Accuracy of Drill Sleeve Housing in 3D-Printed and Milled Implant Surgical Guides: A 3D Analysis Considering Machine Type, Layer Thickness, Sleeve Position, and Steam Sterilization
Abstract
1. Introduction
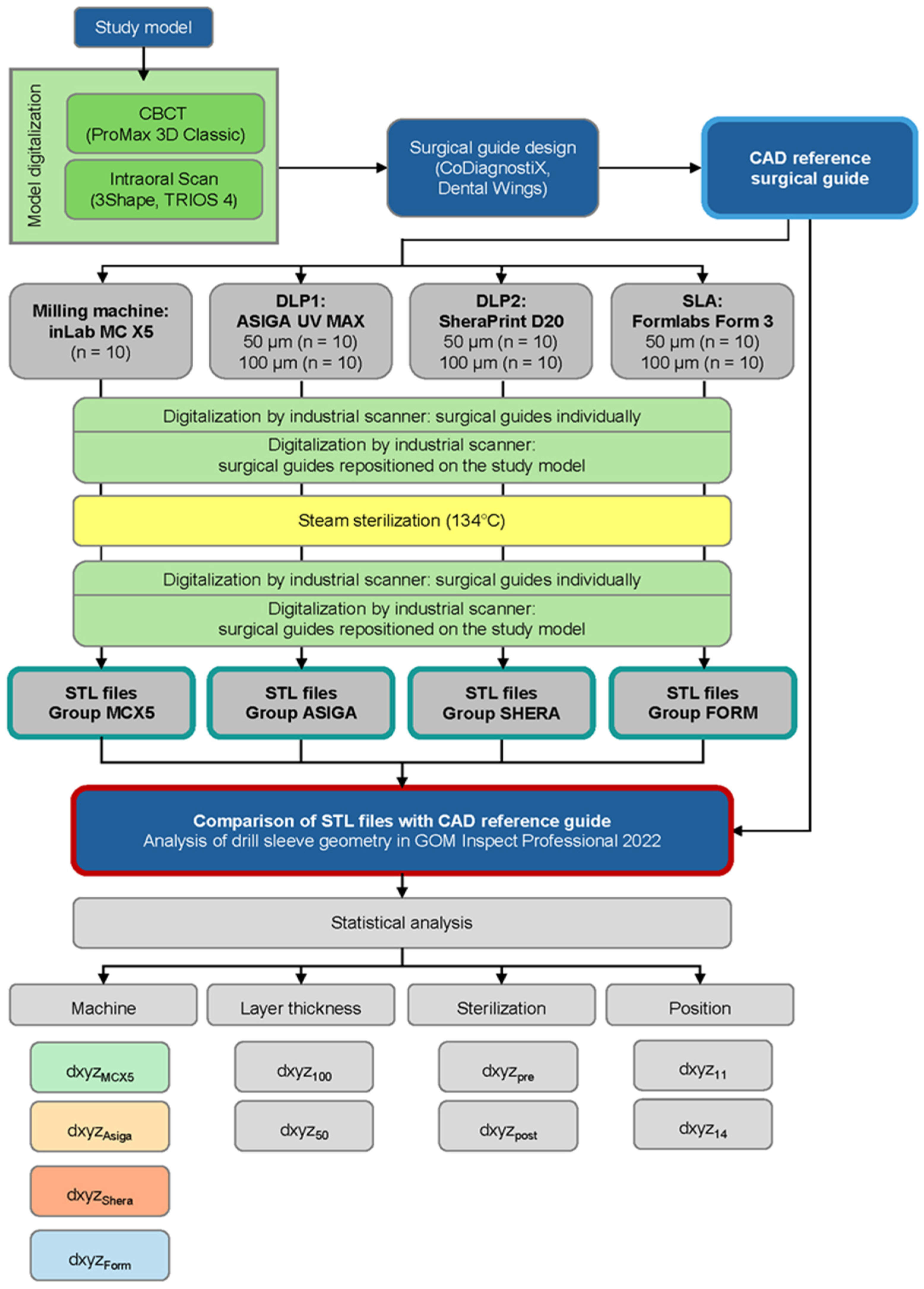
2. Materials and Methods
- There is a statistically significant difference in the three-dimensional deviation of the drill sleeve housing position of surgical guides between different machine types.
