S. Convolutional neural network for apple bruise detection based on hyperspectral. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 279, 121432. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Sun, J.; Wang, S.; Xu, M.; Yao, K.; Zhou, X. Nondestructive evaluation of Zn content in rape leaves using MSSAE and hyperspectral imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 281, 121641. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, J.; Yao, K.; Xu, M.; Wang, S.; Fu, L. Development of multi-disturbance bagging Extreme Learning Machine method for cadmium content prediction of rape leaf using hyperspectral imaging technology. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 279, 121479. [Google Scholar] [CrossRef]
- Luo, W.; Fan, G.; Tian, P.; Dong, W.; Zhang, H.; Zhan, B. Spectrum classification of citrus tissues infected by fungi and multispectral image identification of early rotten oranges. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 279, 121412. [Google Scholar] [CrossRef]
- Shen, F.; Deng, H.; Yu, L.; Cai, F. Open-source mobile multispectral imaging system and its applications in biological sample sensing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121504. [Google Scholar] [CrossRef]
- Schmidt, V.-M.; Zelger, P.; Wöss, C.; Fodor, M.; Hautz, T.; Schneeberger, S.; Huck, C.W.; Arora, R.; Brunner, A.; Zelger, B.; et al. Handheld hyperspectral imaging as a tool for the post-mortem interval estimation of human skeletal remains. Heliyon 2024, 10, e25844. [Google Scholar] [CrossRef]
- Cooney, G.S.; Barberio, M.; Diana, M.; Sucher, R.; Chalopin, C.; Köhler, H. Comparison of spectral characteristics in human and pig biliary system with hyperspectral imaging (HSI). Curr. Dir. Biomed. Eng. 2020, 6, 6–10. [Google Scholar] [CrossRef]
- Ishizawa, T.; Bandai, Y.; Kokudo, N. Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: An initial experience. Arch. Surg. 2009, 144, 381–382. [Google Scholar] [CrossRef]
- Brooke Smith, M.; Figueras, J.; Ullah, S.; Rees, M.; Vauthey, J.N.; Hugh, T.J.; Garden, O.J.; Fan, S.T.; Crawford, M.; Makuuchi, M.; et al. Prospective evaluation of the International Study Group for Liver Surgery definition of bile leak after a liver resection and the role of routine operative drainage: An international multicentre study. HPB 2015, 17, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- Sucher, R.; Wagner, T.; Köhler, H.; Sucher, E.; Quice, H.; Recknagel, S.; Lederer, A.; Hau, H.M.; Rademacher, S.; Schneeberger, S.; et al. Hyperspectral Imaging (HSI) of Human Kidney Allografts. Ann. Surg. 2022, 276, e48–e55. [Google Scholar] [CrossRef]
- Fodor, M.; Lanser, L.; Hofmann, J.; Otarashvili, G.; Pühringer, M.; Cardini, B.; Oberhuber, R.; Resch, T.; Weissenbacher, A.; Maglione, M.; et al. Hyperspectral Imaging as a Tool for Viability Assessment During Normothermic Machine Perfusion of Human Livers: A Proof of Concept Pilot Study. Transpl. Int. 2022, 35, 10355. [Google Scholar] [CrossRef]
- Eurotransplant. ET Liver Allocation System (ELAS). In Eurotransplant Manual; Eurotransplant: Leiden, The Netherlands, 2022; Available online: https://webshare.zenya.work/s17g8g5kzsxksz0c/Document.aspx?websharedocumentid=d74755e4-150a-444b-a3a8-37609c2015a0 (accessed on 29 July 2025).
