1. Introduction
1.1. Melanoma Context and Diagnosis
Melanoma is one of the most aggressive forms of skin cancer, originating in melanocytes, the cells responsible for synthesizing melanin. Although it accounts for a relatively small percentage of all skin cancers, it is responsible for the majority of deaths related to these diseases due to its high metastatic potential and resistance to conventional therapies [
1]. According to the Global Cancer Observatory, in 2020 more than 325,000 new cases of melanoma were diagnosed worldwide, with over 57,000 associated deaths [
2].
Early diagnosis is a critical factor in patient prognosis. When melanoma is detected in its early stages, five-year survival rates exceed 90%; however, these rates drop drastically in advanced stages [
3]. Traditionally, diagnosis is performed through clinical and dermatoscopic evaluation, followed by histopathological confirmation after biopsy. Histopathology, particularly using standard stains such as H&E, remains the gold standard for the classification of skin lesions [
4].
Nevertheless, histopathological assessment presents significant limitations, such as inter-observer variability even among expert dermatopathologists and an increasing workload in pathology laboratories [
5]. This context has driven growing interest in computer-aided diagnosis (CAD) methods, especially those based on deep learning.
1.2. Digital Histopathology and Machine Learning
The digitization of histological samples using high-resolution scanners has given rise to the field known as digital pathology, which enables the storage, visualization, and analysis of tissue sections through images known as Whole Slide Images (WSI). This evolution has created new opportunities for the development of computer-aided diagnostic tools by facilitating systematic access to large volumes of histopathological data [
6].
In this context, deep learning has proven especially promising. Models such as Convolutional Neural Networks (CNN) have reached performance levels comparable to those of human experts in tasks such as tumor classification, metastasis detection, and cellular structure segmentation [
7,
8]. In the case of melanoma, several studies have shown that it is possible to train classification models with H&E-stained images, achieving clinically relevant results, particularly when distinguishing malignant melanomas from melanocytic nevi (
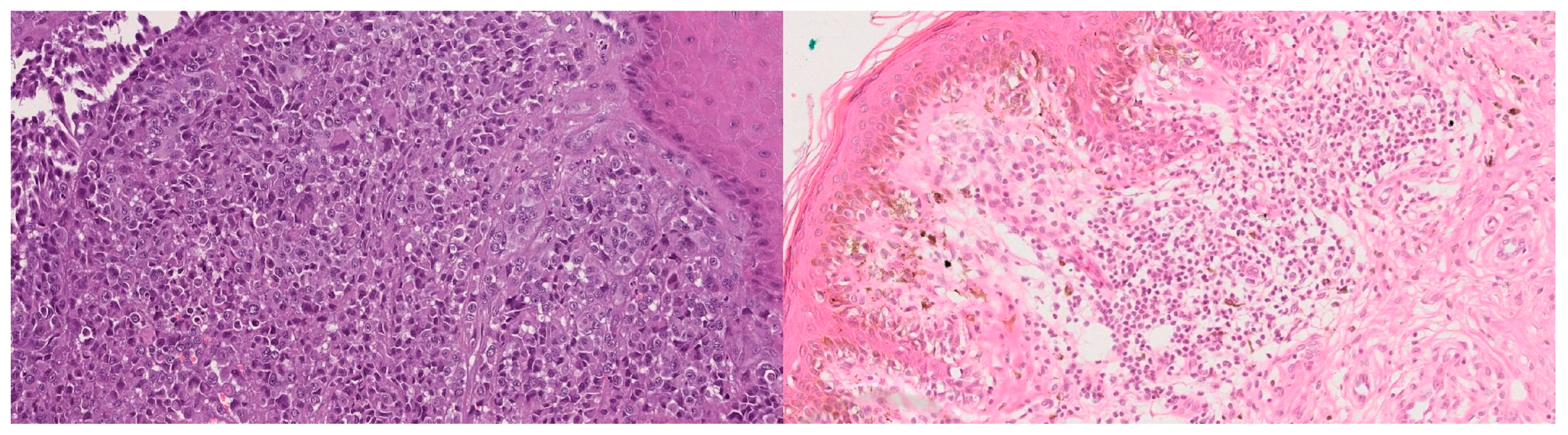
Figure 1)—the main diagnostic challenge in pigmented lesions [
9].
However, the performance of these models depends critically on the quantity and quality of the data available. In biomedical contexts, the number of labeled images is often limited, as annotation requires expert input. Moreover, datasets tend to be imbalanced, reflecting the low number of certain classes (such as melanocytic nevi) compared to others that are more frequent (such as invasive melanomas because these lesions are almost always biopsied). These conditions can induce overfitting and limit the generalization ability of trained models [
10].
1.3. Limitations of Biomedical Data
Developing deep learning models in the biomedical domain faces structural limitations inherent to the available data. Unlike other domains such as computer vision for everyday objects, where massive datasets like ImageNet exist, in the clinical environment obtaining large volumes of labeled images is much more challenging.
First, biomedical image annotation requires specialist intervention (e.g., pathologists or dermatologists), which involves high time and resource costs. In addition, clinical data are subject to ethical, legal, and privacy restrictions, which limit their accessibility and sharing across institutions [
11].
