Introducing Holographic Surgical Navigation in Pediatric Wilms’ Tumor Patients: A Feasibility Study During Total Nephrectomy
Abstract
1. Introduction
2. Materials and Methods
- The error of the accuracy of the registration of the hologram reaches a median accuracy of less than 5 mm;
- The System Usability Scale (SUS, range 0–100) should have a median of 60 or higher to confirm usability and comfort for the surgeons;
- The technique should be consistent and accurate. Therefore, in this study, we required five successful registrations within 10 min in five consecutive patients.
2.1. Study Procedure
2.1.1. Preoperative Planning
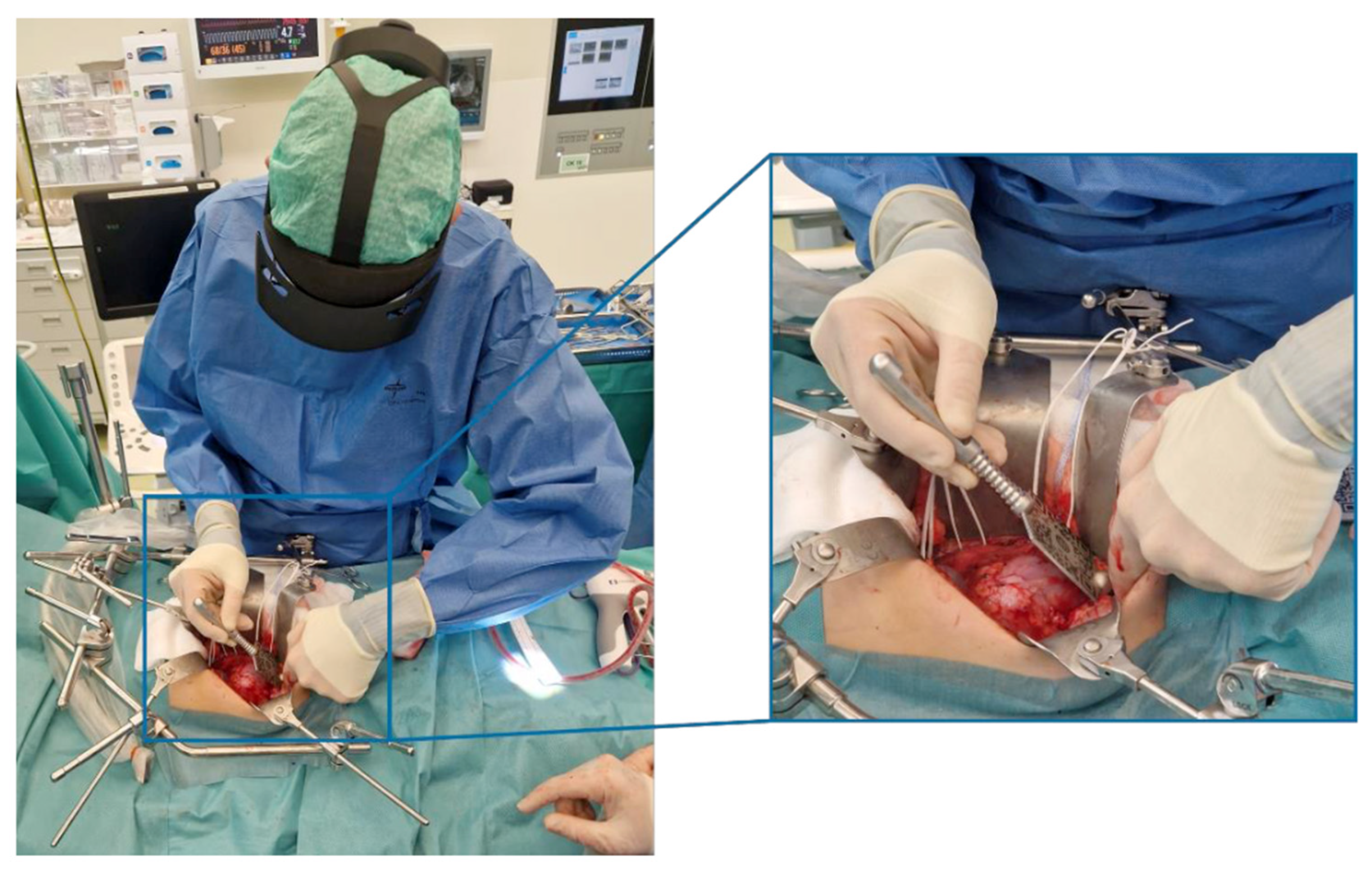
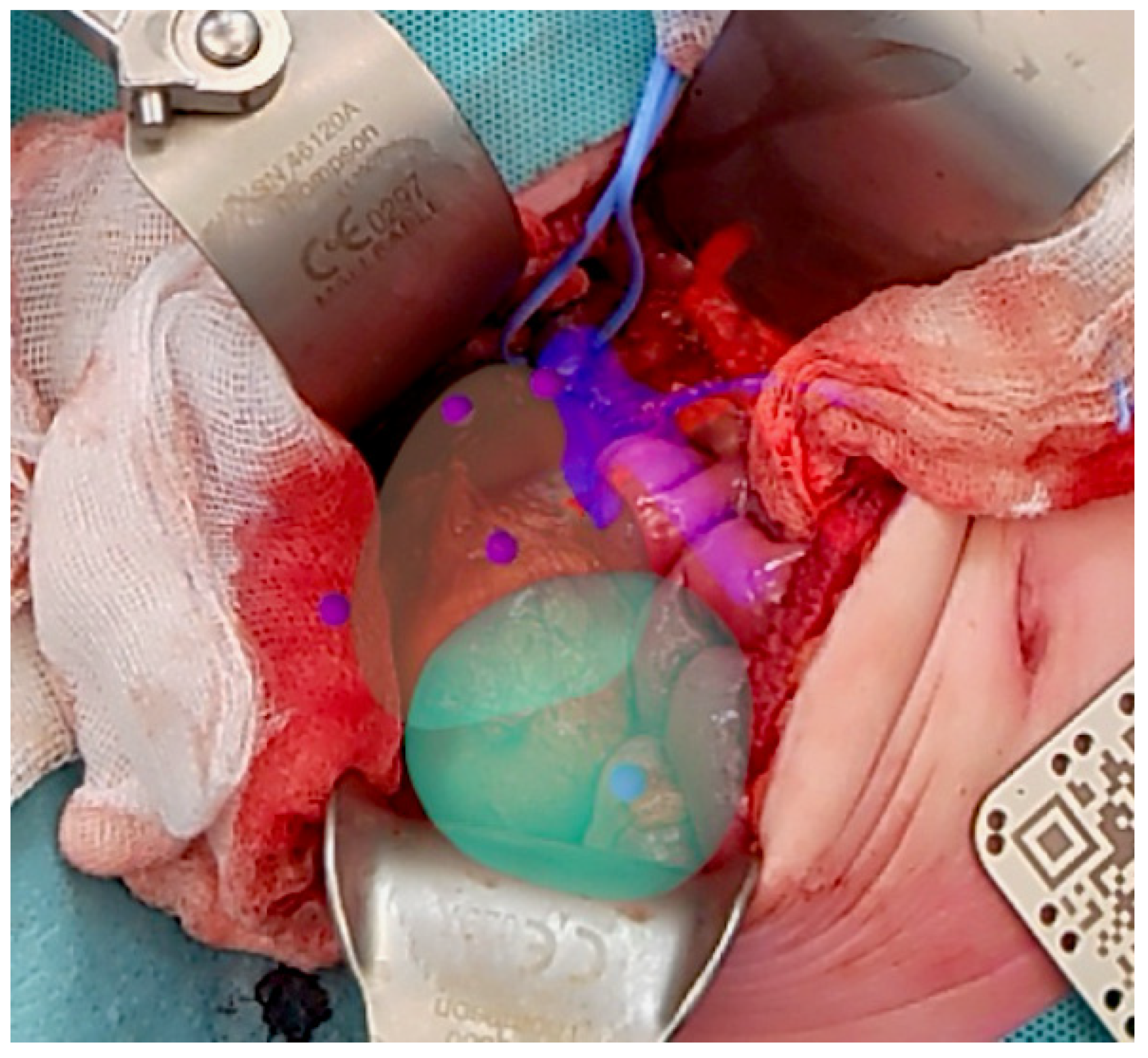
2.1.2. Intraoperative Protocol
2.1.3. Postoperative Analysis
2.2. Data Analysis
3. Results
3.1. Patient Characteristics
3.2. Quantative Results
3.3. Qualitative Results
| # | Question | Case 1 (Surgeon 4) | Case 2 (Surgeon 2) | Case 10 (Surgeon 1) | Median Score (IQR) |
|---|---|---|---|---|---|
| 1 | Determining the position of the target landmarks on the kidney of the patient was straightforward. | 7 | 2 | 8 | 7 (4.5–7.5) |
| 2 | The user interface of the HoloLens software was straightforward to use. | 8 | 8 | 8 | 8 (8–8) |
| 3 | The hologram remained stable on the kidney after positioning. | 10 | 4 | 10 | 10 (7–10) |
| 4 | The holographic overlay improves my sense of depth. | 7 | 8 | 8 | 8 (7.5–8) |
| 5 | The holographic overlay improves my understanding of the anatomical relationships of the patient. | 8 | 8 | 10 | 8 (8–9) |
| 6 | The holographic overlay visualized the position of the intraparenchymal vessels reliably. | 7 | Not defined, as vessels were not present in 3D model | 8 | 7.5 (7.25–7.5) |
| 7 | The holographic overlay of the tumor and vessels is useful for NSS. | 8 | Not defined, as vessels were not present in 3D model | 8 | 9 (8.5–9.5) |
| 8 | The holographic overlay is worth the additional time for NSS. | 8 | 5 | 10 | 8 (6.5–9) |
3.4. Observed Causes for Non-Successful Registration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| AR | Augmented reality |
| IQR | Interquartile Range |
| MRI | Magnetic Resonance Imaging |
| NSS | Nephron-sparing surgery |