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J.; Shaked, A.; Christie, J.D. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010, 16, 943–949. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Dutkowski, P.; Oberkofler, C.E.; Slankamenac, K.; Puhan, M.A.; Schadde, E.; Müllhaupt, B.; Geier, A.; Clavien, P.A. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. 2011, 254, 745–753, discussion 753. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.A. The comprehensive complication index: A novel continuous scale to measure surgical morbidity. Ann Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef]
- Felli, E.; Al-Taher, M.; Collins, T.; Nkusi, R.; Felli, E.; Baiocchini, A.; Lindner, V.; Vincent, C.; Barberio, M.; Geny, B.; et al. Automatic Liver Viability Scoring with Deep Learning and Hyperspectral Imaging. Diagnostics 2021, 11, 1527. [Google Scholar] [CrossRef]
- Signoroni, A.; Savardi, M.; Baronio, A.; Benini, S. Deep Learning Meets Hyperspectral Image Analysis: A Multidisciplinary Review. J. Imaging 2019, 5, 52. [Google Scholar] [CrossRef]
- Sommer, F.; Sun, B.; Fischer, J.; Goldammer, M.; Thiele, C.; Malberg, H.; Markgraf, W. Hyperspectral Imaging during Normothermic Machine Perfusion-A Functional Classification of Ex Vivo Kidneys Based on Convolutional Neural Networks. Biomedicines 2022, 10, 397. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Q.; Bu, S. Two-Stage Liver and Tumor Segmentation Algorithm Based on Convolutional Neural Network. Diagnostics 2021, 11, 1806. [Google Scholar] [CrossRef]
- Fabelo, H.; Halicek, M.; Ortega, S.; Szolna, A.; Morera, J.; Sarmiento, R.; Callico, G.M.; Fei, B. Surgical Aid Visualization System for Glioblastoma Tumor Identification based on Deep Learning and In-Vivo Hyperspectral Images of Human Patients. Proc. SPIE Int. Soc. Opt. Eng. 2019, 10951, 1095110. [Google Scholar] [CrossRef] [PubMed]
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Würdinger, M.; Meierhofer, D.; Clavien, P.A.; Dutkowski, P. Novel Real-time Prediction of Liver Graft Function During Hypothermic Oxygenated Machine Perfusion Before Liver Transplantation. Ann Surg. 2019, 270, 783–790. [Google Scholar] [CrossRef]
- Schlegel, A.; Kron, P.; Graf, R.; Clavien, P.A.; Dutkowski, P. Hypothermic Oxygenated Perfusion (HOPE) downregulates the immune response in a rat model of liver transplantation. Ann Surg. 2014, 260, 931–937, discussion 937–938. [Google Scholar] [CrossRef]
- Schlegel, A.; Dutkowski, P. Impact of Machine Perfusion on Biliary Complications after Liver Transplantation. Int. J. Mol. Sci. 2018, 19, 3567. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.T.P.R.; Isaac, J.R.; Clavien, P.A.; Muiesan, P.; Dutkowski, P. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J. Hepatol. 2019, 70, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Porte, R.; Dutkowski, P. Protective mechanisms and current clinical evidence of hypothermic oxygenated machine perfusion (HOPE) in preventing post-transplant cholangiopathy. J. Hepatol. 2022, 76, 1330–1347. [Google Scholar] [CrossRef]
- Schlegel, A.; Dutkowski, P. Role of hypothermic machine perfusion in liver transplantation. Transpl. Int. 2015, 28, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transplant. 2018, 18, 2005–2020. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Jochmans, I. From “Gut Feeling” to Objectivity: Machine Preservation of the Liver as a Tool to Assess Organ Viability. Curr. Transplant. Rep. 2018, 5, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Hunt, F.; Messer, S.; Currie, I.; Large, S.; Sutherland, A.; Crick, K.; Wigmore, S.J.; Fear, C.; Cornateanu, S.; et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am. J. Transplant. 2019, 19, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- de Jong, I.E.M.; Bodewes, S.B.; van Leeuwen, O.B.; Oosterhuis, D.; Lantinga, V.A.; Thorne, A.M.; Lascaris, B.; van den Heuvel, M.C.; Wells, R.G.; Olinga, P.; et al. Restoration of Bile Duct Injury of Donor Livers During Ex Situ Normothermic Machine Perfusion. Transplantation 2023, 107, e161–e172. [Google Scholar] [CrossRef]