Second, these datasets are often imbalanced, reflecting the natural prevalence of diseases. In the case of melanoma, malignant samples are often much more frequent than melanocytic nevi, creating a bias that may impair model training [
12]. This imbalance can lead to models with apparently high performance that fail to generalize well to underrepresented cases, which are often the most clinically relevant.
Another common challenge is technical variability: differences in acquisition conditions, scanner type, staining protocols, or image processing can introduce non-biological noise that affects model performance. This is known as the
batch effect problem and can hinder model reproducibility in settings different from those in which it was originally trained [
13].
To address these limitations, the use of artificially generated synthetic data has been proposed as a complementary solution to increase dataset diversity, balance, and size, thereby improving the robustness and generalization of trained models.
1.4. Motivation for Using Synthetic Images
The scarcity, imbalance, and variability of biomedical datasets have driven the exploration of strategies to artificially expand training sets without the need to acquire new clinical data. One of the most promising approaches in this field is the use of synthetic images generated through deep learning models, particularly GAN [
14].
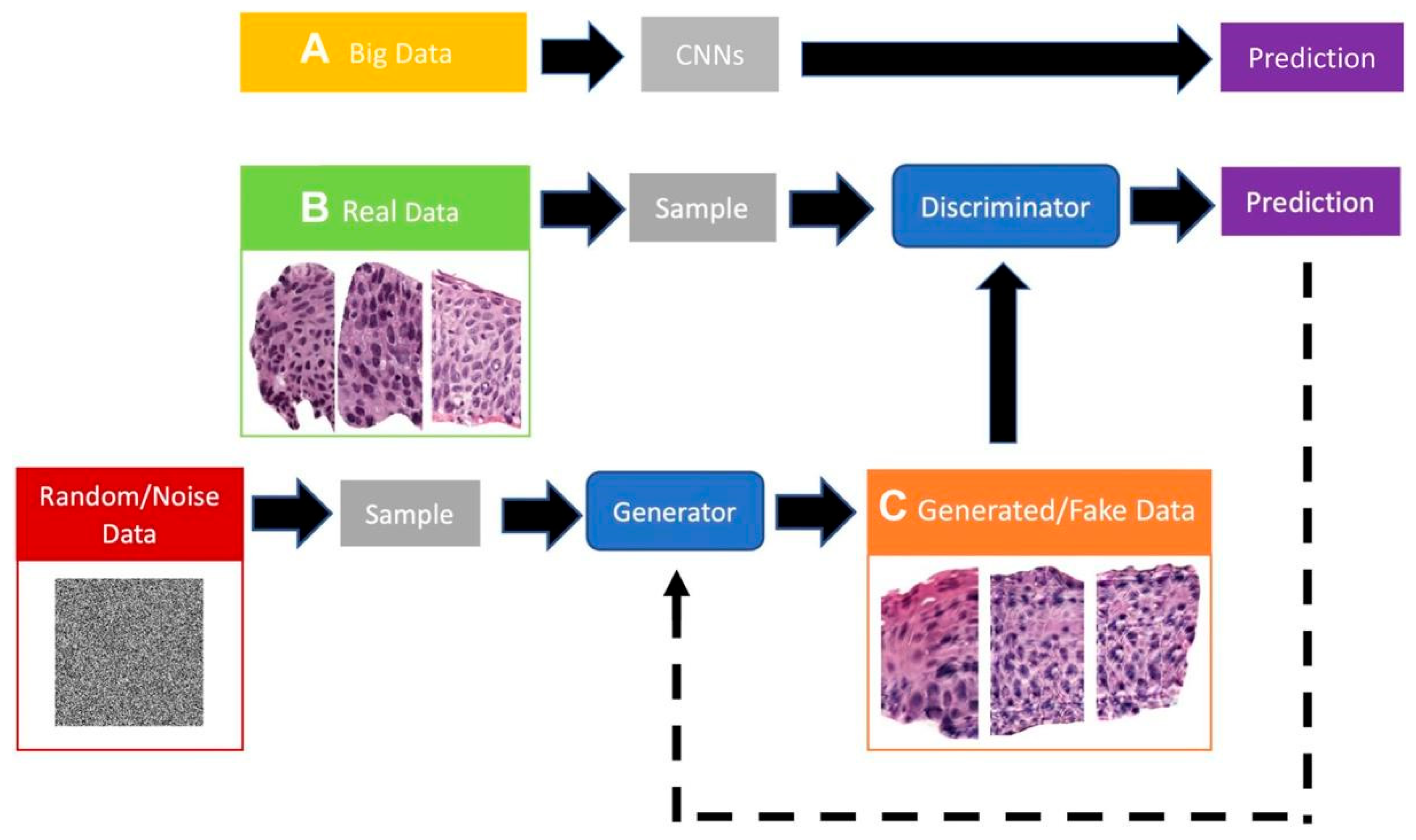
GANs allow the learning of the implicit probability distribution of a real dataset to generate new samples that, both visually and statistically, resemble the originals. This is especially valuable in histology, where images exhibit complex yet repetitive textural and structural patterns that can be effectively modeled by deep generative networks [
15,
16]. To improve accessibility for non-expert readers (e.g., clinicians), we retain a compact schematic of a GAN to visually anchor the concept (
Figure 2).
