Clinical Evidence of Wear Occurrence in CFR-PEEK and Metallic Osteosynthesis Implants: A Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
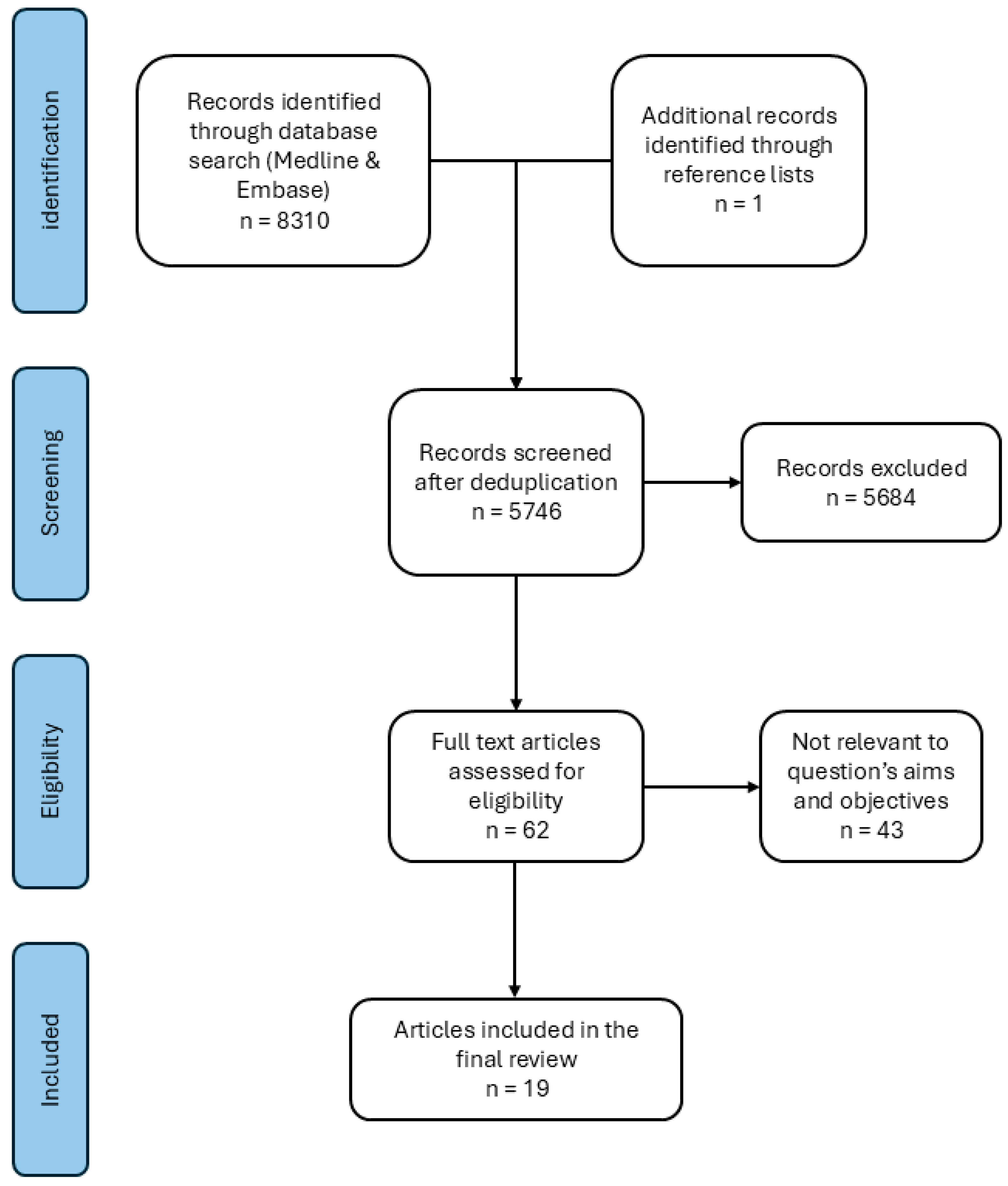
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Critical Appraisal
2.4. Data Extraction
3. Results
3.1. Study Characteristics and Critical Appraisal
3.2. Reported Wear in Plate Fixation
3.3. Reported Wear of IMN for Fracture Fixation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ORIF | Open reduction and internal fixation |
| SS | Stainless steel |
| CFR | Carbon fiber-reinforced |
| PEEK | Polyetheretherketone |
| IMN | Intramedullary nail |
Appendix A. Systematic Search Terms
- (Metallosis OR metalosis OR wear OR debris)
- AND
- (ORIF OR open reduction and internal fixation OR IRF OR internal rigid fixation OR break OR nonunion OR non-union OR malunion OR fractur* OR osteosynthesis)
- OR
- (carbon fiber reinforced poly* OR carbon fibre reinforced poly* OR carbon fiber reinforced poly ether* OR carbon fibre reinforced poly ether* OR Carbon Fiber Reinforced PEEK OR Carbon Fibre Reinforced PEEK OR CFR PEEK OR CFRPEEK OR CF/PEEK OR Carbon PEEK OR Carbon fiber plate* OR Carbon fibre plate* OR CF plate* OR CFR plate* OR Carbon plate*OR PEEK plate OR radiolucent plate* OR Carbon fiber implant* OR Carbon fibre implant* OR CF implant* OR CFR implant* OR Carbon implant*OR PEEK implant* OR radiolucent implant* OR Carbon fiber nail* OR Carbon fibre nail* OR CF nail* OR CFR nail* OR Carbon nail* OR PEEK nail* OR radiolucent nail*)
References
- Nilssen, P.; McKelvey, K.; Lin, C. Revision Surgery Risk After Open Reduction and Internal Fixation Versus Acute Total Hip Arthroplasty in Geriatric Acetabular Fractures: A Nationwide Study. JAAOS—J. Am. Acad. Orthop. Surg. 2024, 32, e533–e541. [Google Scholar] [CrossRef] [PubMed]
- Lunn, K.; Hurley, E.T.; Adu-Kwarteng, K.; Welch, J.M.; Levin, J.M.; Anakwenze, O.; Boachie-Adjei, Y.; Klifto, C.S. Complications Following Intramedullary Nailing of Proximal Humerus and Humeral Shaft Fractures: A Systematic Review. J. Shoulder Elb. Surg. 2025, 34, 626–638. [Google Scholar] [CrossRef]
- Sandhu, M.; Kumar, N.; Singh Sawhney, R.; Kaur, S.; Singh, K. Materials Used in Orthopedic Implants: A Comprehensive Review Study. Obstet. Gynaecol. Forum 2024, 34, 89–95. [Google Scholar]
- Shaikh, M.; Kahwash, F.; Lu, Z.; Alkhreisat, M.; Mohammad, A.; Shyha, I. Revolutionising Orthopaedic Implants—A Comprehensive Review on Metal 3D Printing with Materials, Design Strategies, Manufacturing Technologies, and Post-Process Machining Advancements. Int. J. Adv. Manuf. Technol. 2024, 134, 1043–1076. [Google Scholar] [CrossRef]
- Edelstein, Y.; Ohm, H.; Rosen, Y. Metallosis and Pseudotumor after Failed ORIF of a Humeral Fracture. Bull. NYU Hosp. Jt. Dis. 2011, 69, 188–191. [Google Scholar]
