Change in Mechanical Property of Rat Brain Suffering from Chronic High Intraocular Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Rat Model with Chronic High IOP
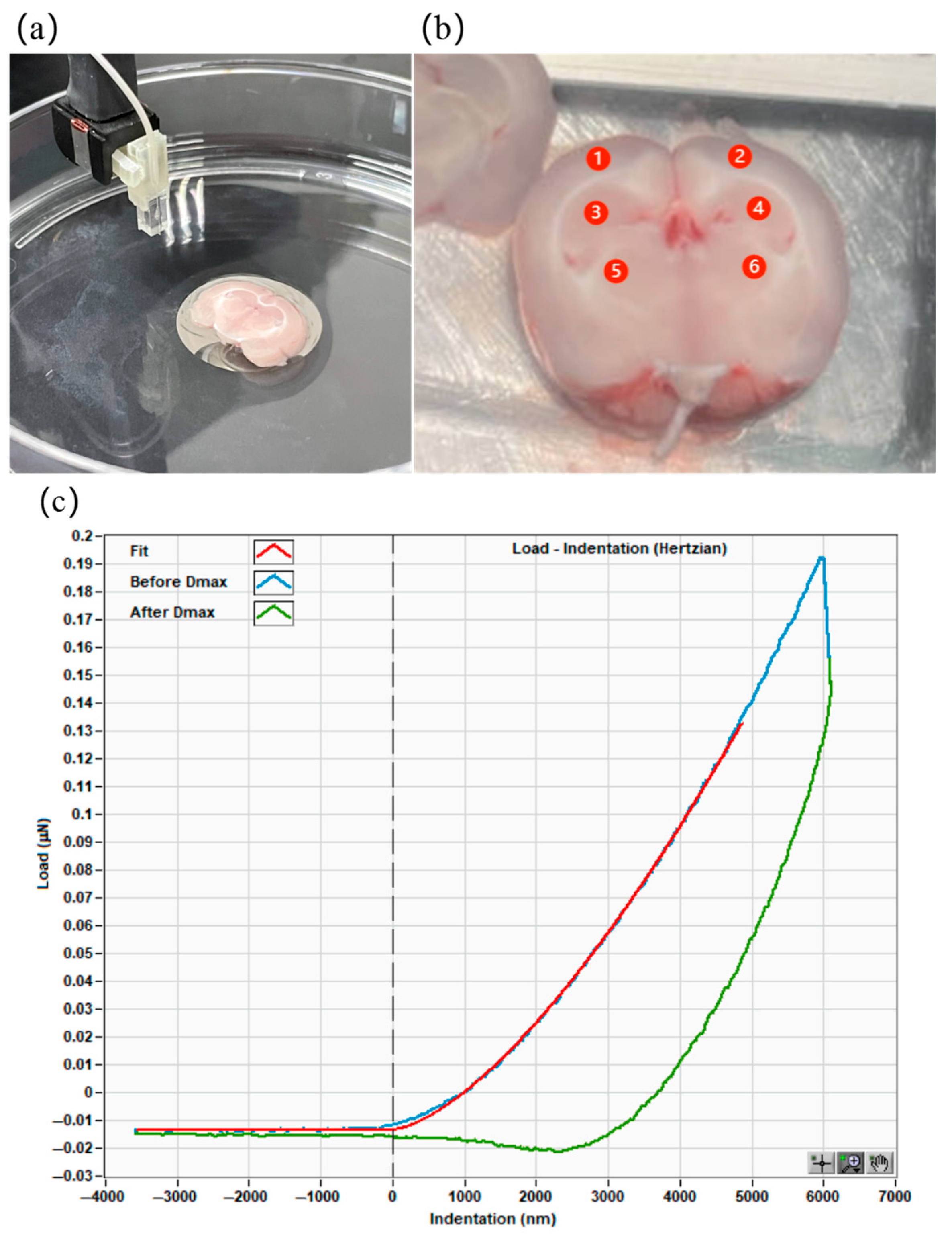