The use of synthetic images offers several potential advantages:
Increase the diversity of the training set, reducing the risk of overfitting.
Balance minority classes by generating more examples of rare lesions, such as invasive melanomas.
Simulate varied clinical conditions, including variability in staining, cell shape, and cell density, thereby improving classifier robustness.
Protect patient privacy, as generated data cannot be directly linked to actual individuals.
Recent studies [
17,
18] have shown that incorporating synthetic images into classifier training can significantly improve performance, particularly in medical classification tasks with limited real data. However, the quality of the generated images and the method of integration into the training workflow are critical factors that must be carefully assessed. Conventional augmentation techniques such as rotations, flips, and color jittering can partially mitigate class imbalance and variability, but they do not generate new morphological structures. GAN-based synthesis offers the possibility of creating images with novel textural and architectural features, potentially providing a complementary benefit beyond traditional methods.
This work is grounded in this motivation, aiming to explore the impact of GAN-generated synthetic images on the performance of melanoma classifiers trained on H&E-stained histological images.
1.5. General Purpose of the Project
The main goal of this work is to evaluate the impact of using synthetic images—generated through GAN—on the performance of automatic melanoma classifiers trained on H&E-stained histological images.
This objective fits within the broader need to address inherent limitations of biomedical datasets—such as sample scarcity, class imbalance, and technical variability—by artificially generating data that complement and enhance real datasets.
This work received a favorable opinion from the Ethics Committee of Euskadi, under approval code PI+CES-BIOEF 2025-01, as recorded in Act No. 03/2025. This approval ensures that the use of anonymized histological data and the methodology applied comply with current ethical standards in biomedical research.
1.6. Hypothesis and Objectives
1.6.1. Main Hypothesis
The hypothesis guiding this work is that the use of synthetic images generated through GAN contributes to improving the performance of melanoma classifiers trained on H&E-stained histological images. Specifically, it is expected that:
The incorporation of synthetic images will increase the variability and size of the training set, thereby reducing overfitting.
The class balance achievable through synthetic generation will improve the sensitivity and specificity of the model, particularly for minority classes.
This hypothesis is based on previous studies in which artificially generated data have proven effective in boosting the performance of models in clinical settings with limited data availability [
16,
17,
18].
1.6.2. Specific Objectives
To test the above hypothesis, the following specific objectives were defined:
Design and train a generative model (GAN) capable of producing synthetic H&E-stained tissue images that maintain visual and structural coherence with real samples.
Train classification models under two different configurations:
Evaluate classifier performance using standard binary classification metrics (accuracy, precision, sensitivity, specificity and F1-score).
Compare and analyze the results obtained for the different configurations, identifying to what extent the use of synthetic images impacts the model’s predictive capabilities.
Reflect on the real-world applicability of the approach, its limitations, and possible extensions to clinical or research contexts.
3. Results and Discussion
3.1. Generated Images and Quality
During the training of the generative model, synthetic images were produced from random latent vectors, with a final resolution of 1024 × 1024 pixels. Over the 700 epochs, a progressive improvement in visual quality, morphological coherence, and structural diversity of the generated samples was observed.
In the early stages of training, the generated images showed evident artifacts, poorly defined cellular contours, and homogeneous regions lacking clear tissue structure. However, from approximately epoch 250 onwards, more sharply defined cell nuclei, architectural patterns typical of melanocytic nevi, and a chromatic distribution closer to that seen in H&E-stained samples began to appear.
Figure 4 shows representative synthetic images generated by the GAN at different training stages.
Although these images do not reach the level of morphological complexity present in real histological samples, many of them correctly reproduce characteristic texture and color patterns of the melanocytic nevus. While a lower cell density and some structural homogeneity are evident, the generated samples provide a plausible approximation to the overall visual distribution of the tissue, which is particularly relevant in the context of training classifiers.
To quantify image quality, the FID metric was used, widely recognized for comparing the statistical similarity between real and synthetic images through distributions of neural activations [
19]. In this case, the generative model achieved a value of 139.56, indicating a considerable distance between the two distributions. This result highlights that, although the generated images may be useful for tasks such as data augmentation, there are still limitations in terms of morphological fidelity and structural diversity.
From a technical standpoint, the generator’s architecture contains 48,319,879 trainable parameters. This number reflects a high representational capacity, necessary for synthesizing large images while maintaining a coherent visual structure. The model’s complexity is justified by its function: transforming a latent vector into a high-resolution image requires multiple convolutional blocks and progressive upsampling operations, which entail a significant computational load during training.
3.2. Classifier Performance
With the aim of analyzing the effect of synthetic images on the performance of the classification system, various models based on the ResNet-18 architecture were trained and evaluated. The configurations varied both in input resolution (224, 512, and 1024 pixels) and in the nature of the dataset (real or mixed). Below, the results obtained for the models trained exclusively with real images and those trained with mixed data, integrating GAN-generated images, are presented separately.
3.2.1. Real Dataset
This section presents the results of the classifiers trained exclusively with real images, using input resolutions of 512 × 512 and 1024 × 1024 pixels. For each resolution, three different models were trained for 10, 25, and 50 epochs, with the aim of observing the evolution of performance throughout training.
