Current Mechanobiological Pathways and Therapies Driving Spinal Health
Abstract
1. Introduction
2. Methodology
2.1. Literature Search Strategy
- Original research articles, systematic reviews, and meta-analyses published in peer-reviewed journals.
- Experimental or computational studies elucidating molecular signaling pathways relevant to spinal tissues.
- Preclinical and clinical investigations on regenerative scaffolds, stem cell therapies, or neurotechnological interventions linked to spinal health.
2.2. Selection and Data Extraction
- Signaling pathways implicated in spinal tissue homeostasis (e.g., integrin-FAK-MAPK, YAP/TAZ, Piezo ion channels, Wnt/β-catenin, IL-6/JAK/STAT, TNF-α/NF-κB, TGF-β/Smad, and MMP-mediated ECM remodeling).
- Experimental models and intervention modalities (bioreactors, mechanical loading systems, scaffold engineering techniques, stem cell conditioning protocols).
- Translational approaches, including clinical trials and emerging brain–computer interface technologies (Neuralink reports up to June 2025).
2.3. Quality Assessment
2.4. Synthesis of Evidence
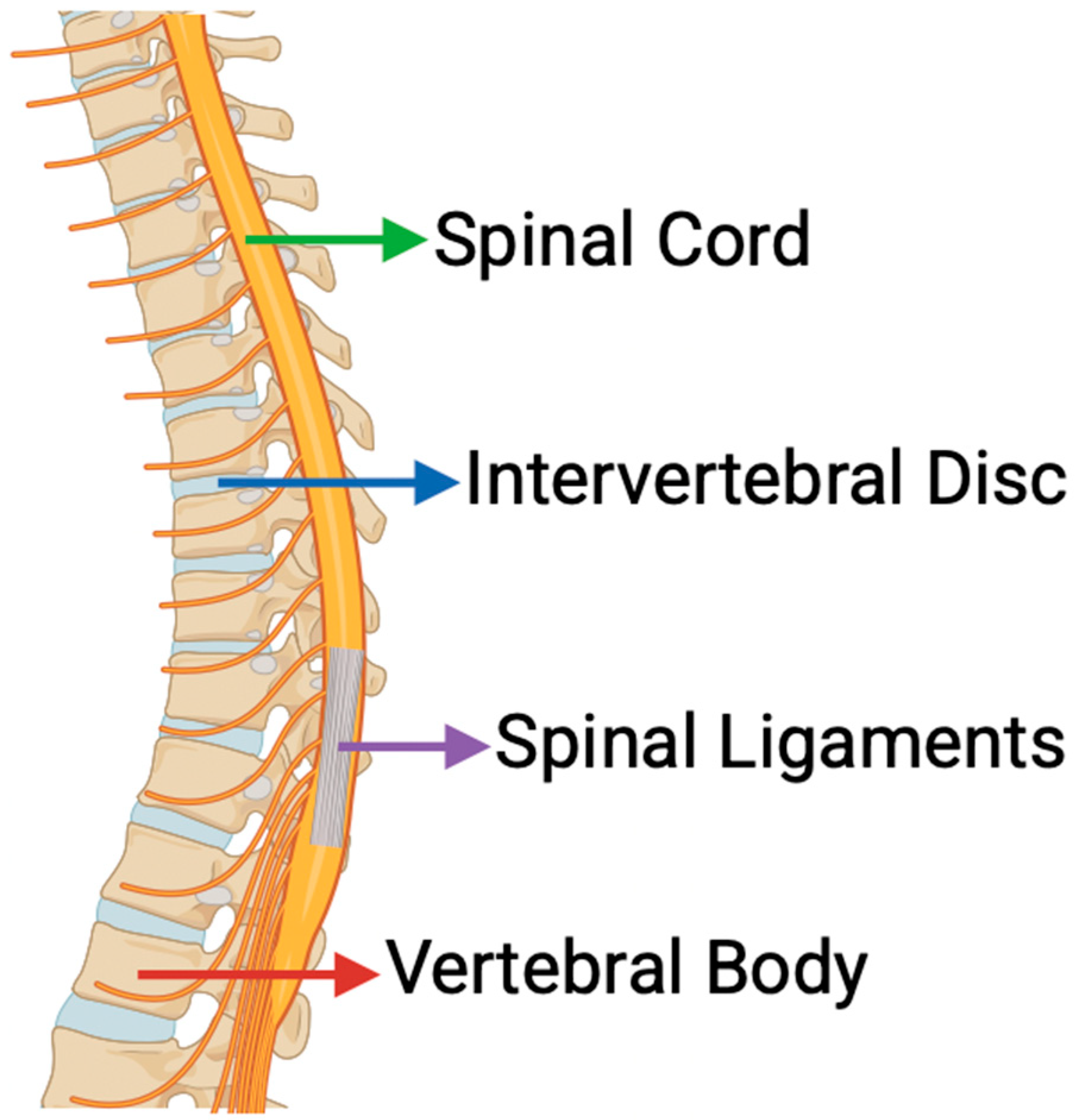
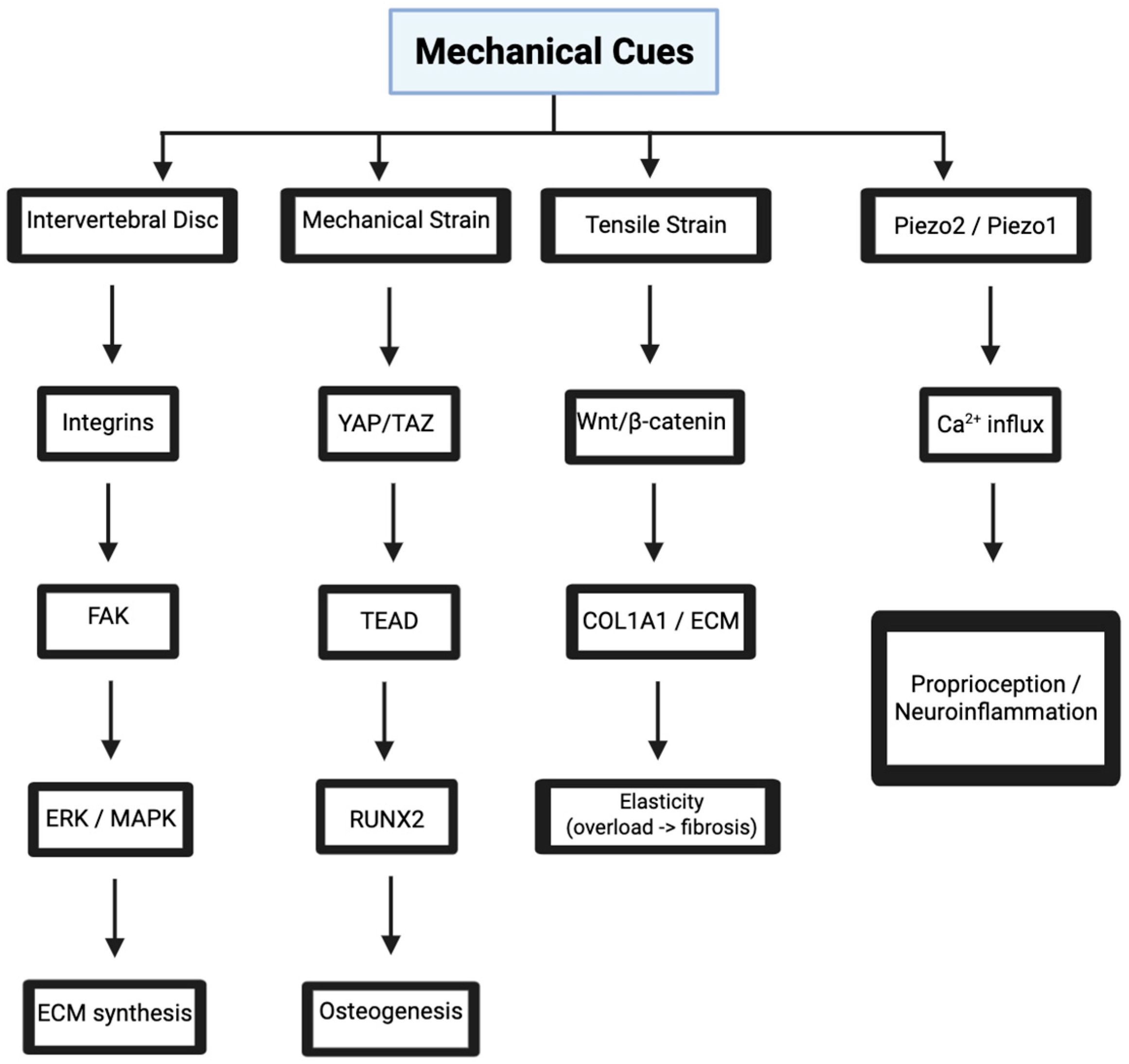
3. Mechanotransduction in Spinal Tissues
3.1. Integrin-Mediated Signaling in Intervertebral Discs
3.2. YAP/TAZ Pathway in Vertebral Bone Mechanobiology
3.2.1. YAP/TAZ in Bone
3.2.2. YAP/TAZ and Inflammation
3.3. Piezo Channels in Neural Mechanosensing
3.3.1. Piezo Signaling in Injury
3.3.2. Piezo in Chronic Pain
3.4. Wnt/β-Catenin Signaling in Ligament Mechanobiology
3.4.1. Role in Ligament Homeostasis, Fibrosis, and Inflammatory Modulation
3.4.2. Wnt-Guided Ligament Regeneration Strategies
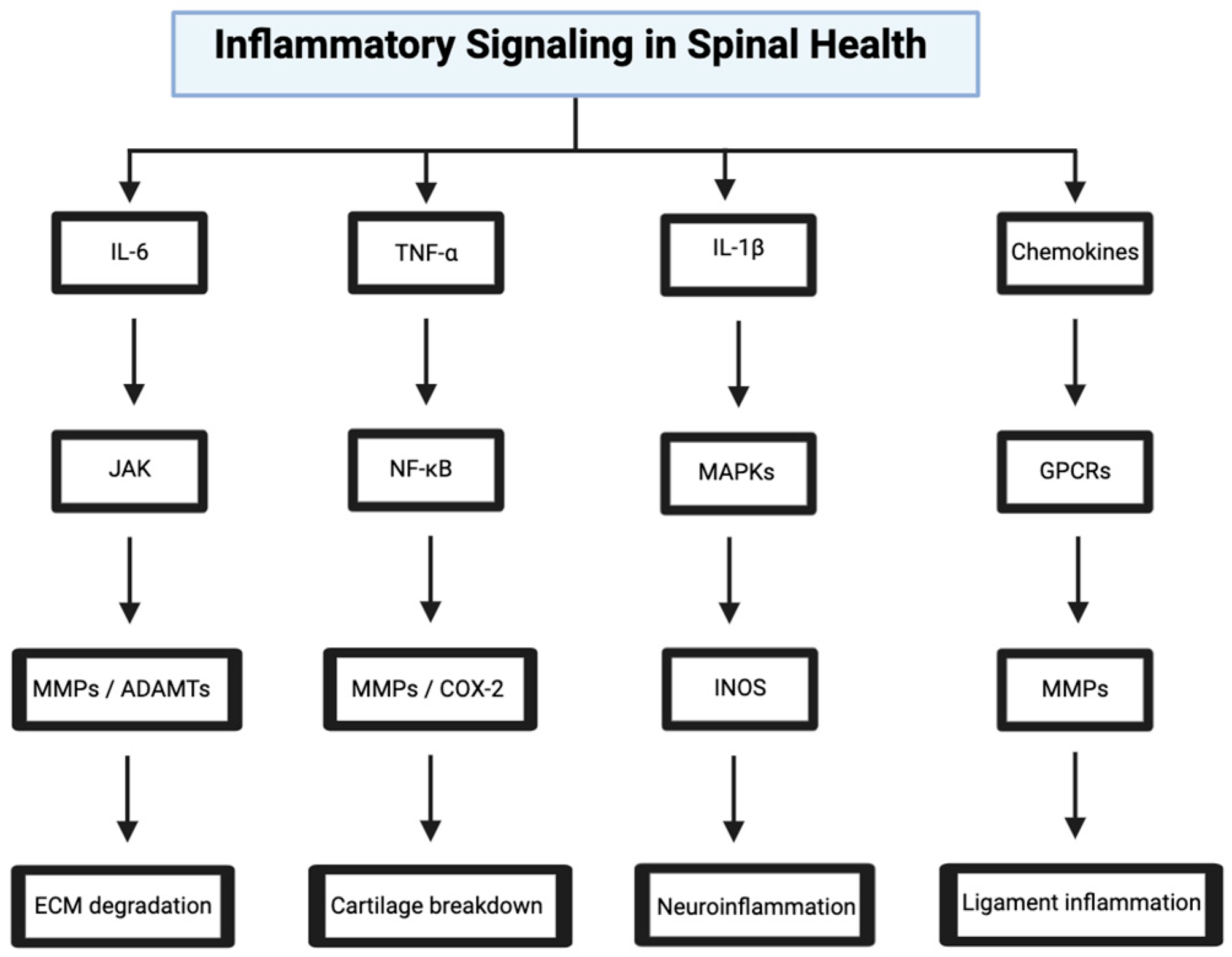
4. Inflammatory Signaling in Spinal Health
4.1. IL-6/JAK/STAT Pathway in Disc Degeneration
4.2. TNF-α/NF-κB Pathway in Osteoarthritis
4.2.1. Mechanotransduction and Catabolic Signaling
4.2.2. Anti-TNF Therapies and Scaffold-Based Modulation
4.2.3. Immunomodulatory Stem Cell Strategies
4.3. IL-1β/MAPK Pathway in Spinal Cord Injury
4.3.1. Neuroinflammatory Cascade and Biomaterial-Based Modulation