- Steven, M. Kurtz PEEK Biomaterials Handbook, 2nd ed.; William Andrew: Norwich, NY, USA, 2019. [Google Scholar]
- Baba, K.; Mori, Y.; Chiba, D.; Kuwahara, Y.; Kurishima, H.; Tanaka, H.; Kogure, A.; Kamimura, M.; Yamada, N.; Ohtsu, S.; et al. TiNbSn Stems with Gradient Changes of Young’s Modulus and Stiffness Reduce Stress Shielding Compared to the Standard Fit-and-Fill Stems. Eur. J. Med. Res. 2023, 28, 214. [Google Scholar] [CrossRef]
- Callister, W.D. Materials Science and Engineering: An Introduction, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 978-0471736967. [Google Scholar]
- Zhang, S.; Patel, D.; Brady, M.; Gambill, S.; Theivendran, K.; Deshmukh, S.; Swadener, J.; Junaid, S.; Leslie, L.J. Experimental Testing of Fracture Fixation Plates: A Review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2022, 236, 1253–1272. [Google Scholar] [CrossRef]
- Rahmitasari, F.; Ishida, Y.; Kurahashi, K.; Matsuda, T.; Watanabe, M.; Ichikawa, T. PEEK with Reinforced Materials and Modifications for Dental Implant Applications. Dent. J. 2017, 5, 35. [Google Scholar] [CrossRef]
- Li, C.S.; Vannabouathong, C.; Sprague, S.; Bhandari, M. The Use of Carbon-Fiber-Reinforced (CFR) PEEK Material in Orthopedic Implants: A Systematic Review. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2015, 8, CMAMD.S20354. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. Br. Med. J. 2016, 355, i4919. [Google Scholar] [CrossRef]
- Patton, M.S.; Lyon, T.D.B.; Ashcroft, G.P. Levels of Systemic Metal Ions in Patients with Intramedullary Nails. Acta Orthop. 2008, 79, 820–825. [Google Scholar] [CrossRef]
- Tanoğlu, O.; Say, F.; Yücens, M.; Alemdaroğlu, K.B.; İltar, S.; Aydoğan, N.H. Titanium Alloy Intramedullary Nails and Plates Affect Serum Metal Ion Levels within the Fracture Healing Period. Biol. Trace Elem. Res. 2020, 196, 60–65. [Google Scholar] [CrossRef]
- Jones, D.M.; Marsh, J.L.; Nepola, J.V.; Jacobs, J.J.; Skipor, A.K.; Urban, R.M.; Gilbert, J.L.; Buckwalter, J.A. Focal Osteolysis at the Junctions of a Modular Stainless-Steel Femoral Intramedullary Nail. J. Bone Jt. Surg. Am. Vol. 2001, 83, 537–548. [Google Scholar] [CrossRef]
- Cotic, M.; Vogt, S.; Hinterwimmer, S.; Feucht, M.J.; Slotta-Huspenina, J.; Schuster, T.; Imhoff, A.B. A Matched-Pair Comparison of Two Different Locking Plates for Valgus-Producing Medial Open-Wedge High Tibial Osteotomy: Peek–Carbon Composite Plate versus Titanium Plate. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Krischak, G.D.; Gebhard, F.; Mohr, W.; Krivan, V.; Ignatius, A.; Beck, A.; Wachter, N.J.; Reuter, P.; Arand, M.; Kinzl, L.; et al. Difference in Metallic Wear Distribution Released from Commercially Pure Titanium Compared with Stainless Steel Plates. Arch. Orthop. Trauma Surg. 2004, 124, 104–113. [Google Scholar] [CrossRef]