Table 2 summarizes the main metrics (accuracy, precision, recall, specificity, and F1-score) obtained by each of the models on the test set.
Based on the results shown in
Table 2, a clear trend can be observed in which models trained with higher-resolution images (1024 × 1024) show progressively better performance as the number of epochs increases. Although models at a resolution of 512 × 512 achieve higher accuracy and F1-score values in less training time, it is evident that the 1024 × 1024 models display continuous and sustained improvement, suggesting a greater generalization capacity as hyperparameters are optimized. Therefore, the 1024 × 1024 resolution model is established as the baseline for exploring different learning rates, with the goal of identifying the optimal configuration for subsequent experiments with mixed data (real + synthetic).
A systematic exploration of different learning rates was conducted to analyze their impact on the performance of the 1024 × 1024 resolution model. For this purpose, models were trained for a fixed number of 50 epochs and evaluated with learning rates of 1 × 10
−4, 2 × 10
−4, 3 × 10
−4, and 5 × 10
−4. The analysis focused mainly on the F1-score metric, due to its ability to balance precision and recall.
Figure 5 shows how this metric evolves as the learning rate increases, allowing the identification of the optimal value for future comparisons with mixed data.
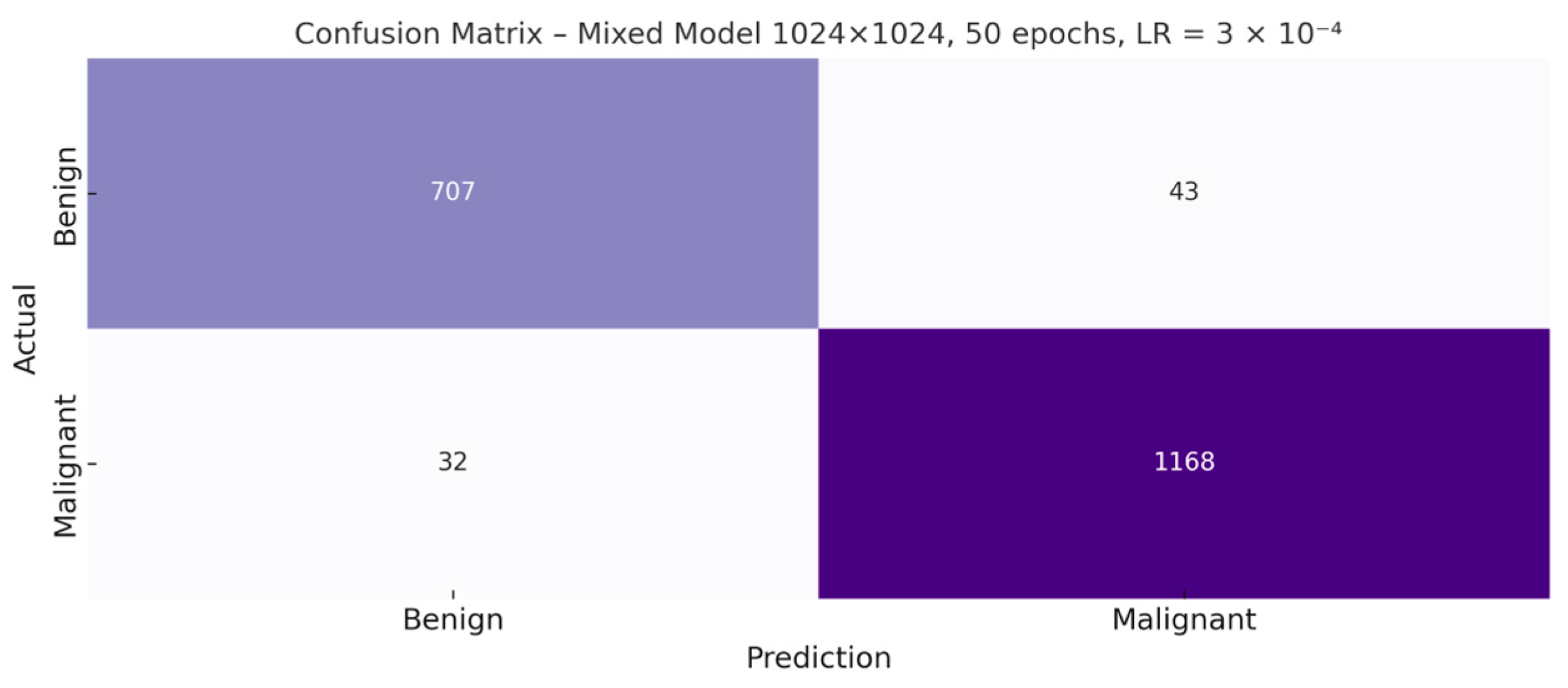
Once the optimal hyperparameter configuration—1024 × 1024 resolution, 50 epochs, and a learning rate of 3 × 10
−4—was identified, the corresponding confusion matrix for the resulting model was generated. This graphical representation makes it possible to examine in greater detail the distribution of correct and incorrect predictions for both classes, providing a more precise view of the types of errors made by the classifier (
Figure 6).
After identifying the optimal learning rate (3 × 10−4) through the comparative analysis presented above, this configuration will be used as the baseline to evaluate the impact of using synthetic images. This decision is justified by the superior performance obtained in the real-data models with this combination of resolution, learning rate, and number of epochs, ensuring that any improvement or deterioration observed when introducing synthetic data can be attributed to the latter and not to poor hyperparameter tuning of the model.