| QR | Quick Response |
| SUS | System Usability Scale |
| TN | Total nephrectomy |
| TRE | Target Registration Error |
| WT | Wilms’ tumor |
References
- Spreafico, F.; van den Heuvel-Eibrink, M.M.; Pritchard-Jones, K.; Bergeron, C.; Godzinski, J.; Smets, A.; Olsen, O.; Vujanic, G.; Rübe, C.; van Tinteren, H.; et al. Paediatric renal tumours: Perspectives from the SIOP–RTSG. Nat. Rev. Urol. 2017, 14, 3–4. [Google Scholar] [CrossRef]
- Van den Heuvel-Eibrink, M.M.; Hol, J.A.; Pritchard-Jones, K.; van Tinteren, H.; Furtwängler, R.; Verschuur, A.C.; Vujanic, G.M.; Leuschner, I.; Brok, J.; Rübe, C.; et al. Rationale for the treatment of wilms tumour in the UMBRELLA SIOP–RTSG 2016 protocol. Nat. Rev. Urol. 2017, 14, 743–752. [Google Scholar] [CrossRef]
- Wilde, J.C.H.; Aronson, D.C.; Sznajder, B.; Van Tinteren, H.; Powis, M.; Okoye, B.; Cecchetto, G.; Audry, G.; Fuchs, J.; Schweinitz, D.V.; et al. Nephron sparing surgery (NSS) for unilateral wilms tumor (UWT): The SIOP 2001 experience. Pediatr. Blood Cancer 2014, 61, 2175–2179. [Google Scholar] [CrossRef]
- Davidoff, A.M.; Interiano, R.B.; Wynn, L.; Delos Santos, N.; Dome, J.S.; Green, D.M.; Brennan, R.C.; McCarville, M.B.; Krasin, M.J.; Kieran, K.; et al. Overall survival and renal function of patients with synchronous bilateral wilms tumor undergoing surgery at a single institution. Ann. Surg. 2015, 262, 570–576. [Google Scholar] [CrossRef]
- Richards, M.K.; Goldin, A.B.; Ehrlich, P.F.; Beierle, E.A.; Doski, J.J.; Goldfarb, M.; Langer, M.; Nuchtern, J.G.; Vasudevan, S.; Gow, K.W. Partial nephrectomy for nephroblastoma: A national cancer data base review. Am. Surg. 2018, 84, 338–343. [Google Scholar] [CrossRef]
- Fitski, M.; Bökkerink, G.M.J.; van Peer, S.E.; Hulsker, C.C.C.; Terwisscha van Scheltinga, S.E.J.; van de Ven, C.P.; Wijnen, M.H.W.A.; Klijn, A.J.; Van den Heuvel-Eibrink, M.M.; van der Steeg, A.F.W. Nephron-sparing surgery for pediatric renal tumors after centralization of pediatric oncology care in the Netherlands: Improved outcomes with 3D modeling. J. Pediatr. Surg. 2025, 60, 162125. [Google Scholar] [CrossRef]