- Park, S.J.; Bae, G.C.; Kwon, D.G. Metallosis after Using Distal Fibular Locking Plate for Lateral Malleolar Fractures: A Retrospective Study. Arch. Orthop. Trauma Surg. 2022, 142, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Shahgaldi, B.F.; Compson, J. Wear and Corrosion of Sliding Counterparts of Stainless-Steel Hip Screw-Plates. Injury 2000, 31, 85–92. [Google Scholar] [CrossRef]
- Yeo, E.D.; Kim, H.J.; Cho, W.I.; Lee, Y.K. A Specialized Fibular Locking Plate for Lateral Malleolar Fractures. J. Foot Ankle Surg. 2015, 54, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Foong, B.; Panagiotopoulou, V.C.; Hothi, H.S.; Henckel, J.; Calder, P.R.; Goodier, D.W.; Hart, A.J. Assessment of Material Loss of Retrieved Magnetically Controlled Implants for Limb Lengthening. Proc. Inst. Mech. Eng. H. 2018, 232, 1129–1136. [Google Scholar] [CrossRef]
- Law, G.W.; Koh, J.S.B.; Yew, A.K.S.; Howe, T.S. Scanning Electron Microscopy Study of Retrieved Implants Suggests a Ratcheting Mechanism behind Medial Migration in Cephalomedullary Nailing of Hip Fractures. Malays. Orthop. J. 2020, 14, 7–17. [Google Scholar] [CrossRef]
- Fleischhacker, E.; Sprecher, C.M.; Milz, S.; Saller, M.M.; Wirz, R.; Zboray, R.; Parrilli, A.; Gleich, J.; Siebenbürger, G.; Böcker, W.; et al. Inflammatory Tissue Response in Human Soft Tissue Is Caused by a Higher Particle Load near Carbon Fiber-Reinforced PEEK Compared to Titanium Plates. Acta Biomater. 2024, 180, 128–139. [Google Scholar] [CrossRef]
- Bertoldi, C.; Zaffe, D.; Bellini, P.; Consolo, U. Metallic Elements in Tissues Surrounding Internal Rigid Fixation (IRF) Devices. Minerva Stomatol. 2001, 50, 121–132. [Google Scholar]
- Kumar, A.; Zaidi, S.M.H.; Sahito, B.; Kumar, D.; Ali, M. A Case Report of Metallosis with a Failed Distal Femur Plate. Cureus 2020, 12, e10361. [Google Scholar] [CrossRef]
- Merolli, A.; Rocchi, L.; De Spirito, M.; Federico, F.; Morini, A.; Mingarelli, L.; Fanfani, F. Debris of Carbon-Fibers Originated from a CFRP (PEEK) Wrist-Plate Triggered a Destruent Synovitis in Human. J. Mater. Sci. Mater. Med. 2016, 27, 50. [Google Scholar] [CrossRef] [PubMed]
- Metikala, S.; Bhogadi, P. Orthogonal Double Plating and Autologous Bone Grafting of Postoperative Humeral Shaft Nonunion—A Rare Case Report and Review of Literature. Orthpaedic Case Rep. J. Orthop. Case Rep. 2015, 5, 50–53. [Google Scholar]
- Tang, C.Q.Y.; Chuah, K.L.; Teoh, L.C. Metallosis Following Titanium Implant Use in the Hand: A Case Report and Review of Current Literature. J. Hand Microsurg. 2023, 15, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Stern, P.J. Humeral Nonunion Associated with Metallosis Secondary to Use of a Titanium Flexible Humeral Intramedullary Nail: A Case Report. J. Bone Jt. Surg. Am. Vol. 2002, 84, 2266–2269. [Google Scholar] [CrossRef]
- Ngo, D.; Todd, M.; Accadbled, F.; Foster, B.; Jellesen, M.S.; Rölfing, J.D.; Rawat, J. Corrosion of a Fassier-Duval Telescopic Nail Causing Pain and Osteolysis: A Case Report. JBJS Case Connect 2024, 14, e23. [Google Scholar] [CrossRef]