4.3.2. MSC Preconditioning for Immunomodulation
4.4. Chemokine Signaling in Ligament Inflammation
5. ECM Remodeling and Spinal Stability
5.1. TGF-β/Smad Pathway in Disc ECM Synthesis
5.2. MMP-13/TNF-α Pathway in Vertebral Cartilage Degradation
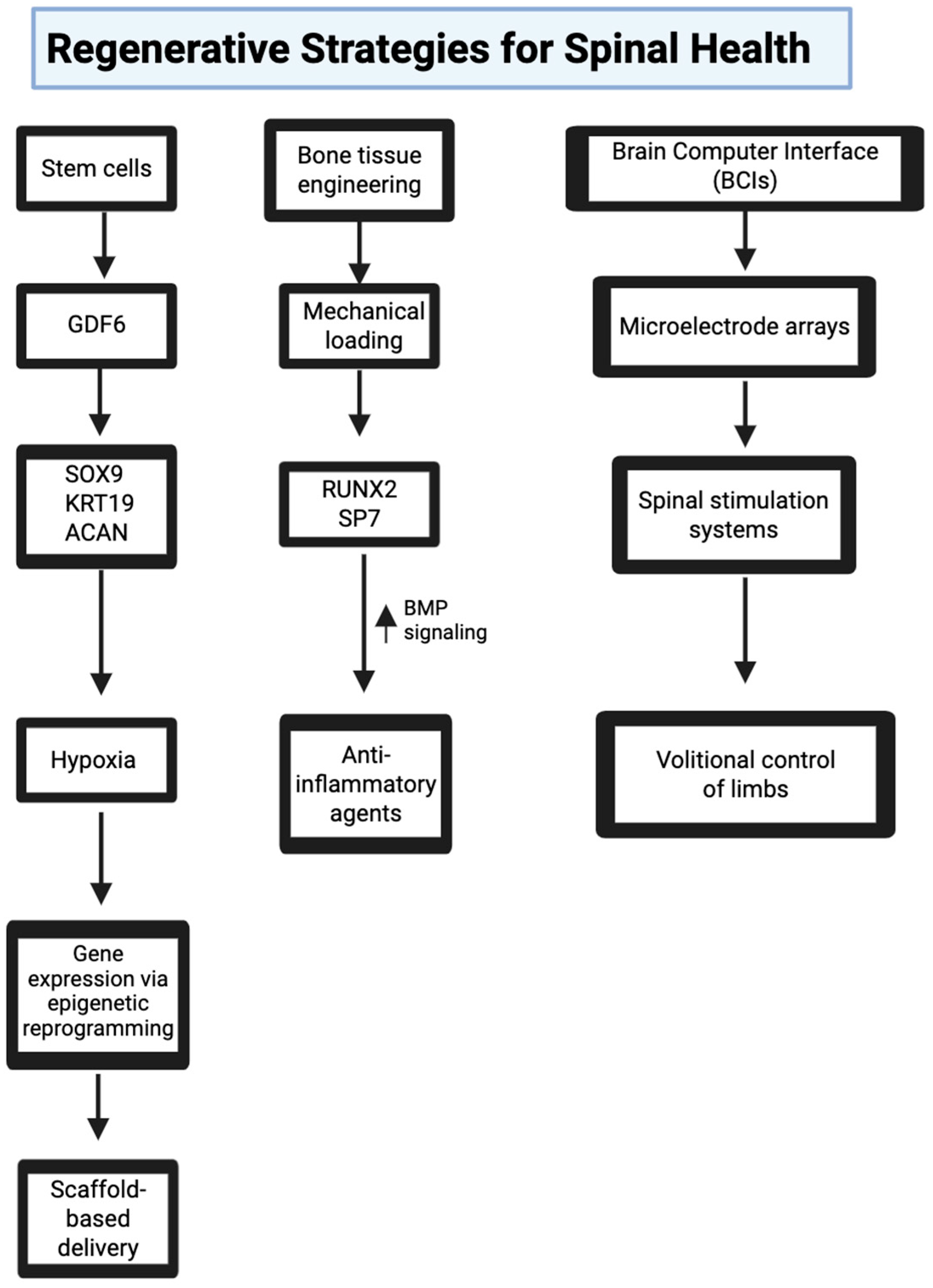
6. Regenerative Strategies for Spinal Health
6.1. Stem Cell Therapies for Disc Regeneration
6.1.1. Morphogen-Induced Discogenic Differentiation
6.1.2. Hypoxic Preconditioning and Epigenetic Modulation
6.1.3. Biomechanically Tuned Scaffolds and Bioreactor Platforms
6.1.4. Genetically Engineered MSCs and Extracellular Vesicles
6.2. Bone Tissue Engineering for Vertebral Fractures
6.3. Neural Technology for Rehabilitation and Support
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diwan, A.D.; Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 2022, 6, e1231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waxenbaum, J.A.; Reddy, V.; Futterman, B. Anatomy, Back, Intervertebral Discs. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470583/ (accessed on 5 June 2025).