3.2.2. Mixed Dataset
This section presents the experiments carried out with the mixed dataset, built by combining real and synthetic images. In this case, the images generated by the GAN corresponded exclusively to the melanocytic nevus class, with the specific aim of mitigating the class imbalance present in the original dataset.
This strategy made it possible to equalize the number of melanocytic nevus samples with that of melanomas, resulting in a more balanced and potentially more robust dataset from the perspective of supervised training.
Table 3 shows the final distribution of images by class and subset, once the synthetic samples were integrated.
Once the models were retrained with the mixed dataset, their performance was evaluated on the test set, composed exclusively of real images not seen during training.
Table 4 presents the main metrics obtained for each experimental configuration, including accuracy, precision, recall, specificity, and F1-score. This comparison makes it possible to assess the actual impact of synthetic images on the classifier’s ability to generalize to clinically relevant data.
To complement the quantitative results presented in
Table 4, the confusion matrix of the best model trained with real and synthetic images is shown below (
Figure 7). This visualization allows a more direct observation of the classifier’s behavior in terms of correct and incorrect predictions between the benign and malignant classes.
Although the mixed model’s metrics were promising, some classification errors were observed. Below are specific examples of false positives and false negatives that illustrate the model’s difficulties in certain borderline cases.
Despite the high quantitative performance shown in the previous metrics,
Figure 8 highlights cases where the classifier fails to distinguish between malignant melanoma and melanocytic nevus. Some false negatives present structures reminiscent of benign tissue, with little cellular atypia or faint chromatic distribution, which can lead to classification errors. On the other hand, certain false positives exhibit areas of hyperchromasia or local disorganization that could resemble typical malignancy patterns. These errors reflect the inherent difficulty of discriminating between histologically similar entities, especially in borderline or morphologically ambiguous regions.