- The layer thickness used by additive manufacturing machines (50 µm and 100 µm) has a statistically significant influence on the three-dimensional deviation of the drill sleeve housing position.
- The position within the surgical guide (regions 11 and 14) has no statistically significant influence on the three-dimensional deviation of the drill sleeve housing position before and after sterilization.
- Steam sterilization has a statistically significant influence on the three-dimensional deviation of the drill sleeve housing position.
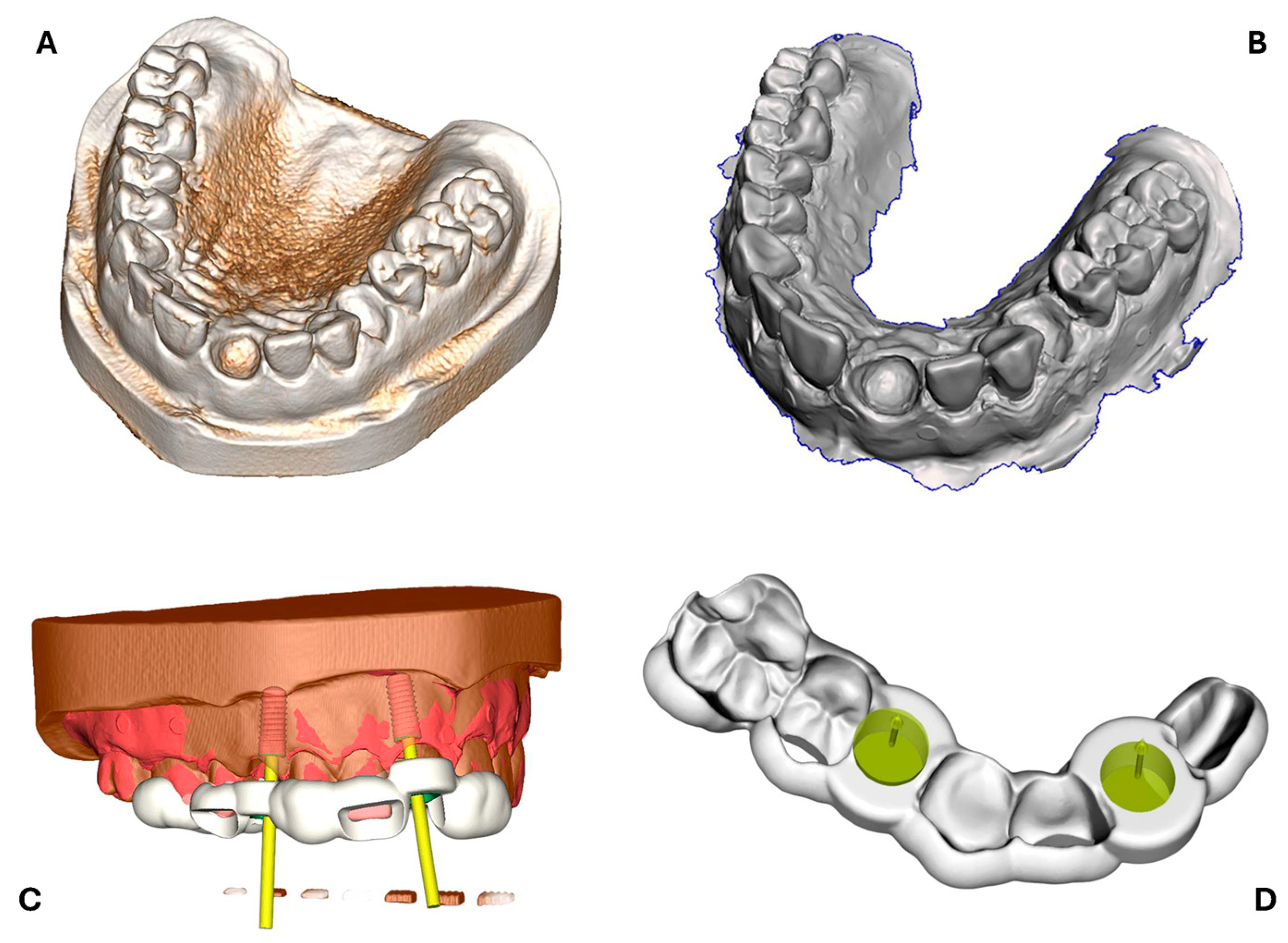
2.1. Design of Surgical Reference Guide
2.2. Surgical Guide Production
2.3. Digitalization and 3D Evaluation of Surgical Guides
2.3.1. Surgical Guide Digitalization
2.3.2. Sterilization Process
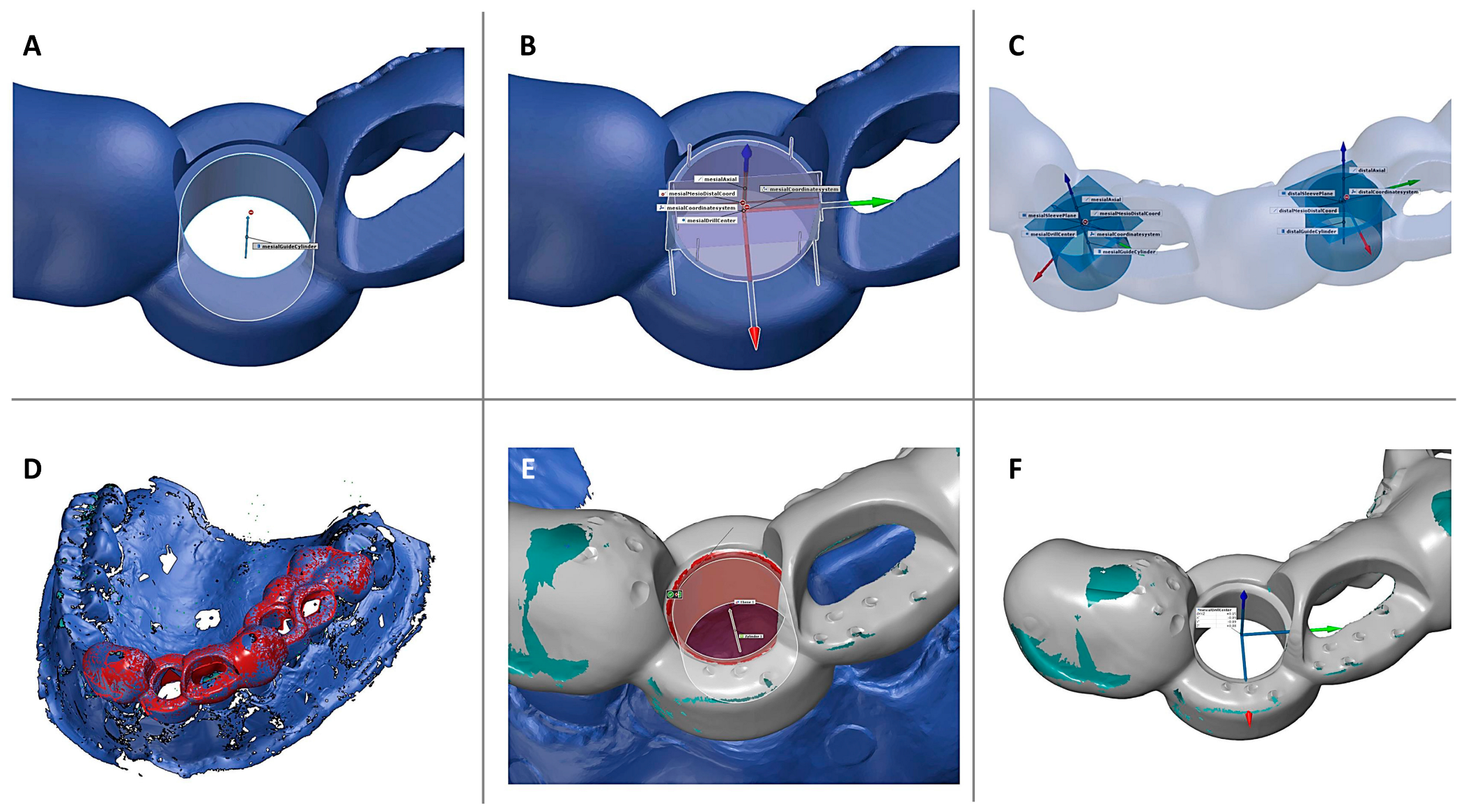
2.3.3. Three-Dimensional Data Analysis and Calculation of Deviations
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAD | Computer-aided design |
| CAM | Computer-aided manufacturing |
| CBCT | Cone beam computed tomography |
| SLA | Stereolithography apparatus |
| DLP | Digital light processing |
| DMD | Direct micromirror device |
| DICOM | Digital imaging and communications in medicine |
| CEREC | Ceramic reconstruction |
| STL | Standard tessellation language |
| s-CAIS | Static computer-aided implant surgery |
| CNC | Computer numeric control |
| IQR | Interquartile range |
References
- Brånemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Tatakis, D.N.; Chien, H.H.; Parashis, A.O. Guided implant surgery risks and their prevention. Periodontology 2000 2019, 81, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.; Chen, S.T.; Martin, W.C.; Levine, R.A.; Buser, D. Consensus statements and recommended clinical procedures regarding optimizing esthetic outcomes in implant dentistry. Int. J. Oral Maxillofac. Implant. 2014, 29, 216–220. [Google Scholar] [CrossRef]
- Wismeijer, D.; Joda, T.; Flügge, T.; Fokas, G.; Tahmaseb, A.; Bechelli, D.; Bohner, L.; Bornstein, M.; Burgoyne, A.; Caram, S.; et al. Group 5 ITI Consensus Report: Digital technologies. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 436–442. [Google Scholar] [CrossRef]
- Tattan, M.; Chambrone, L.; González-Martín, O.; Avila-Ortiz, G. Static computer-aided, partially guided, and free-handed implant placement: A systematic review and meta-analysis of randomized controlled trials. Clin. Oral Implant. Res. 2020, 31, 889–916. [Google Scholar] [CrossRef] [PubMed]