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strohmeyer, N.; Bharadwaj, M.; Costell, M.; Fässler, R.; Müller, D.J. Fibronectin-bound α5β1 integrins sense load and signal to reinforce adhesion in less than a second. Nat. Mater. 2017, 16, 1262–1270, Erratum in Nat. Mater. 2017, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- Sawai, H.; Okada, Y.; Funahashi, H.; Matsuo, Y.; Takahashi, H.; Takeyama, H.; Manabe, T. Activation of focal adhesion kinase enhances the adhesion and invasion of pancreatic cancer cells via extracellular signal-regulated kinase-1/2 signaling pathway activation. Mol. Cancer 2005, 4, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayes, A.J.; Melrose, J. Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity. Biomolecules 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neidlinger-Wilke, C.; Galbusera, F.; Pratsinis, H.; Mavrogonatou, E.; Mietsch, A.; Kletsas, D.; Wilke, H.-J. Mechanical loading of the intervertebral disc: From the macroscopic to the cellular level. Eur. Spine J. 2014, 23 (Suppl. S3), S333–S343. [Google Scholar] [CrossRef]
- Schmidt, C.; Pommerenke, H.; Dürr, F.; Nebe, B.; Rychly, J. Mechanical stressing of integrin receptors induces enhanced tyrosine phosphorylation of cytoskeletally anchored proteins. J. Biol. Chem. 1998, 273, 5081–5085. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K. Integrin and Its Associated Proteins as a Mediator for Mechano-Signal Transduction. Biomolecules 2025, 15, 166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maggi, A.; Li, H.; Greer, J.R. Three-dimensional nano-architected scaffolds with tunable stiffness for efficient bone tissue growth. Acta Biomater. 2017, 63, 294–305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. S4), 467–479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Park, E.J.; Myint, P.K.; Ito, A.; Appiah, M.G.; Darkwah, S.; Kawamoto, E.; Shimaoka, M. Integrin-Ligand Interactions in Inflammation, Cancer, and Metabolic Disease: Insights into the Multifaceted Roles of an Emerging Ligand Irisin. Front. Cell Dev. Biol. 2020, 8, 588066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chavez, L.; Meguro, J.; Chen, S.; de Paiva, V.N.; Zambrano, R.; Eterno, J.M.; Kumar, R.; Duncan, M.R.; Benny, M.; Young, K.C.; et al. Circulating extracellular vesicles activate the pyroptosis pathway in the brain following ventilation-induced lung injury. J. Neuroinflamm. 2021, 18, 310. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Imran, S.A.M.; Ahmad Amin Noordin, K.B.; Zaman, W.S.W.K.; Nordin, F. Mechanotransduction in Mesenchymal Stem Cells (MSCs) Differentiation: A Review. Int. J. Mol. Sci. 2022, 23, 4580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.N.; Huang, Y.C.; Ni, G.X. Mechanotransduction of stem cells for tendon repair. World J. Stem Cells 2020, 12, 952–965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pattnaik, A.; Sanket, A.S.; Pradhan, S.; Sahoo, R.; Das, S.; Pany, S.; Douglas, T.E.L.; Dandela, R.; Liu, Q.; Rajadas, J.; et al. Designing of gradient scaffolds and their applications in tissue regeneration. Biomaterials 2023, 296, 122078. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Han, W.M.; Hou, L.Y.; Lin, D.D.; Li, J.Y.; Lin, S.T.; Yang, J.P.; Liao, L.; Zeng, X.A. Glutenin-chitosan 3D porous scaffolds with tunable stiffness and systematized microstructure for cultured meat model. Int. J. Biol. Macromol. 2024, 267 Pt 1, 131438. [Google Scholar] [CrossRef] [PubMed]
- Kegelman, C.D.; Collins, J.M.; Nijsure, M.P.; Eastburn, E.A.; Boerckel, J.D. Gone Caving: Roles of the Transcriptional Regulators YAP and TAZ in Skeletal Development. Curr. Osteoporos. Rep. 2020, 18, 526–540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heng, B.C.; Zhang, X.; Aubel, D.; Bai, Y.; Li, X.; Wei, Y.; Fussenegger, M.; Deng, X. An overview of signaling pathways regulating YAP/TAZ activity. Cell. Mol. Life Sci. 2021, 78, 497–512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, J.; Wu, T.; Lin, Q. Non-hippo kinases: Indispensable roles in YAP/TAZ signaling and implications in cancer therapy. Mol. Biol. Rep. 2023, 50, 4565–4578. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, J.; Zhang, X.; Luo, W.; Liu, L.; Zhu, Y.; Liu, Q.; Zhang, X.A. Emerging role and function of Hippo-YAP/TAZ signaling pathway in musculoskeletal disorders. Stem Cell Res. Ther. 2024, 15, 386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thampatty, B.P.; Li, H.; Im, H.J.; Wang, J.H. EP4 receptor regulates collagen type-I, MMP-1, and MMP-3 gene expression in human tendon fibroblasts in response to IL-1 beta treatment. Gene 2007, 386, 154–161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kegelman, C.D.; Coulombe, J.C.; Jordan, K.M.; Horan, D.J.; Qin, L.; Robling, A.G.; Ferguson, V.L.; Bellido, T.M.; Boerckel, J.D. YAP and TAZ Mediate Osteocyte Perilacunar/Canalicular Remodeling. J. Bone Miner. Res. 2020, 35, 196–210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shin, S.M.; Moehring, F.; Itson-Zoske, B.; Fan, F.; Stucky, C.L.; Hogan, Q.H.; Yu, H. Piezo2 mechanosensitive ion channel is located to sensory neurons and nonneuronal cells in rat peripheral sensory pathway: Implications in pain. Pain 2021, 162, 2750–2768. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woo, S.H.; Lukacs, V.; de Nooij, J.C.; Zaytseva, D.; Criddle, C.R.; Francisco, A.; Jessell, T.M.; Wilkinson, K.A.; Patapoutian, A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 2015, 18, 1756–1762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, H.; Hu, J.; Zheng, Q.; Feng, X.; Zhan, F.; Wang, X.; Xu, G.; Hua, F. Piezo1 Channels as Force Sensors in Mechanical Force-Related Chronic Inflammation. Front. Immunol. 2022, 13, 816149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, L.; Tian, D.; Su, Z.; Zhang, L.; Jie, L.; Guo, S.; Zhu, W.; Zhang, N.; Wang, P. Mechanical stress overload promotes NF-κB/NLRP3-mediated osteoarthritis synovitis and fibrosis through Piezo1. Cell. Signal. 2025, 132, 111786. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Mhalhel, K.; Pansera, L.; Montalbano, G.; Guerrera, M.C.; Levanti, M.; Laurà, R.; Abbate, F.; Vega, J.A.; Germanà, A. Localization of Piezo 1 and Piezo 2 in Lateral Line System and Inner Ear of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2024, 25, 9204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, D.; Zhao, J.; Zheng, J.; Zhao, Y.; Le, M.; Qin, D.; Huang, Q.; Huang, J.; Zhao, Q.; Wang, L.; et al. LOX-mediated ECM mechanical stress induces Piezo1 activation in hypoxic-ischemic brain damage and identification of novel inhibitor of LOX. Redox Biol. 2024, 76, 103346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-Castorena, J.M.; Riester, R.; Gamino-Ornelas, M.; Ada, N.; Guilak, F.; Danalache, M. PIEZO1-mediated calcium influx transiently alters nuclear mechanical properties via actin remodeling in chondrocytes. Biochem. Biophys. Res. Commun. 