3.3. Complementary Statistical Analysis
To reinforce the validity of the results obtained, complementary statistical tests were applied to verify whether the differences observed between the models trained with real and mixed data are statistically significant and robust.
3.3.1. McNemar’s Test
McNemar’s test was used to compare the patterns of correct and incorrect predictions between both models on the same test set. A 2 × 2 contingency table was constructed, considering only the cases in which one model predicted correctly and the other failed.
As shown in
Table 5, both models achieve very similar performance in terms of accuracy and F1-score, with a slight advantage for the mixed model. However, these overall metrics do not allow us to determine whether the observed differences are statistically significant.
To assess whether there is a significant change in the classification patterns between the two models, the aforementioned test was applied.
Table 6 shows the distribution of these discordant cases.
From the discordant values, the McNemar statistic with continuity correction is calculated, allowing estimation of whether the differences are attributable to chance:
With the obtained values b = 12 and c = 17, a χ2 statistic of 0.55 and a p-value of 0.46 were obtained. This result does not indicate a statistically significant difference between the two models at the α = 0.05 level.
3.3.2. Bootstrap and Confidence Intervals
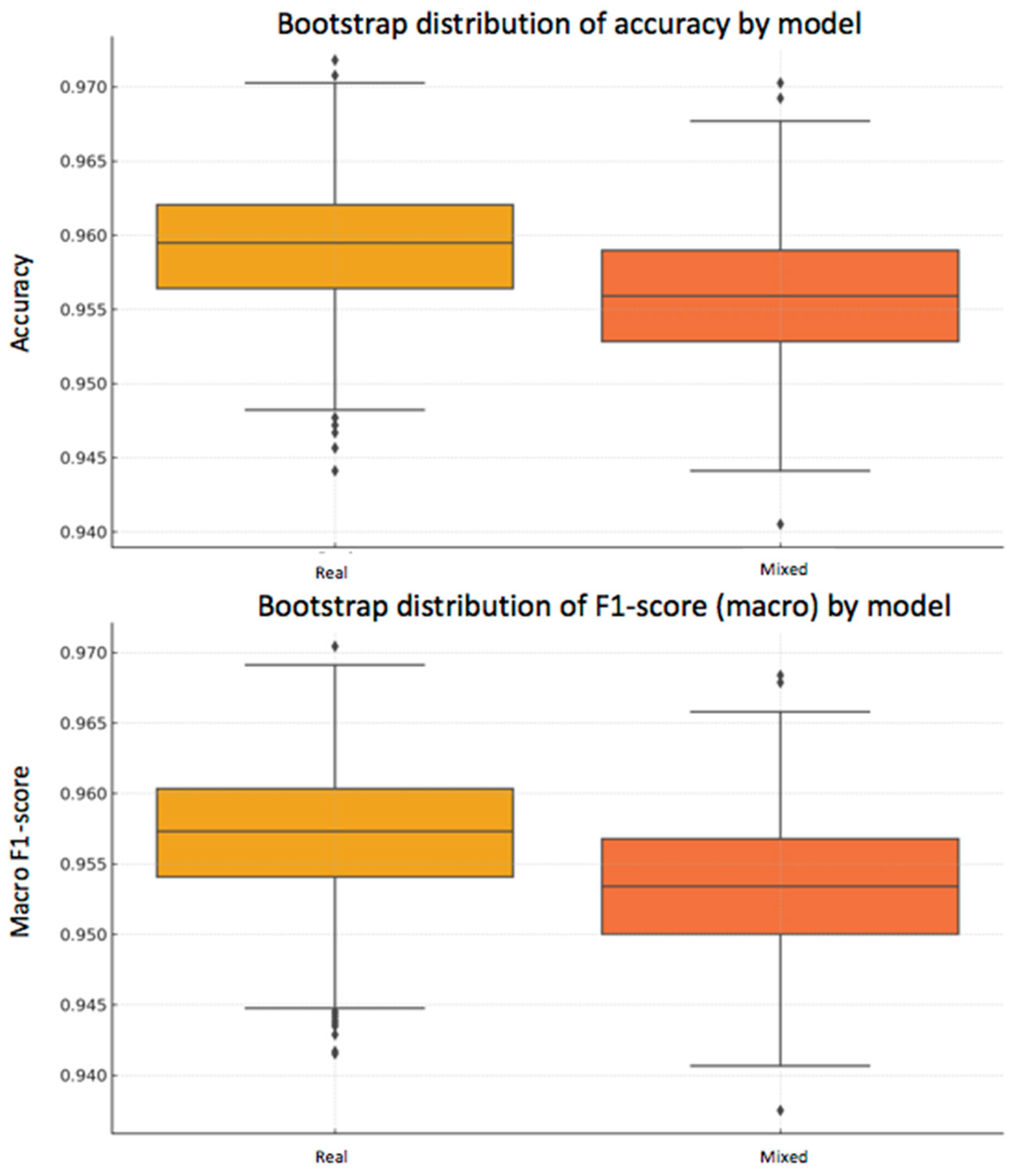
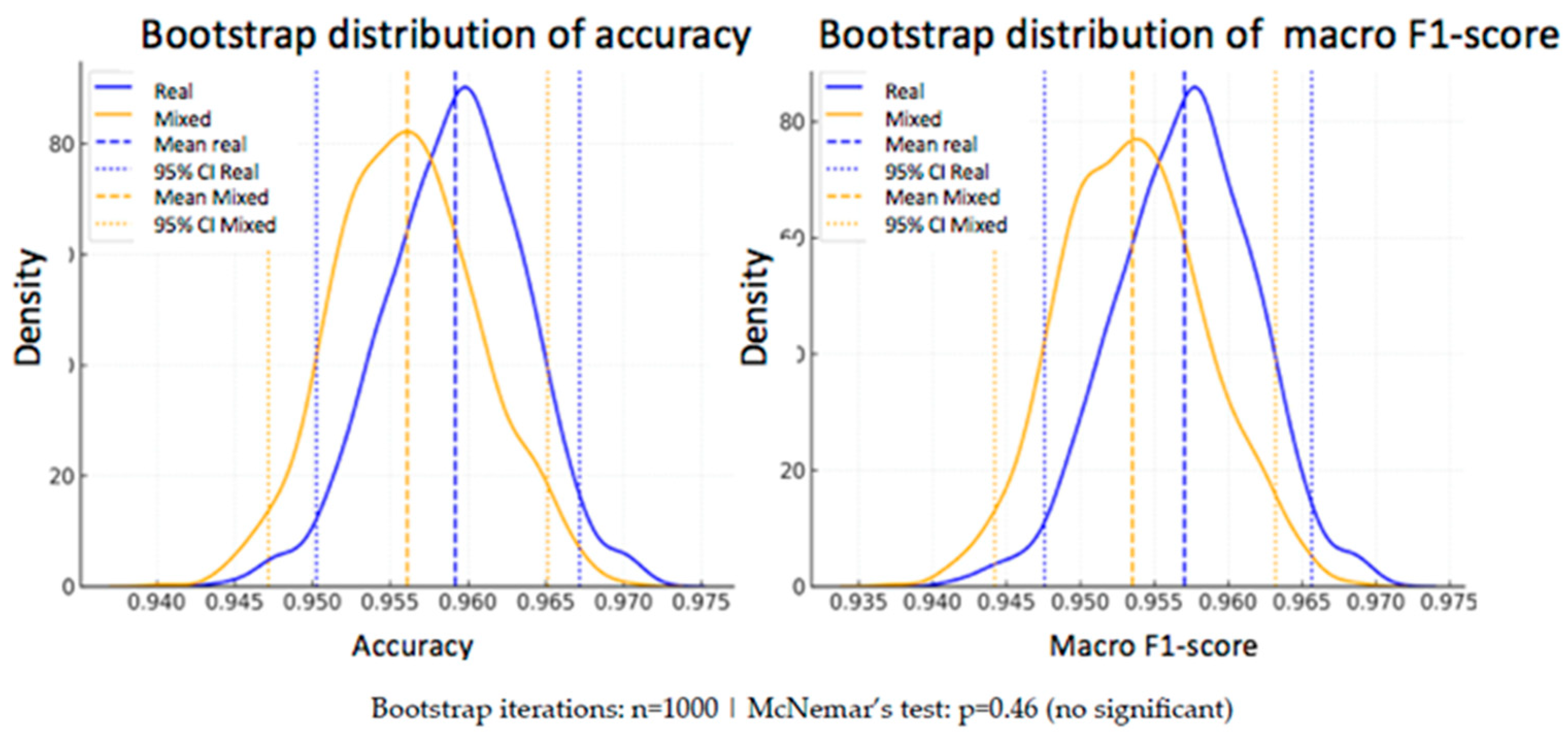
In addition to the statistical analysis using McNemar’s test, a bootstrap strategy was applied to estimate the variability of the performance metrics and assess their robustness. This technique consists of generating subsets of the test set through resampling with replacement, repeating the evaluation process multiple times (n = 1000 in this case).