- Bozic, K.J.; Browne, J.; Dangles, C.J.; Manner, P.A.; Yates, A.J.J.; Weber, K.L.; Boyer, K.M.; Zemaitis, P.; Woznica, A.; Turkelson, C.M.; et al. Modern Metal-on-Metal Hip Implants. JAAOS—J. Am. Acad. Orthop. Surg. 2012, 20, 402–406. [Google Scholar] [CrossRef]
- Stratton-Powell, A.A.; Pasko, K.M.; Brockett, C.L.; Tipper, J.L. The Biologic Response to Polyetheretherketone (PEEK) Wear Particles in Total Joint Replacement: A Systematic Review. Clin. Orthop. Relat. Res. 2016, 474, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.; Skipor, A.K.; Patterson, L.M.; Hallab, N.J.; Paprosky, W.G.; Black, J.; Galante, J.O. Metal Release in Patients Who Have Had a Primary Total Hip Arthroplasty. A Prospective, Controlled, Longitudinal Study. J. Bone Jt. Surg. 1998, 80, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Vertesich, K.; Staats, K.; Böhler, C.; Koza, R.; Lass, R.; Giurea, A. Long Term Results of a Rotating Hinge Total Knee Prosthesis with Carbon-Fiber Reinforced Poly-Ether-Ether-Ketone (CFR-PEEK) as Bearing Material. Front. Bioeng. Biotechnol. 2022, 10, 845859. [Google Scholar] [CrossRef] [PubMed]
| Confounding | Classification | Selection | Deviation | Missing Data | Outcome Measures | Reporting | Overall | |
|---|---|---|---|---|---|---|---|---|
| Cotic | Low | Low | Low | Low | Low | Low | Low | Low |
| Fleschhacker | Low | Low | Serious | Low | Low | Moderate | Low | Serious |
| Krischak | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Park | Low | Low | Serious | Low | Low | Moderate | Low | Serious |
| Tanoğlu | Low | Low | Low | Low | Low | Low | Low | Low |
| Shahgaldi | Low | Low | Serious | Low | Low | Low | Low | Serious |
| Yeo | Low | Low | Low | Low | Low | Low | Low | Low |
| Foong | Low | Low | Low | Low | Low | Low | Low | Low |
| Patton | Low | Low | Low | Low | Low | Serious | Low | Serious |
| Jones | Low | Low | Low | Low | Low | Low | Low | Low |
| Law | Low | Low | serious | Low | Low | Low | Low | Serious |
| JBI Checklist Questions | Bertoldi | Edelstein | Kumar | Merolli | Metikala | Tang | Kang | Ngo |
|---|---|---|---|---|---|---|---|---|
| 1. Were the patient’s demographic characteristics clearly described? | N | Y | Y | Y | Y | Y | Y | Y |
| 2. Was the patient’s history clearly described and presented as a timeline? | N | Y | Y | Y | Y | Y | Y | Y |
| 3. Was the current clinical condition of the patient on presentation clearly described? | Y | Y | Y | Y | Y | Y | Y | Y |
| 4. Were diagnostic tests or assessment methods and the results clearly described? | Y | Y | Y | Y | Y | Y | Y | Y |
| 5. Was the intervention(s) or treatment procedure(s) clearly described? | Y | Y | Y | Y | Y | Y | Y | Y |
| 6. Was the post-intervention clinical condition clearly described? | Y | NA | Y | U | Y | Y | Y | Y |