- Henprasert, P.; Dawson, D.V.; El-Kerdani, T.; Song, X.; Couso-Queiruga, E.; Holloway, J.A. Comparison of the Accuracy of Implant Position Using Surgical Guides Fabricated by Additive and Subtractive Techniques. J. Prosthodont. 2020, 29, 534–541. [Google Scholar] [CrossRef]
- Azevedo, M.; Correia, F.; Faria Almeida, R. Accuracy of Implant Guided Surgery in Fully Edentulous Patients: Prediction vs. Actual Outcome—Systematic Review. J. Clin. Med. 2024, 13, 5178. [Google Scholar] [CrossRef]
- Eftekhar Ashtiani, R.; Ghasemi, Z.; Nami, M.; Mighani, F.; Namdari, M. Accuracy of static digital surgical guides for dental implants based on the guide system: A systematic review. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 600–607. [Google Scholar] [CrossRef]
- Alharbi, N.; Wismeijer, D.; Osman, R.B. Additive Manufacturing Techniques in Prosthodontics: Where Do We Currently Stand? A Critical Review. Int. J. Prosthodont. 2017, 30, 474–484. [Google Scholar] [CrossRef]
- Duret, F.; Blouin, J.-L.; Duret, B. CAD-CAM in dentistry. J. Am. Dent. Assoc. 1988, 117, 715–720. [Google Scholar] [CrossRef]
- Blatz, M.B.; Conejo, J. The Current State of Chairside Digital Dentistry and Materials. Dent. Clin. N. Am. 2019, 63, 175–197. [Google Scholar] [CrossRef]
- Kirsch, C.; Ender, A.; Attin, T.; Mehl, A. Trueness of four different milling procedures used in dental CAD/CAM systems. Clin. Oral Investig. 2017, 21, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wei, H.; Li, D. Additive manufacturing technologies in the oral implant clinic: A review of current applications and progress. Front. Bioeng. Biotechnol. 2023, 11, 1100155. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Hickel, R.; Reymus, M. 3D Printing in Dentistry—State of the Art. Oper. Dent. 2019, 45, 30–40. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.V.; Hernandez, A. A Review of Additive Manufacturing. ISRN Mech. Eng. 2012, 2012, 208760. [Google Scholar] [CrossRef]
- Jockusch, J.; Özcan, M. Additive manufacturing of dental polymers: An overview on processes, materials and applications. Dent. Mater. J. 2020, 39, 345–354. [Google Scholar] [CrossRef]
- Johansson, C.; Dibes, J.; Rodriguez, L.E.L.; Papia, E. Accuracy of 3D printed polymers intended for models and surgical guides printed with two different 3D printers. Dent. Mater. J. 2021, 40, 339–347. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Muelhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Felzmann, R.; Gruber, S.; Mitteramskogler, G.; Tesavibul, P.; Boccaccini, A.R.; Liska, R.; Stampfl, J. Lithography-Based Additive Manufacturing of Cellular Ceramic Structures. Adv. Eng. Mater. 2012, 14, 1052–1058. [Google Scholar] [CrossRef]