From the predictions of both models —one trained exclusively with real data and the other with a mixed dataset of real and synthetic data— accuracy and macro F1-score were calculated in each iteration.
- -
Real model: mean accuracy = 95.92% [95% CI: 95.03–96.72%], macro F1-score = 95.70% [95% CI: 94.76–96.57%]
- -
Mixed model: mean accuracy = 95.61% [95% CI: 94.72–96.51%], macro F1-score = 95.35% [95% CI: 94.42–96.32%]
As can be seen, the differences in the mean metrics between the two models are small, and their confidence intervals overlap, suggesting that there is no statistically conclusive advantage for either of them. This conclusion reinforces the result obtained with McNemar’s test.
To visually illustrate this variability, two complementary plots are included below (
Figure 9 and
Figure 10):
3.3.3. Comparative Analysis
When comparing the two models, the mixed-data approach consistently outperformed the real-only model across multiple performance metrics. As described in
Section 3.3.1 and
Section 3.3.2, the mixed-data model achieved higher accuracy, sensitivity, specificity, and F1-score, indicating a better balance between correctly identifying malignant lesions and avoiding false positives in benign cases.
3.4. Comparison Between Scenarios
The comparison between models reveals that the incorporation of synthetic images did not lead to a generalized improvement in performance, especially when using as reference the metrics obtained by the model trained exclusively with real data at 512 × 512 pixels. However, the mixed models showed consistent and competitive results, particularly in higher-resolution configurations (1024 × 1024), where a slight improvement in specificity and a reduction in false negatives were observed.
This behavior suggests that, although synthetic images do not substantially increase overall performance, they do help strengthen the classifier’s ability to correctly discriminate between classes in unbalanced or high-variability situations. Thus, their usefulness as a support tool is partially validated, especially in contexts where obtaining additional real samples is costly or limited.
3.5. Critical Analysis and Limitations
Despite the promising results, this study presents several relevant limitations. The quality of the generated images, although useful for classifier training, remains inferior to that of real tissue, as reflected by the FID value of 139.56. This value indicates a considerable statistical distance between the distributions of real and synthetic images, suggesting that the generative model fails to adequately replicate the morphological complexity and structural diversity present in authentic samples. This limitation directly affects the classifier’s ability to generalize, although the generated images remain useful as a complementary tool to enrich the training set.
Furthermore, although the classifier’s performance was evaluated exclusively on real images from the test set, its behavior when synthetic images also act as evaluation data was not explored. This methodological decision responds to an issue of experimental integrity: since the synthetic images generated represent only the melanocytic nevus class, introducing them into the test set would have disrupted class balance and biased the evaluation. By exposing only one class to artificial generation, the model could learn to indirectly associate the visual style of synthetic images with a specific class, regardless of its tissue morphology. This would introduce an uncontrolled technical bias, as the test distribution would no longer be a faithful representation of the real clinical problem. Therefore, evaluation was conducted exclusively on unseen real images, thus ensuring an objective and comparable assessment between models.
In addition, the generation of synthetic images was applied solely to the melanocytic nevus class, aiming to correct the original class imbalance. However, this decision introduces an artificial bias into the composition of the mixed dataset, limiting the analysis of how a more balanced generation of both classes would affect performance. The exclusion of the malignant class as a generative target restricts the evaluation of potential benefits in scenarios of reverse imbalance or multiclass settings.
The computational burden associated with both training the generative model and using high resolutions (1024 × 1024) should also be considered, as these could pose a barrier to practical implementation in clinical environments with limited resources. Finally, although the possibility of applying more advanced generative models such as StyleGAN2 or diffusion models was considered, technical restrictions of the computing cluster prevented the installation of the required dependencies, limiting the architectural exploration in this work.
Unlike real histology, which naturally incorporates inter-center variability (scanner type, staining protocols, tissue handling), synthetic images tend to be more homogeneous. While this uniformity can stabilize training, it may also reduce robustness when facing heterogeneous external data. Future work should explore conditional generation or diffusion-based synthesis to explicitly model staining and acquisition variability.
3.6. Comparison with Previous Studies
The findings of this work are consistent with prior research demonstrating the potential of GAN-based synthetic augmentation to enhance histopathological image classification under data scarcity or class imbalance. Frid et al. [
17] showed that the use of synthetic images can improve the robustness of classification models in histopathology, especially under data scarcity conditions and Baur et al. (MelanoGANs) showed that high-resolution melanoma synthesis (256 × 256 px) could effectively address severe class imbalance, improving classification robustness despite a limited dataset [
20]. Compared to their approach, our study extends the resolution to 1024 × 1024 px and focuses on H&E-stained histopathology, which poses different textural and structural complexity challenges.