- Ma, L.; Huang, T.; Wang, J.; Liao, H. Visualization, registration and tracking techniques for augmented reality guided surgery: A review. Phys. Med. Biol. 2023, 68, 04TR02. [Google Scholar] [CrossRef]
- Spijkerboer, K.G.P.; Fitski, M.; Siepel, F.J.; van de Ven, C.P.; van der Steeg, A.F.W. Augmented reality-guided localization of a chest wall tumor in a pediatric patient. Eur. J. Cancer 2022, 170, 103–105. [Google Scholar] [CrossRef]
- Van der Woude, R.; Fitski, M.; van der Zee, J.M.; van de Ven, C.P.; Bökkerink, G.M.J.; Wijnen, M.H.W.A.; Meulstee, J.W.; van Doormaal, T.P.C.; Siepel, F.J.; van der Steeg, A.F.W. Clinical application and further development of augmented reality guidance for the surgical localization of pediatric chest wall tumors. J. Pediatr. Surg. 2024, 59, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Fitski, M.; Meulstee, J.W.; Littooij, A.S.; van de Ven, C.P.; van der Steeg, A.F.W.; Wijnen, M.H.W.A. MRI-based 3-Dimensional visualization workflow for the preoperative planning of nephron-sparing surgery in wilms’ tumor surgery: A pilot study. J. Healthc. Eng. 2020, 2020, 8899049. [Google Scholar] [CrossRef]
- (PDF) SUS: A Quick and Dirty Usability Scale. Available online: https://www.researchgate.net/publication/228593520_SUS_A_quick_and_dirty_usability_scale (accessed on 12 May 2025).
- Bangor, A.; Kortum, P.; Miller, J. Determining what individual SUS scores mean: Adding an adjective rating scale. JUX J. User Exp. 2009, 4, 114–123. [Google Scholar]
- Malhotra, S.; Halabi, O.; Dakua, S.P.; Padhan, J.; Paul, S.; Palliyali, W. Augmented reality in surgical navigation: A review of evaluation and validation metrics. Appl. Sci. 2023, 13, 1629. [Google Scholar] [CrossRef]
- Konovalov, A.N.; Okishev, D.N.; Pilipenko, Y.V.; Eliava, S.S.; Artemyev, A.A.; Abzalov, T.Y.; Knyazev, A.V.; Ivanov, V.M.; Smirnov, A.Y.; Strelkov, S.V. Augmented reality for external ventricular drain placement: Model alignment and integration software. Surg. Neurol. Int. 2025, 16, 93. [Google Scholar] [CrossRef]
- Manfredi, M.; Piramide, F.; Amparore, D.; Burgio, M.; Busacca, G.; Colombo, M.; Meziere, J.; Granato, S.; Piana, A.; Cillis, S.D.; et al. Augmented reality: The smart way to guide robotic urologic surgery. Mini-Invasive Surg. 2022, 6, 40. [Google Scholar] [CrossRef]