- Commission for Hospital Hygiene and Infection Prevention (KRINKO); Federal Institute for Drugs and Medical Devices (BfArM). [Hygiene requirements for the reprocessing of medical devices. Recommendation of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI) and the Federal Institute for Drugs and Medical Devices (BfArM)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2012, 55, 1244–1310. [Google Scholar] [CrossRef]
- Bayarsaikhan, E.; Lim, J.H.; Shin, S.H.; Park, K.H.; Park, Y.B.; Lee, J.H.; Kim, J.E. Effects of Postcuring Temperature on the Mechanical Properties and Biocompatibility of Three-Dimensional Printed Dental Resin Material. Polymers 2021, 13, 1180. [Google Scholar] [CrossRef]
- Shaheen, E.; Alhelwani, A.; Van De Casteele, E.; Politis, C.; Jacobs, R. Evaluation of Dimensional Changes of 3D Printed Models After Sterilization: A Pilot Study. Open Dent. J. 2018, 12, 72–79. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Valente, F.; Schiroli, G.; Sbrenna, A. Accuracy of Computer-Aided Oral Implant Surgery: A Clinical and Radiographic Study. Int. J. Oral Maxillofac. Implant. 2008, 24, 234–242. [Google Scholar]
- Motel, C.; Kirchner, E.; Adler, W.; Wichmann, M.; Matta, R.E. Impact of Different Scan Bodies and Scan Strategies on the Accuracy of Digital Implant Impressions Assessed with an Intraoral Scanner: An In Vitro Study. J. Prosthodont. 2020, 29, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Holst, S.; Karl, M.; Wichmann, M.; Matta, R.E. A new triple-scan protocol for 3D fit assessment of dental restorations. Quintessence Int. 2011, 42, 651–657. [Google Scholar] [PubMed]
- Lo Russo, L.; Ercoli, C.; Guida, L.; Merli, M.; Laino, L. Surgical guides for dental implants: Measurement of the accuracy using a freeware metrology software program. J. Prosthodont. Res. 2023, 67, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Matta, R.E.; Bergauer, B.; Adler, W.; Wichmann, M.; Nickenig, H.J. The impact of the fabrication method on the three-dimensional accuracy of an implant surgery template. J. Cranio-Maxillo-Facial Surg. 2017, 45, 804–808. [Google Scholar] [CrossRef]