2025, 742, 151135. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, N.; Linley, J.E.; Torres, J.M.; Bee, L.; Dickenson, A.H.; Gringhuis, M.; Minett, M.S.; Hong, G.S.; Lee, E.; Oh, U.; et al. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat. Commun. 2013, 4, 1682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Obeidat, A.M.; Wood, M.J.; Adamczyk, N.S.; Ishihara, S.; Li, J.; Wang, L.; Ren, D.; Bennett, D.A.; Miller, R.J.; Malfait, A.M.; et al. Piezo2 expressing nociceptors mediate mechanical sensitization in experimental osteoarthritis. Nat. Commun. 2023, 14, 2479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, R.; Sporn, K.; Borole, A.; Khanna, A.; Gowda, C.; Paladugu, P.; Ngo, A.; Jagadeesan, R.; Zaman, N.; Tavakkoli, A. Biomarker-Guided Imaging and AI-Augmented Diagnosis of Degenerative Joint Disease. Diagnostics 2025, 15, 1418. [Google Scholar] [CrossRef]
- Xie, Z.; Feng, J.; Hibberd, T.J.; Chen, B.N.; Zhao, Y.; Zang, K.; Hu, X.; Yang, X.; Chen, L.; Brookes, S.J.; et al. Piezo2 channels expressed by colon-innervating TRPV1-lineage neurons mediate visceral mechanical hypersensitivity. Neuron 2023, 111, 526–538.e4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murthy, S.E.; Loud, M.C.; Daou, I.; Marshall, K.L.; Schwaller, F.; Kühnemund, J.; Francisco, A.G.; Keenan, W.T.; Dubin, A.E.; Lewin, G.R.; et al. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med. 2018, 10, eaat9897. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wan, Y.; Zhou, J.; Li, H. The Role of Mechanosensitive Piezo Channels in Chronic Pain. J. Pain Res. 2024, 17, 4199–4212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devos, H.; Zoidakis, J.; Roubelakis, M.G.; Latosinska, A.; Vlahou, A. Reviewing the Regulators of COL1A1. Int. J. Mol. Sci. 2023, 24, 10004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wadhwa, H.; Rohde, M.; Koltsov, J.C.B.; Cabell, A.; Smuck, M.; Hu, S.S.; Kleimeyer, J.P. Incidence and risk factors for complications following cervical epidural steroid injections. Spine J. 2025, 25, 886–902. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Sakai, D.; Risbud, M.V.; Tanaka, M.; Arai, F.; Abe, K.; Mochida, J. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010, 62, 3036–3047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, L.; Chen, W.; Qian, A.; Li, Y.P. Wnt/β-catenin signaling components and mechanisms in bone formation, homeostasis, and disease. Bone Res. 2024, 12, 39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Premaraj, S.; Souza, I.; Premaraj, T. Mechanical loading activates β-catenin signaling in periodontal ligament cells. Angle Orthod. 2011, 81, 592–599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tigchelaar, S.S.; Wadhwa, H.; Mathur, M.B.; He, Z.; Tharin, S. Spinal cord injury: A systematic review and meta-analysis of microRNA alterations. BioRxiv 2023. [Google Scholar] [CrossRef]
- Bastakoty, D.; Saraswati, S.; Cates, J.; Lee, E.; Nanney, L.B.; Young, P.P. Inhibition of Wnt/β-catenin pathway promotes regenerative repair of cutaneous and cartilage injury. FASEB J. 2015, 29, 4881–4892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ravi, K.; Paidas, M.J.; Saad, A.; Jayakumar, A.R. Astrocytes in rare neurological conditions: Morphological and functional considerations. J. Comp. Neurol. 2021, 529, 2676–2705. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Nengroo, M.A.; Datta, D.; Katti, D.S. Converse modulation of Wnt/β-catenin signaling during expansion and differentiation phases of Infrapatellar fat pad-derived MSCs for improved engineering of hyaline cartilage. Biomaterials 2023, 302, 122296. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, A.; Tadagavadi, R.K.; Swafford, D.; Manicassamy, S. Modulation of Inflammatory Responses by Wnt/β-Catenin Signaling in Dendritic Cells: A Novel Immunotherapy Target for Autoimmunity and Cancer. Front. Immunol. 2016, 7, 460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, Z.; Guo, J.; Wu, T.Y.; Chen, X.; Xu, L.L.; Lin, S.E.; Sun, Y.X.; Chan, K.M.; Ouyang, H.; Li, G. Stepwise Differentiation of Mesenchymal Stem Cells Augments Tendon-Like Tissue Formation and Defect Repair In Vivo. Stem Cells Transl. Med. 2016, 5, 1106–1116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, P.T.; Handorf, A.M.; Jeon, W.B.; Li, W.J. Stem cell-based tissue engineering approaches for musculoskeletal regeneration. Curr. Pharm. Des. 2013, 19, 3429–3445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakai, T.; Kumagai, K. Molecular dissection of tendon development and healing: Insights into tenogenic phenotypes and functions. J. Biol. Chem. 2025, 301, 108353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yilgor, C.; Yilgor Huri, P.; Huri, G. Tissue engineering strategies in ligament regeneration. Stem Cells Int. 2012, 2012, 374676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoang, V.T.; Nguyen, Q.T.; Phan, T.T.K.; Pham, T.H.; Dinh, N.T.H.; Anh, L.P.H.; Dao, L.T.M.; Bui, V.D.; Dao, H.N.; Le, D.S.; et al. Tissue Engineering and Regenerative Medicine: Perspectives and Challenges. MedComm 2025, 6, e70192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ajalik, R.E.; Alenchery, R.G.; Cognetti, J.S.; Zhang, V.Z.; McGrath, J.L.; Miller, B.L.; Awad, H.A. Human Organ-on-a-Chip Microphysiological Systems to Model Musculoskeletal Pathologies and Accelerate Therapeutic Discovery. Front. Bioeng. Biotechnol. 2022, 10, 846230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kishimoto, Y.; Ohkawara, B.; Sakai, T.; Ito, M.; Masuda, A.; Ishiguro, N.; Shukunami, C.; Docheva, D.; Ohno, K. Wnt/β-catenin signaling suppresses expressions of Scx, Mkx, and Tnmd in tendon-derived cells. PLoS ONE 2017, 12, e0182051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciardulli, M.C.; Marino, L.; Lovecchio, J.; Giordano, E.; Forsyth, N.R.; Selleri, C.; Maffulli, N.; Porta, G.D. Tendon and Cytokine Marker Expression by Human Bone Marrow Mesenchymal Stem Cells in a Hyaluronate/Poly-Lactic-Co-Glycolic Acid (PLGA)/Fibrin Three-Dimensional (3D) Scaffold. Cells 2020, 9, 1268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiegertjes, R.; van de Loo, F.A.J.; Blaney Davidson, E.N. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology 2020, 59, 2681–2694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tateiwa, D.; Yoshikawa, H.; Kaito, T. Cartilage and Bone Destruction in Arthritis: Pathogenesis and Treatment Strategy: A Literature Review. Cells 2019, 8, 818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; van Boxel-Dezaire, A.H.; Cheon, H.; Yang, J.; Stark, G.R. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 16975–16980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, P.C.; Feist, E.; Pope, J.E.; Nash, P.; Sibilia, J.; Caporali, R.; Balsa, A. What have we learnt from the inhibition of IL-6 in RA and what are the clinical opportunities for patient outcomes? Ther. Adv. Musculoskelet. Dis. 2024, 16, 1759720X241283340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrari, L.F.; Khomula, E.V.; Araldi, D.; Levine, J.D. CD44 Signaling Mediates High Molecular Weight Hyaluronan-Induced Antihyperalgesia. J. Neurosci. 2018, 38, 308–321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, H.; Yeo, M.; Kang, Y.; Kim, H.J.; Park, S.G.; Jang, E.; Park, S.H.; Kim, E.; Kang, S. Lactate oxidase/catalase-displaying nanoparticles efficiently consume lactate in the tumor microenvironment to effectively suppress tumor growth. J. Nanobiotechnol. 2023, 21, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, N.; Wang, X.; Wang, Z.; Kan, Y.; Fang, Y.; Gao, J.; Kong, X.; Wang, J. Nanomaterials-driven in situ vaccination: A novel frontier in tumor immunotherapy. J. Hematol. Oncol. 2025, 18, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, Y.; Tang, Q.; Wang, B.; Yang, Q.; Zhang, Y.; Lei, L.; Li, S. Targeting the tumor microenvironment with biomaterials for enhanced immunotherapeutic efficacy. J. Nanobiotechnol. 2024, 22, 737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moazzam, M.; Zhang, M.; Hussain, A.; Yu, X.; Huang, J.; Huang, Y. The landscape of nanoparticle-based siRNA delivery and therapeutic development. Mol. Ther. 2024, 32, 284–312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, Z.I.; Schoepflin, Z.R.; Choi, H.; Shapiro, I.M.; Risbud, M.V. Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur. Cells Mater. 2015, 30, 104–116; discussion 116–117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Haseeb, A.; Chen, D.; Haqqi, T.M. Delphinidin inhibits IL-1β-induced activation of NF-κB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes. Rheumatology 2013, 52, 998–1008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Velnar, T.; Gradisnik, L. Endplate role in the degenerative disc disease: A brief review. World J. Clin. Cases 2023, 11, 17–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kievit, W.; Fransen, J.; Oerlemans, A.J.; Kuper, H.H.; van der Laar, M.A.; de Rooij, D.J.; De Gendt, C.M.; Ronday, K.H.; Jansen, T.L.; van Oijen, P.C.; et al. The efficacy of anti-TNF in rheumatoid arthritis, a comparison between randomised controlled trials and clinical practice. Ann. Rheum. Dis. 2007, 66, 1473–1478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, N.J.; Ha, S.K.; Sati, P.; Absinta, M.; Nair, G.; Luciano, N.J.; Leibovitch, E.C.; Yen, C.C.; Rouault, T.A.; Silva, A.C.; et al. Potential role of iron in repair of inflammatory demyelinating lesions. J. Clin. Investig. 2019, 129, 4365–4376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donadieu, M.; Lee, N.J.; Gaitán, M.I.; Ha, S.K.; Luciano, N.J.; Roy, S.; Ineichen, B.; Leibovitch, E.C.; Yen, C.C.; Pham, D.L.; et al. In vivo MRI is sensitive to remyelination in a nonhuman primate model of multiple sclerosis. eLife 2023, 12, e73786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brovold, M.; Almeida, J.I.; Pla-Palacín, I.; Sainz-Arnal, P.; Sánchez-Romero, N.; Rivas, J.J.; Almeida, H.; Dachary, P.R.; Serrano-Aulló, T.; Soker, S.; et al. Naturally-Derived Biomaterials for Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1077, 421–449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, M.; Wang, J.; Xu, X.; Li, E.; Xu, P. Engineering gene-activated bioprinted scaffolds for enhancing articular cartilage repair. Mater. Today Bio 2024, 29, 101351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kacprzak, B.; Stańczak, M.; Bielenda, B.; Yarmohammadi, A.A.; Hagner-Derengowska, M. Molecular Aspects of Cartilage Microfracturation: Rehabilitation Insights. Orthop. Rev. 2025, 17, 129917. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McClurg, O.; Tinson, R.; Troeberg, L. Targeting Cartilage Degradation in Osteoarthritis. Pharmaceuticals 2021, 14, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tolstova, T.; Dotsenko, E.; Luzgina, N.; Rusanov, A. Preconditioning of Mesenchymal Stem Cells Enhances the Neuroprotective Effects of Their Conditioned Medium in an Alzheimer’s Disease In Vitro Model. Biomedicines 2024, 12, 2243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haider, K.H. Priming mesenchymal stem cells to develop “super stem cells”. World J. Stem Cells 2024, 16, 623–640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, H.; Yang, H.; Wang, Q.; Ji, H.; Qian, T.; Qiao, Y.; Shi, J.; Cong, M. Mesenchymal stem cells and their extracellular vesicles: New therapies for cartilage repair. Front. Bioeng. Biotechnol. 2025, 13, 1591400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paladugu, P.; Kumar, R.; Hage, T.; Vaja, S.; Sekhar, T.; Weisberg, S.; Sporn, K.; Waisberg, E.; Ong, J.; Vadhera, A.; et al. Leveraging lower body negative pressure for enhanced outcomes in orthopedic arthroplasty—Insights from NASA’s bone health research. Life Sci. Space Res. 2025, 46, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, D.; Yen, J.H.; Joseph, D.J.; Friedman, W. Cell type-specific interleukin-1beta signaling in the CNS. J. Neurosci. 2004, 24, 6482–6488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Z.; Wang, Y.; Zhou, R.; Li, Y.; Gao, Y.; Tu, D.; Wilson, B.; Song, S.; Feng, J.; Hong, J.-S.; et al. A novel role of NLRP3-generated IL-1β in the acute-chronic transition of peripheral lipopolysaccharide-elicited neuroinflammation: Implications for sepsis-associated neurodegeneration. J. Neuroinflamm. 2020, 17, 64. [Google Scholar] [CrossRef]