- Piana, A.; Amparore, D.; Sica, M.; Volpi, G.; Checcucci, E.; Piramide, F.; De Cillis, S.; Busacca, G.; Scarpelli, G.; Sidoti, F.; et al. Automatic 3D augmented-reality robot-assisted partial nephrectomy using machine learning: Our pioneer experience. Cancers 2024, 16, 1047. [Google Scholar] [CrossRef]
- Bernhardt, S.; Nicolau, S.A.; Soler, L.; Doignon, C. The status of augmented reality in laparoscopic surgery as of 2016. Med. Image Anal. 2017, 37, 66–90. [Google Scholar] [CrossRef]
- Birlo, M.; Edwards, P.J.E.; Clarkson, M.; Stoyanov, D. Utility of optical see-through head mounted displays in augmented reality-assisted surgery: A systematic review. Med. Image Anal. 2022, 77, 102361. [Google Scholar] [CrossRef]
- Michiels, C.; Khene, Z.-E.; Prudhomme, T.; Boulenger de Hauteclocque, A.; Cornelis, F.H.; Percot, M.; Simeon, H.; Dupitout, L.; Bensadoun, H.; Capon, G.; et al. 3D-Image guided robotic-assisted partial nephrectomy: A multi-institutional propensity score-matched analysis (UroCCR Study 51). World J. Urol. 2023, 41, 303–313. [Google Scholar] [CrossRef]
- Porpiglia, F.; Checcucci, E.; Amparore, D.; Piramide, F.; Volpi, G.; Granato, S.; Verri, P.; Manfredi, M.; Bellin, A.; Piazzolla, P.; et al. Three-dimensional augmented reality robot-assisted partial nephrectomy in case of complex tumours (PADUA ≥10): A new intraoperative tool overcoming the ultrasound guidance. Eur. Urol. 2020, 78, 229–238. [Google Scholar] [CrossRef]
- Iqbal, H.; Rodriguez y Baena, F. Semi-automatic infrared calibration for augmented reality systems in surgery. In Proceedings of the 2022 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Kyoto, Japan, 23 October 2022; pp. 4957–4964. [Google Scholar]
- Groenenberg, A.; Brouwers, L.; Bemelman, M.; Maal, T.J.J.; Heyligers, J.M.M.; Louwerse, M.M. Feasibility and accuracy of a real-time depth-based markerless navigation method for hologram-guided surgery. BMC Digit. Health 2024, 2, 11. [Google Scholar] [CrossRef]
- Padovan, E.; Marullo, G.; Tanzi, L.; Piazzolla, P.; Moos, S.; Porpiglia, F.; Vezzetti, E. A deep learning framework for real-time 3D model registration in robot-assisted laparoscopic surgery. Int. J. Med. Robot. 2022, 18, e2387. [Google Scholar] [CrossRef] [PubMed]