- Keßler, A.; Dosch, M.; Reymus, M.; Folwaczny, M. Influence of 3D-printing method, resin material, and sterilization on the accuracy of virtually designed surgical implant guides. J. Prosthet. Dent. 2022, 128, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Gjelvold, B.; Mahmood, D.J.H.; Wennerberg, A. Accuracy of surgical guides from 2 different desktop 3D printers for computed tomography-guided surgery. J. Prosthet. Dent. 2019, 121, 498–503. [Google Scholar] [CrossRef]
- Anunmana, C.; Ueawitthayasuporn, C.; Kiattavorncharoen, S.; Thanasrisuebwong, P. In Vitro Comparison of Surgical Implant Placement Accuracy Using Guides Fabricated by Three Different Additive Technologies. Appl. Sci. 2020, 10, 7791. [Google Scholar] [CrossRef]
- Morón-Conejo, B.; Berrendero, S.; Salido, M.P.; Zarauz, C.; Pradíes, G. Accuracy of surgical guides manufactured with four different 3D printers. A comparative in vitro study. J. Dent. 2024, 148, 105226. [Google Scholar] [CrossRef] [PubMed]
- Wegmüller, L.; Halbeisen, F.; Sharma, N.; Kühl, S.; Thieringer, F.M. Consumer vs. High-End 3D Printers for Guided Implant Surgery-An In Vitro Accuracy Assessment Study of Different 3D Printing Technologies. J. Clin. Med. 2021, 10, 4894. [Google Scholar] [CrossRef] [PubMed]
- Lo Russo, L.; Guida, L.; Mariani, P.; Ronsivalle, V.; Gallo, C.; Cicciù, M.; Laino, L. Effect of Fabrication Technology on the Accuracy of Surgical Guides for Dental-Implant Surgery. Bioengineering 2023, 10, 875. [Google Scholar] [CrossRef]
- Marei, H.F.; Alshaia, A.; Alarifi, S.; Almasoud, N.; Abdelhady, A. Effect of Steam Heat Sterilization on the Accuracy of 3D Printed Surgical Guides. Implant. Dent. 2019, 28, 372–377. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, J.; Ma, C.; Shen, J.; Dong, X.; Lin, D. A systematic review of the accuracy of digital surgical guides for dental implantation. Int. J. Implant. Dent. 2023, 9, 38. [Google Scholar] [CrossRef]
- Strunz, A.; Berger, L.; Seidel, A.; Ries, J.; Adler, W.; Wichmann, M.; Matta, R.E. The Impact of Three-Dimensional Printer Technology on the Accuracy of Dental Implant Models. Materials 2025, 18, 1902. [Google Scholar] [CrossRef]
- Rubayo, D.D.; Phasuk, K.; Vickery, J.M.; Morton, D.; Lin, W.S. Influences of build angle on the accuracy, printing time, and material consumption of additively manufactured surgical templates. J. Prosthet. Dent. 2020, 126, 658–663. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Bui, P.H.; Schille, C.; Geis-Gerstorfer, J.; Huettig, F.; Spintzyk, S. Objects build orientation, positioning, and curing influence dimensional accuracy and flexural properties of stereolithographically printed resin. Dent. Mater. 2018, 34, e324–e333. [Google Scholar] [CrossRef]