Similarly, Xue et al. (HistoGAN) proposed a selective synthetic augmentation framework that filters GAN-generated images based on label confidence and feature similarity to real samples, achieving significant performance gains in cervical and lymph node histopathology [
21]. While our study does not incorporate a selective inclusion mechanism, it shares the emphasis on quantitative quality assessment through the FID and on evaluating the functional impact of synthetic images on classifier performance rather than relying solely on visual inspection.
Broader reviews by Alajaji et al. [
16] and Jose et al. [
22] highlight that GAN in digital histopathology have primarily been applied to color normalization, virtual staining, and moderate-resolution image synthesis. In this context, our work contributes by combining high-resolution synthesis with a direct evaluation in a clinically relevant melanoma vs. melanocytic nevus classification task. Furthermore, recent methodological advances, such as integrating explainable artificial intelligence (XAI) into GAN training to improve image diversity and reduce FID [
23], point to future directions that could further enhance our approach.
These observations are consistent with recent literature on GANs in medical imaging. In particular, a comprehensive review by Hussain et al. [
24] highlighted the wide application of GAN-based methods in image enhancement, reconstruction, and augmentation across different medical domains, while also underlining their current limitations in terms of fidelity and generalizability. This reinforces our conclusion that although synthetic histopathology images cannot yet replace real data, they may serve as a valuable complementary tool to mitigate class imbalance and enrich training datasets.
Moreover, Cho et al. [
25] demonstrated that GAN-based synthetic images can enhance melanoma/nevus classification in dermatoscopic datasets; our study extends this concept to histopathological images with rigorous statistical testing.
Finally, ensemble machine-learning approaches have also shown promise in other oncological histopathology contexts. For instance, Sahoo et al. [
26] predicted breast cancer relapse using ensemble models on H&E-stained slides, reporting high prognostic accuracy and balanced performance across sensitivity and specificity. Although this work addresses a different tumor type and endpoint, it highlights the broader applicability of advanced ML frameworks to both prognostic and diagnostic settings, supporting the relevance of our melanoma-focused approach.
Overall, although our results show that mixed models do not consistently outperform those trained exclusively on real data, they align with previous evidence [
20,
21] that GAN-generated images can improve specific performance metrics—in our case, specificity and reduction in false negatives—and can be a valuable tool when acquiring additional real samples is not feasible.
4. Conclusions
This study demonstrates that the incorporation of synthetic images generated by a GAN can serve as a valuable complement to real histopathological data in the training of melanoma classifiers. Although models trained with mixed datasets did not consistently outperform those trained exclusively with real data, they reached comparable performance and showed a modest but clinically meaningful improvement in some key metrics, such as a reduction in false negatives. In our best configuration (1024 × 1024 pixels, 50 epochs), the mixed model achieved an accuracy of 96% and specificity of 97%, while reducing false negatives from 17 to 12 cases compared with the real-only model. This highlights the potential of synthetic augmentation to decrease clinically critical errors, particularly missed melanoma diagnoses.
The advantages of this approach are several. First, GAN-based generation effectively mitigated class imbalance by expanding the minority nevus class, allowing for a more balanced and stable training process. Second, the use of high-resolution synthetic images (1024 × 1024 px) provided detailed structural patterns that enriched the variability of the training set. Third, the strategy contributed to maintaining diagnostic performance without requiring additional real data, which are often scarce, costly to obtain, and ethically restricted. Together, these strengths illustrate the utility of synthetic augmentation as a support tool in biomedical image analysis.
Nevertheless, this work also presents important limitations. The morphological fidelity of synthetic images remains inferior to that of real histological samples, as evidenced by an FID of 139.56, which reflects a considerable distributional gap. Furthermore, synthetic images tend to be more homogeneous than real-world histology, lacking the variability introduced by different scanners, staining protocols, and acquisition settings. While this uniformity can stabilize training, it may reduce robustness in external datasets, limiting generalizability. In addition, the exclusive generation of nevus images addressed the imbalance but introduced a selection bias, as malignant cases were not augmented. Finally, the lack of direct comparison with conventional augmentation techniques (rotations, flips, and stain variations) prevents us from quantifying the added value of GAN-based synthesis over simpler approaches.
Future work should therefore aim to overcome these constraints by adopting more advanced generative architectures such as StyleGAN3 or diffusion models, which are expected to achieve greater morphological fidelity and lower FID scores. Conditional and class-aware generation could also enable balanced synthesis of both benign and malignant classes, or even more complex diagnostic categories such as atypical proliferations, dysplastic nevi, or Spitz tumors. Moreover, the integration of expert-in-the-loop curation and explainable AI mechanisms could enhance the realism and reliability of synthetic datasets. Finally, comparative studies directly contrasting GAN-based augmentation with classical techniques would provide stronger evidence for its clinical utility.
In conclusion, although synthetic images cannot yet replace real histopathological data, they represent a promising complementary tool to alleviate class imbalance, increase variability, and modestly improve clinically relevant outcomes in melanoma diagnosis. The findings of this work reinforce the potential of synthetic augmentation as a bridge between limited real datasets and the development of robust, clinically applicable AI systems in dermatopathology.