- Vinodkumar, P.K.; Karabulut, D.; Avots, E.; Ozcinar, C.; Anbarjafari, G. Deep learning for 3D reconstruction, augmentation, and registration: A review paper. Entropy 2024, 26, 235. [Google Scholar] [CrossRef] [PubMed]
- Thabit, A.; Benmahdjoub, M.; van Veelen, M.-L.C.; Niessen, W.J.; Wolvius, E.B.; van Walsum, T. Augmented reality navigation for minimally invasive craniosynostosis surgery: A phantom study. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Groot, N.T.; van der Zee, J.M.; Bökkerink, G.M.J.; Littooij, A.S.; Hulsker, C.C.C.; Terwisscha van Scheltinga, C.E.J.; van de Ven, C.P.; Wortel, R.C.; Klijn, A.J.; Wijnen, M.H.W.A.; et al. Introducing Holographic Surgical Navigation in Pediatric Wilms’ Tumor Patients: A Feasibility Study During Total Nephrectomy. Bioengineering 2025, 12, 896. https://doi.org/10.3390/bioengineering12080896
de Groot NT, van der Zee JM, Bökkerink GMJ, Littooij AS, Hulsker CCC, Terwisscha van Scheltinga CEJ, van de Ven CP, Wortel RC, Klijn AJ, Wijnen MHWA, et al. Introducing Holographic Surgical Navigation in Pediatric Wilms’ Tumor Patients: A Feasibility Study During Total Nephrectomy. Bioengineering. 2025; 12(8):896. https://doi.org/10.3390/bioengineering12080896
Chicago/Turabian Stylede Groot, Nick T., Jasper M. van der Zee, Guus M. J. Bökkerink, Annemieke S. Littooij, Caroline C. C. Hulsker, Cecilia E. J. Terwisscha van Scheltinga, Cornelis P. van de Ven, Ruud C. Wortel, Aart J. Klijn, Marc H. W. A. Wijnen, and et al. 2025. "Introducing Holographic Surgical Navigation in Pediatric Wilms’ Tumor Patients: A Feasibility Study During Total Nephrectomy" Bioengineering 12, no. 8: 896. https://doi.org/10.3390/bioengineering12080896
APA Stylede Groot, N. T., van der Zee, J. M., Bökkerink, G. M. J., Littooij, A. S., Hulsker, C. C. C., Terwisscha van Scheltinga, C. E. J., van de Ven, C. P., Wortel, R. C., Klijn, A. J., Wijnen, M. H. W. A., Fitski, M., & van der Steeg, A. F. W. (2025). Introducing Holographic Surgical Navigation in Pediatric Wilms’ Tumor Patients: A Feasibility Study During Total Nephrectomy. Bioengineering, 12(8), 896. https://doi.org/10.3390/bioengineering12080896