- Jang, G.; Kim, S.K.; Heo, S.J.; Koak, J.Y. Fit analysis of stereolithography-manufactured three-unit resin prosthesis with different 3D-printing build orientations and layer thicknesses. J. Prosthet. Dent. 2024, 131, 301–312. [Google Scholar] [CrossRef]
- Liravi, F.; Das, S.; Zhou, C. Separation force analysis and prediction based on cohesive element model for constrained-surface Stereolithography processes. Comput.-Aided Des. 2015, 69, 134–142. [Google Scholar] [CrossRef]
- Alharbi, N.; Osman, R.B.; Wismeijer, D. Factors Influencing the Dimensional Accuracy of 3D-Printed Full-Coverage Dental Restorations Using Stereolithography Technology. Int. J. Prosthodont. 2016, 29, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Cao, S.; Msallem, B.; Kunz, C.; Brantner, P.; Honigmann, P.; Thieringer, F.M. Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides—An Accuracy Assessment Study. J. Clin. Med. 2020, 9, 1506. [Google Scholar] [CrossRef] [PubMed]


| Group | Technology | Machine | Nesting Software | Material | Post-Processing Units |
|---|---|---|---|---|---|
| MCX5 | Milling | inLab MC X5 | inLab CAM SW 2019 (Dentsply Sirona, Bensheim, Germany) | Splint Plus BioStar (ERNST HINRICHS DENTAL) | |
| FORM | SLA | Asiga Max UV | PreForm Version 3.17 (Formlabs Inc., Somerville, MA, USA) | Imprimo LC MJF (SCHEU-DENTAL) | Imprimo Clean and Imprimo Cure (SCHEU-DENTAL); nitrogen atmosphere |
| ASIGA | DLP | Shera D20 | Asiga Composer Version 1.37 (Asiga, Sydney, Australia) | SHERAprint ortho plus UV (Shera Werkstoff-Technologie) | BioSonic UC150 (Coltene), Otoflash G171 (NK-Optik); nitrogen atmosphere |
| SHERA | DLP | Form 3 | Netfabb Premium 2020 (Autodesk, San Francisco, CA, USA) | Surgical Guide Resin V1 (Formlabs) | Form Wash and Form Cure (Formlabs) |
| Deviation [mm] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Sterilization | Post-Sterilization | ||||||||||
| Machine | Evaluation Axis | Layer Thickness | Sleeve Position | Mean | SD | Min | Max | Mean | SD | Min | Max |
| MCX5 | dxyz | - | total | 0.352 | ±0.075 | 0.18 | 0.47 | 0.372 | ±0.055 | 0.27 | 0.49 |
| dxyz | 11 | 0.307 | ±0.069 | 0.18 | 0.38 | 0.352 | ±0.045 | 0.27 | 0.42 | ||
| dxyz | 14 | 0.396 | ±0.057 | 0.32 | 0.47 | 0.392 | ±0.062 | 0.28 | 0.49 | ||
| x | total | −0.149 | ±0.057 | −0.26 | −0.09 | −0.173 | ±0.063 | −0.29 | −0.09 | ||
| y | total | 0.108 | ±0.073 | −0.04 | 0.25 | 0.111 | ±0.060 | −0.02 | 0.24 | ||