| 7. Were adverse events (harms) or unanticipated events identified and described? | Y | N | Y | Y | Y | Y | Y | Y |
| 8. Does the case report provide takeaway lessons? | Y | Y | Y | Y | Y | Y | Y | Y |
| Article | Material | n | Anatomical Site(s) | Wear Incidence/Indication |
|---|---|---|---|---|
| [24] | CFR-PEEK Ti | 16 | Humerus | CFR-PEEK: 8/8 (100%) Ti: 8/8 (100%) |
| [29] | Ti | 1 | Fourth and fifth metacarpals | 1/1 (100%) |
| [19] | SS | 69 | Fibula | 4/69 (5.8%) |
| [26] | Metallic | 1 | Femur | 1/1 (100%) Preceding trauma 6 months prior to examination |
| [15] † | Ti | 10 | Femur (4 plate) Tibia (6 plate) | Significant increase in serum Ti, Al, V, and Mo ions |
| [27] | CFR-PEEK | 1 | Radius | 1/1 (100%) |
| [17] | CFR-PEEK | 26 | Tibia | CFR-PEEK: 15/26 (58%) |
| [28] | Ti | 1 | Humerus | 1/1 (100%) |
| [21] | SS | 27 | Fibula | 5/27 (18.5%) |
| [5] | Metallic | 1 | Humerus | 1/1 (100%) |
| [18] | Ti SS | 22 | Ti: fibula (4), clavicula (1), radius (3) SS: fibula (11), tibia (1), radius (2) | Ti: 5/8 (62.5%) SS: 14/14 (100%) |
| [25] | Metallic | 18 | Unknown | |
| [20] | SS | 15 | Femur | Wear identified on the screws and barrels |
| First Author | Material | n | Anatomical Site(s) | Wear Incidence/Indication |
|---|---|---|---|---|
| [31] | SS | 1 | Femur | 1/1 (100%) |
| [23] | Ti | 4 | Femur | 2/4 (50%) |
| [15] † | Ti | 10 | Femur (4) Tibia (6) | Significant increase in the serum Ti, Al, V, and Mo ion levels |
| [22] | Ti | 11 | Femur | 11/11 (100%) |
| [14] | Metallic | 41 | Tibia | Significant increase in the serum Ti and Cr |
| [30] | Ti | 1 | Humerus | 1/1 (100%) |
| [16] | SS | 25 | Femur | Increased Cr levels in modular IMN (1.04 ± 0.57 ng/mL) compared to one-piece IMNs (0.26 ± 0.40 ng/mL) or control (0.05 0.06 ng/mL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doodkorte, R.; Kuske, R.; Arts, J. Clinical Evidence of Wear Occurrence in CFR-PEEK and Metallic Osteosynthesis Implants: A Systematic Literature Review. Bioengineering 2025, 12, 965. https://doi.org/10.3390/bioengineering12090965
Doodkorte R, Kuske R, Arts J. Clinical Evidence of Wear Occurrence in CFR-PEEK and Metallic Osteosynthesis Implants: A Systematic Literature Review. Bioengineering. 2025; 12(9):965. https://doi.org/10.3390/bioengineering12090965
Chicago/Turabian StyleDoodkorte, Remco, Rachèl Kuske, and Jacobus Arts. 2025. "Clinical Evidence of Wear Occurrence in CFR-PEEK and Metallic Osteosynthesis Implants: A Systematic Literature Review" Bioengineering 12, no. 9: 965. https://doi.org/10.3390/bioengineering12090965
APA StyleDoodkorte, R., Kuske, R., & Arts, J. (2025). Clinical Evidence of Wear Occurrence in CFR-PEEK and Metallic Osteosynthesis Implants: A Systematic Literature Review. Bioengineering, 12(9), 965. https://doi.org/10.3390/bioengineering12090965