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cota Quintero, J.L.; Ramos-Payán, R.; Romero-Quintana, J.G.; Ayala-Ham, A.; Bermúdez, M.; Aguilar-Medina, E.M. Hydrogel-Based Scaffolds: Advancing Bone Regeneration Through Tissue Engineering. Gels 2025, 11, 175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Hu, J.; Chen, C.; Li, X.; Zhang, H.; Xin, Y.; Tian, Q.; Wang, S. Emerging advances in hydrogel-based therapeutic strategies for tissue regeneration. Regen. Ther. 2023, 24, 459–471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, R.; Chen, F.; Chen, K.; Xu, J. Advances in the application of hydrogel-based scaffolds for tendon repair. Genes Dis. 2023, 11, 101019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, Y.; Liu, Z.; Wang, H.; Meng, H.; Cao, Y. Mesoporous Silica Nanoparticles Mediate SiRNA Delivery for Long-Term Multi-Gene Silencing in Intact Plants. Adv. Sci. 2024, 11, e2301358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Deng, G.; Hu, X.; Li, C.; Wang, X.; Zhu, Q.; Zheng, K.; Xiong, W.; Wu, H. Recent advances in mesoporous silica nanoparticle-based targeted drug-delivery systems for cancer therapy. Nanomedicine 2022, 17, 1253–1279. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, N.U.; Lee, J.; Kim, J.; Kim, Y.; Yu, S.; Kim, J.; Kim, S.; Sung, D.; Kim, H. Mesoporous Silica Nanoparticles as a Gene Delivery Platform for Cancer Therapy. Pharmaceutics 2023, 15, 1432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saparov, A.; Ogay, V.; Nurgozhin, T.; Jumabay, M.; Chen, W.C. Preconditioning of Human Mesenchymal Stem Cells to Enhance Their Regulation of the Immune Response. Stem Cells Int. 2016, 2016, 3924858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, P.; Wang, Y.; Lv, L.; Wang, D.; Wang, Y. Roles of Chemokines in Intervertebral Disk Degeneration. Curr. Pain Headache Rep. 2024, 28, 95–108. [Google Scholar] [CrossRef]
- Hicks, M.R.; Cao, T.V.; Campbell, D.H.; Standley, P.R. Mechanical strain applied to human fibroblasts differentially regulates skeletal myoblast differentiation. J. Appl. Physiol. 2012, 113, 465–472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bess, S.N.; Greening, G.J.; Rajaram, N.; Muldoon, T.J. Macrophage-targeted anti-CCL2 immunotherapy enhances tumor sensitivity to 5-fluorouracil in a Balb/c-CT26 murine colon carcinoma model measured using diffuse reflectance spectroscopy. BMC Immunol. 2022, 23, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sullivan, C.B.; Porter, R.M.; Evans, C.H.; Ritter, T.; Shaw, G.; Barry, F.; Murphy, J.M. TNFα and IL-1β influence the differentiation and migration of murine MSCs independently of the NF-κB pathway. Stem Cell Res. Ther. 2014, 5, 104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katanov, C.; Lerrer, S.; Liubomirski, Y.; Leider-Trejo, L.; Meshel, T.; Bar, J.; Feniger-Barish, R.; Kamer, I.; Soria-Artzi, G.; Kahani, H.; et al. Regulation of the inflammatory profile of stromal cells in human breast cancer: Prominent roles for TNF-α and the NF-κB pathway. Stem Cell Res Ther. 2015, 6, 87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuang, P.P.; Liu, X.Q.; Li, C.G.; He, B.X.; Xie, Y.C.; Wu, Z.C.; Li, C.L.; Deng, X.H.; Fu, Q.L. Mesenchymal stem cells overexpressing interleukin-10 prevent allergic airway inflammation. Stem Cell Res. Ther. 2023, 14, 369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diekman, B.O.; Wu, C.L.; Louer, C.R.; Furman, B.D.; Huebner, J.L.; Kraus, V.B.; Olson, S.A.; Guilak, F. Intra-articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents posttraumatic arthritis. Cell Transplant. 2013, 22, 1395–1408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sukubo, N.G.; Bigini, P.; Morelli, A. Nanocarriers and macrophage interaction: From a potential hurdle to an alternative therapeutic strategy. Beilstein J. Nanotechnol. 2025, 16, 97–118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, V.W.; Akaishi, S.; Longaker, M.T.; Gurtner, G.C. Pushing back: Wound mechanotransduction in repair and regeneration. J. Investig. Dermatol. 2011, 131, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Qiu, S.; Huang, R.; Wang, Y.; Wang, Y.; Li, M.; Ye, Q.; Zhang, S.; Qi, Z.; et al. IL-33/ST2 drives inflammatory pain via CCL2 signaling and activation of TRPV1 and TRPM8. Commun. Biol. 2025, 8, 724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, R.; Sporn, K.; Paladugu, P.; Khanna, A.; Gowda, C.; Ngo, A.; Waisberg, E.; Ong, J.; Jagadeesan, R.; Tavakkoli, A. Emerging Diagnostic Approaches for Musculoskeletal Disorders: Advances in Imaging, Biomarkers, and Clinical Assessment. Diagnostics 2025, 15, 1648. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Z.; Wang, K.; Chen, Z.; Shen, H. Suppression of microglial Ccl2 reduces neuropathic pain associated with chronic spinal compression. Front. Immunol. 2023, 14, 1191188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, R.; Sporn, K.; Gowda, C.; Khanna, A.; Prabhakar, P.; Paladugu, P.; Jagadeesan, R.; Clarkson, L.; Chandrahasegowda, S.; Kumar, T.; et al. Advancing Spine Connectomics and Neural Integration through Machine Learning and Neuroengineering. Preprints 2025, 2025060518. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakao, A.; Imamura, T.; Souchelnytskyi, S.; Kawabata, M.; Ishisaki, A.; Oeda, E.; Tamaki, K.; Hanai, J.; Heldin, C.H.; Miyazono, K.; et al. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997, 16, 5353–5362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-beta signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J. Investig. Dermatol. 2002, 118, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, T.; Pajarinen, J.; Nabeshima, A.; Lu, L.; Nathan, K.; Yao, Z.; Goodman, S.B. Establishment of NF-κB sensing and interleukin-4 secreting mesenchymal stromal cells as an “on-demand” drug delivery system to modulate inflammation. Cytotherapy 2017, 19, 1025–1034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoshi, H.; Akagi, R.; Yamaguchi, S.; Muramatsu, Y.; Akatsu, Y.; Yamamoto, Y.; Sasaki, T.; Takahashi, K.; Sasho, T. Effect of inhibiting MMP13 and ADAMTS5 by intra-articular injection of small interfering RNA in a surgically induced osteoarthritis model of mice. Cell Tissue Res. 2017, 368, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pizzute, T.; Pei, M. Anti-inflammatory strategies in cartilage repair. Tissue Eng. Part B Rev. 2014, 20, 655–668. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, X.; Yue, T.; Gu, W.; Cheng, W.; He, L.; Ren, W.; Li, F.; Piao, J.G. Recent Advances in Mesoporous Silica Nanoparticles Delivering siRNA for Cancer Treatment. Pharmaceutics 2023, 15, 2483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yap, K.M.; Sekar, M.; Fuloria, S.; Wu, Y.S.; Gan, S.H.; Mat Rani, N.N.I.; Subramaniyan, V.; Kokare, C.; Lum, P.T.; Begum, M.Y.; et al. Drug Delivery of Natural Products Through Nanocarriers for Effective Breast Cancer Therapy: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 7891–7941. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Desai, P.R.; Marepally, S.; Patel, A.R.; Voshavar, C.; Chaudhuri, A.; Singh, M. Topical delivery of anti-TNFα siRNA and capsaicin via novel lipid-polymer hybrid nanoparticles efficiently inhibits skin inflammation in vivo. J. Control. Release 2013, 170, 51–63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, C.M.; Macdonald, C.D.; Litherland, G.J.; Wilkinson, D.J.; Skelton, A.; Europe-Finner, G.N.; Rowan, A.D. Cytokine-induced MMP13 Expression in Human Chondrocytes Is Dependent on Activating Transcription Factor 3 (ATF3) Regulation. J. Biol. Chem. 2017, 292, 1625–1636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, Y.; Yuan, W.; Fujita, N.; Wang, J.; Wang, H.; Shapiro, I.M.; Risbud, M.V. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-κB. Am. J. Pathol. 2013, 182, 2310–2321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, Q.; Sun, Q.; Xu, H.; Chen, J.; Ling, H.; Ge, Q.; Zou, K.; Wang, X.; Jin, H.; Li, J.; et al. Amygdalin Delays Cartilage Endplate Degeneration and Improves Intervertebral Disc Degeneration by Inhibiting NF-κB Signaling Pathway and Inflammatory Response. J. Inflamm. Res. 2023, 16, 3455–3468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tabeian, H.; Betti, B.F.; Dos Santos Cirqueira, C.; de Vries, T.J.; Lobbezoo, F.; Ter Linde, A.V.; Zandieh-Doulabi, B.; Koenders, M.I.; Everts, V.; Bakker, A.D. IL-1β Damages Fibrocartilage and Upregulates MMP-13 Expression in Fibrochondrocytes in the Condyle of the Temporomandibular Joint. Int. J. Mol. Sci. 2019, 20, 2260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masuda, K.; Lotz, J.C. New challenges for intervertebral disc treatment using regenerative medicine. Tissue Eng. Part B Rev. 2010, 16, 147–158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Sun, T.; Zhang, W.; Yang, M.; Li, Z. Autologous cultured adipose derived mesenchymal stem cells combined with hyaluronic acid hydrogel in the treatment of discogenic low back pain: A study protocol for a phase II randomised controlled trial. BMJ Open 2022, 12, e063925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clarke, L.E.; McConnell, J.C.; Sherratt, M.J.; Derby, B.; Richardson, S.M.; Hoyland, J.A. Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res. Ther. 2014, 16, R67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, A.; Shen, B.; Williams, L.; Diwan, A. Mesenchymal stem cells: Potential application in intervertebral disc regeneration. Transl. Pediatr. 2014, 3, 71–90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.; Lee, E.H.; Yang, Z. Hypoxia-Conditioned Mesenchymal Stem Cells in Tissue Regeneration Application. Tissue Eng. Part B Rev. 2022, 28, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Shi, X.; Darabi, R.; Li, Y. Hypoxia in Cell Reprogramming and the Epigenetic Regulations. Front. Cell Dev. Biol. 2021, 9, 609984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camuzi, D.; de Amorim, Í.S.S.; Ribeiro Pinto, L.F.; Oliveira Trivilin, L.; Mencalha, A.L.; Soares Lima, S.C. Regulation Is in the Air: The Relationship between Hypoxia and Epigenetics in Cancer. Cells 2019, 8, 300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Cai, W.; Xiang, P.; Liu, Y.; Xu, H.; Zhang, W.; Han, F.; Luo, Z.; Liang, T. Viscoelastic hydrogel combined with dynamic compression promotes osteogenic differentiation of bone marrow mesenchymal stem cells and bone repair in rats. Regen. Biomater. 2024, 12, rbae136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peffers, M.J.; Thorpe, C.T.; Collins, J.A.; Eong, R.; Wei, T.K.; Screen, H.R.; Clegg, P.D. Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation. J. Biol. Chem. 2014, 289, 25867–25878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gillespie, E.F.; Santos, P.M.G.; Curry, M.; Salz, T.; Chakraborty, N.; Caron, M.; Fuchs, H.E.; Vicioso, N.L.; Mathis, N.; Kumar, R.; et al. Implementation Strategies to Promote Short-Course Radiation for Bone Metastases. JAMA Netw. Open 2024, 7, e2411717. [Google Scholar] [CrossRef]
- Kegelman, C.D.; Nijsure, M.P.; Moharrer, Y.; Pearson, H.B.; Dawahare, J.H.; Jordan, K.M.; Qin, L.; Boerckel, J.D. YAP and TAZ Promote Periosteal Osteoblast Precursor Expansion and Differentiation for Fracture Repair. J. Bone Miner. Res. 2021, 36, 143–157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Binaymotlagh, R.; Chronopoulou, L.; Palocci, C. Peptide-Based Hydrogels: Template Materials for Tissue Engineering. J. Funct. Biomater. 2023, 14, 233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, Q.; Sandhurst, E.S.; Liu, Y.; Sun, H. BBP-Functionalized Biomimetic Nanofibrous Scaffold Can Capture BMP2 and Promote Osteogenic Differentiation. J. Mater. Chem. B 2017, 5, 5196–5205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barakat, H.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Alshimali, S.I.; Abdellatif, A.A.H. Amygdalin: A Review on Its Characteristics, Antioxidant Potential, Gastrointestinal Microbiota Intervention, Anticancer Therapeutic and Mechanisms, Toxicity, and Encapsulation. Biomolecules 2022, 12, 1514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, K.; Han, J.; Wang, Z. Histone modifications centric-regulation in osteogenic differentiation. Cell Death Discov. 2021, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Waisberg, E.; Ong, J.; Lee, A.G. The potential power of Neuralink-how brain-machine interfaces can revolutionize medicine. Expert Rev. Med. Devices 2025, 22, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Musk, E.; Neuralink. An Integrated Brain-Machine Interface Platform with Thousands of Channels. J. Med. Internet Res. 2019, 21, e16194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ognard, J.; El Hajj, G.; Verma, O.; Ghozy, S.; Kadirvel, R.; Kallmes, D.F.; Brinjikji, W. Advances in endovascular brain computer interface: Systematic review and future implications. J. Neurosci. Methods 2025, 420, 110471. [Google Scholar] [CrossRef] [PubMed]
- Neuralink. Neuralink Raises $650 Million Series E. 2025. Available online: https://neuralink.com/updates/neuralink-raises-650m-series-e/ (accessed on 4 June 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Sporn, K.; Kaur, H.; Khanna, A.; Paladugu, P.; Zaman, N.; Tavakkoli, A. Current Mechanobiological Pathways and Therapies Driving Spinal Health. Bioengineering 2025, 12, 886. https://doi.org/10.3390/bioengineering12080886
Kumar R, Sporn K, Kaur H, Khanna A, Paladugu P, Zaman N, Tavakkoli A. Current Mechanobiological Pathways and Therapies Driving Spinal Health. Bioengineering. 2025; 12(8):886. https://doi.org/10.3390/bioengineering12080886
Chicago/Turabian StyleKumar, Rahul, Kyle Sporn, Harlene Kaur, Akshay Khanna, Phani Paladugu, Nasif Zaman, and Alireza Tavakkoli. 2025. "Current Mechanobiological Pathways and Therapies Driving Spinal Health" Bioengineering 12, no. 8: 886. https://doi.org/10.3390/bioengineering12080886
APA StyleKumar, R., Sporn, K., Kaur, H., Khanna, A., Paladugu, P., Zaman, N., & Tavakkoli, A. (2025). Current Mechanobiological Pathways and Therapies Driving Spinal Health. Bioengineering, 12(8), 886. https://doi.org/10.3390/bioengineering12080886