| z | total | 0.287 | ±0.067 | 0.13 | 0.40 | 0.297 | ±0.060 | 0.19 | 0.43 | ||
| ASIGA | dxyz | 50 µm | total | 0.195 | ±0.136 | 0.04 | 0.56 | 0.205 | ±0.147 | 0.03 | 0.55 |
| dxyz | 11 | 0.246 | ±0.146 | 0.13 | 0.56 | 0.266 | ±0.137 | 0.14 | 0.55 | ||
| dxyz | 14 | 0.144 | ±0.120 | 0.04 | 0.43 | 0.143 | ±0.146 | 0.03 | 0.49 | ||
| x | total | −0.079 | ±0.085 | −0.21 | 0.06 | −0.080 | ±0.100 | −0.23 | 0.07 | ||
| y | total | 0.057 | ±0.040 | −0.02 | 0.15 | 0.061 | ±0.041 | −0.02 | 0.15 | ||
| z | total | 0.089 | ±0.175 | −0.09 | 0.52 | 0.113 | ±0.169 | −0.06 | 0.49 | ||
| dxyz | 100 µm | total | 0.129 | ±0.067 | 0.03 | 0.22 | 0.151 | ±0.061 | 0.06 | 0.23 | |
| dxyz | 11 | 0.189 | ±0.022 | 0.16 | 0.22 | 0.202 | ±0.025 | 0.17 | 0.23 | ||
| dxyz | 14 | 0.068 | ±0.036 | 0.03 | 0.13 | 0.099 | ±0.043 | 0.06 | 0.18 | ||
| x | total | −0.075 | ±0.093 | −0.21 | 0.04 | −0.057 | ±0.010 | −0.21 | 0.10 | ||
| y | total | 0.067 | ±0.039 | 0.0 | 0.13 | 0.070 | ±0.046 | −0.02 | 0.15 | ||
| z | total | 0.011 | ±0.027 | −0.03 | 0.05 | 0.044 | ±0.041 | −0.03 | 0.13 | ||
| SHERA | dxyz | 50 µm | total | 0.091 | ±0.043 | 0.04 | 0.17 | 0.133 | ±0.052 | 0.04 | 0.24 |
| dxyz | 11 | 0.098 | ±0.052 | 0.04 | 0.17 | 0.128 | ±0.048 | 0.04 | 0.15 | ||
| dxyz | 14 | 0.083 | ±0.034 | 0.05 | 0.19 | 0.138 | ±0.060 | 0.04 | 0.24 | ||
| x | total | −0.048 | ±0.046 | −0.13 | 0.03 | −0.034 | ±0.083 | −0.15 | 0.09 | ||
| y | total | 0.009 | ±0.061 | −0.10 | 0.11 | 0.026 | ±0.036 | −0.02 | 0.11 | ||
| z | total | 0.014 | ±0.039 | −0.04 | 0.11 | 0.079 | ±0.062 | −0.02 | 0.23 | ||
| dxyz | 100 µm | total | 0.183 | ±0.070 | 0.09 | 0.40 | 0.205 | ±0.069 | 0.07 | 0.35 | |
| dxyz | 11 | 0.230 | ±0.070 | 0.17 | 0.40 | 0.261 | ±0.047 | 0.20 | 0.35 | ||
| dxyz | 14 | 0.136 | ±0.034 | 0.09 | 0.19 | 0.149 | ±0.036 | 0.07 | 0.20 | ||
| x | total | −0.097 | ±0.069 | −0.19 | −0.01 | −0.094 | ±0.095 | −0.23 | 0.02 | ||
| y | total | 0.018 | ±0.048 | −0.06 | 0.09 | 0.025 | ±0.034 | −0.06 | 0.09 | ||
| z | total | 0.132 | ±0.064 | 0.05 | 0.35 | 0.156 | ±0.048 | 0.06 | 0.27 | ||
| FORM | dxyz | 50 µm | total | 0.290 | ±0.115 | 0.07 | 0.49 | 0.294 | ±0.114 | 0.12 | 0.48 |
| dxyz | 11 | 0.271 | ±0.121 | 0.07 | 0.46 | 0.234 | ±0.092 | 0.12 | 0.48 | ||
| dxyz | 14 | 0.308 | ±0.118 | 0.11 | 0.49 | 0.353 | ±0.111 | 0.19 | 0.48 | ||
| x | total | −0.126 | ±0.053 | −0.24 | −0.05 | −0.078 | ±0.049 | −0.19 | 0.02 | ||
| y | total | −0.007 | ±0.070 | −0.14 | 0.13 | −0.005 | ±0.071 | −0.15 | 0.11 | ||