- Boyer, J.L. Bile formation and secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [CrossRef]
- Nachabé, R.; Evers, D.J.; Hendriks, B.H.; Lucassen, G.W.; van der Voort, M.; Wesseling, J.; Ruers, T.J. Effect of bile absorption coefficients on the estimation of liver tissue optical properties and related implications in discriminating healthy and tumorous samples. Biomed. Opt. Express 2011, 2, 600–614. [Google Scholar] [CrossRef]
- Zuzak, K.J.; Naik, S.C.; Alexandrakis, G.; Hawkins, D.; Behbehani, K.; Livingston, E. Intraoperative bile duct visualization using near-infrared hyperspectral video imaging. Am. J. Surg. 2008, 195, 491–497. [Google Scholar] [CrossRef] [PubMed]
| Paramter | Total (n = 7) |
|---|---|
| Donor and operative data | |
| Age (y) * | 61 (51–68) |
| Gender | |
| Man | 5 (71) |
| Woman | 2 (29) |
| CIT (h) * | 6 (5–8) |
| Cause of death | |
| Cerebrovascular | 2 (29) |
| Circulatory | 1 (14) |
| Trauma | 2 (29) |
| Other | 2 (29) |
| Donor Type | |
| ECD | 6 (86) |
| DBD | 6 (86) |
| DCD | 1 (14) |
| NLMP indication | |
| Complex recipient | 1 (14) |
| Marginal donor | 3 (43) |
| Logistics | 4 (57) |
| NLMP time (h) * | 19 (11–21) |
| Total preservation time (h) * | 22 (18–27) |
| Recipient data and postoperative outcome | |
| Age (y) * | 59 (58–65) |
| Gender | |
| Man | 5 (71) |
| Woman | 2 (29) |
| BMI (kg/m2) * | 25 (23–30) |
| MELD * | 16 (13–19) |
| BAR score * | 7 (5–8) |
| BAR score ≥ 8 | 2 (29) |
| Total hospital stay (d) * | 16 (15–26) |
| ICU stay (d) * | 3 (3–5) |
| Early allograft dysfunction | 3 (43) |
| Clavien Dindo ≥ 3 | 5 (71) |
| 30—days readmission rate (unplanned) | 1 (14) |
| Biliary complications | 2 (29) |
| ≤30 d | 2 (29) |
| >30 d | 1 (14) |
| Biliary leakage | 1 (14) |
| Anastomotic stricture | 2 (29) |
| Arterial complication | 3 (43) |
| Acute rejection | 1 (14) |
| Infectious complication | 5 (71) |
| Mortality rate | 0 (0) |
| Re-transplantation rate | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelger, P.; Jenewein, B.; Sovago, M.; Krendl, F.J.; Meszaros, A.T.; Cardini, B.; Gehwolf, P.; Pallua, J.D.; Graf, S.; Schneeberger, S.; et al. Spectral Analysis of Extrahepatic Bile Ducts During Normothermic Liver Machine Perfusion. Bioengineering 2025, 12, 966. https://doi.org/10.3390/bioengineering12090966
Zelger P, Jenewein B, Sovago M, Krendl FJ, Meszaros AT, Cardini B, Gehwolf P, Pallua JD, Graf S, Schneeberger S, et al. Spectral Analysis of Extrahepatic Bile Ducts During Normothermic Liver Machine Perfusion. Bioengineering. 2025; 12(9):966. https://doi.org/10.3390/bioengineering12090966
Chicago/Turabian StyleZelger, Philipp, Benjamin Jenewein, Magdalena Sovago, Felix J. Krendl, Andras T. Meszaros, Benno Cardini, Philipp Gehwolf, Johannes D. Pallua, Simone Graf, Stefan Schneeberger, and et al. 2025. "Spectral Analysis of Extrahepatic Bile Ducts During Normothermic Liver Machine Perfusion" Bioengineering 12, no. 9: 966. https://doi.org/10.3390/bioengineering12090966
APA StyleZelger, P., Jenewein, B., Sovago, M., Krendl, F. J., Meszaros, A. T., Cardini, B., Gehwolf, P., Pallua, J. D., Graf, S., Schneeberger, S., Fodor, M., & Oberhuber, R. (2025). Spectral Analysis of Extrahepatic Bile Ducts During Normothermic Liver Machine Perfusion. Bioengineering, 12(9), 966. https://doi.org/10.3390/bioengineering12090966