| z | total | 0.242 | ±0.122 | 0.01 | 0.44 | 0.261 | ±0.127 | 0.04 | 0.44 | ||
| dxyz | 100 µm | total | 0.233 | ±0.090 | 0.10 | 0.47 | 0.262 | ±0.085 | 0.11 | 0.45 | |
| dxyz | 11 | 0.199 | ±0.086 | 0.10 | 0.41 | 0.222 | ±0.099 | 0.11 | 0.45 | ||
| dxyz | 14 | 0.266 | ±0.090 | 0.14 | 0.47 | 0.302 | ±0.052 | 0.25 | 0.40 | ||
| x | total | −0.065 | ±0.078 | −0.24 | 0.08 | −0.032 | ±0.046 | −0.12 | 0.08 | ||
| y | total | 0.028 | ±0.073 | −0.15 | 0.15 | −0.024 | ±0.100 | −0.20 | 0.13 | ||
| z | total | 0.187 | ±0.100 | 0.04 | 0.42 | 0.225 | ±0.107 | 0.05 | 0.44 | ||
| Pre-Sterilization | Post-Sterilization | |||
|---|---|---|---|---|
| Machine Type | Median [mm] | IQR [mm] | Median [mm] | IQR [mm] |
| MCX5 | 0.365 | 0.075 | 0.375 | 0.072 |
| ASIGA | 0.145 | 0.105 | 0.170 | 0.128 |
| SHERA | 0.135 | 0.093 | 0.155 | 0.065 |
| FORM | 0.240 | 0.140 | 0.260 | 0.163 |
| p-Values | |||
|---|---|---|---|
| Reference | Comparison | Pre-Sterilization | Post-Sterilization |
| MCX5 | FORM | =0.022 | =0.007 |
| ASIGA | <0.001 | <0.001 | |
| SHERA | <0.001 | <0.001 | |
| FORM | ASIGA | <0.001 | <0.001 |
| SHERA | <0.001 | <0.001 | |
| ASIGA | SHERA | =0.404 | =0.774 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidel, A.; Zerrahn, K.; Wichmann, M.; Matta, R.E. Accuracy of Drill Sleeve Housing in 3D-Printed and Milled Implant Surgical Guides: A 3D Analysis Considering Machine Type, Layer Thickness, Sleeve Position, and Steam Sterilization. Bioengineering 2025, 12, 799. https://doi.org/10.3390/bioengineering12080799
Seidel A, Zerrahn K, Wichmann M, Matta RE. Accuracy of Drill Sleeve Housing in 3D-Printed and Milled Implant Surgical Guides: A 3D Analysis Considering Machine Type, Layer Thickness, Sleeve Position, and Steam Sterilization. Bioengineering. 2025; 12(8):799. https://doi.org/10.3390/bioengineering12080799
Chicago/Turabian StyleSeidel, Anna, Kai Zerrahn, Manfred Wichmann, and Ragai Edward Matta. 2025. "Accuracy of Drill Sleeve Housing in 3D-Printed and Milled Implant Surgical Guides: A 3D Analysis Considering Machine Type, Layer Thickness, Sleeve Position, and Steam Sterilization" Bioengineering 12, no. 8: 799. https://doi.org/10.3390/bioengineering12080799
APA StyleSeidel, A., Zerrahn, K., Wichmann, M., & Matta, R. E. (2025). Accuracy of Drill Sleeve Housing in 3D-Printed and Milled Implant Surgical Guides: A 3D Analysis Considering Machine Type, Layer Thickness, Sleeve Position, and Steam Sterilization. Bioengineering, 12(8), 799. https://doi.org/10.3390/bioengineering12080799