2.3. Mechanical Property Measurement of Rat Brain
2.4. Immunofluorescence Staining
2.5. Luxol Fast Blue (LFB) Staining
2.6. Statistical Analysis
3. Results
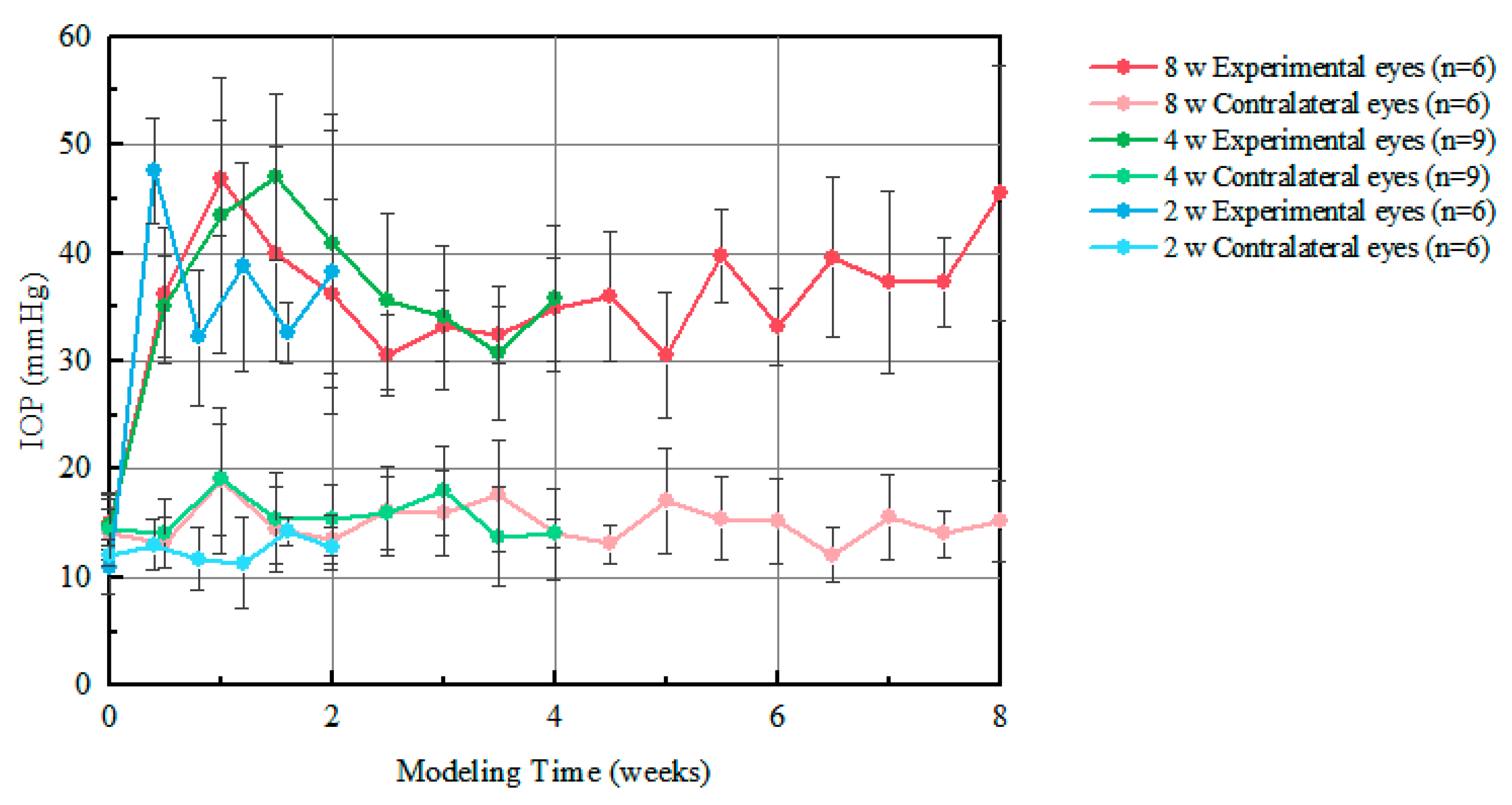
3.1. IOP Measurement Results
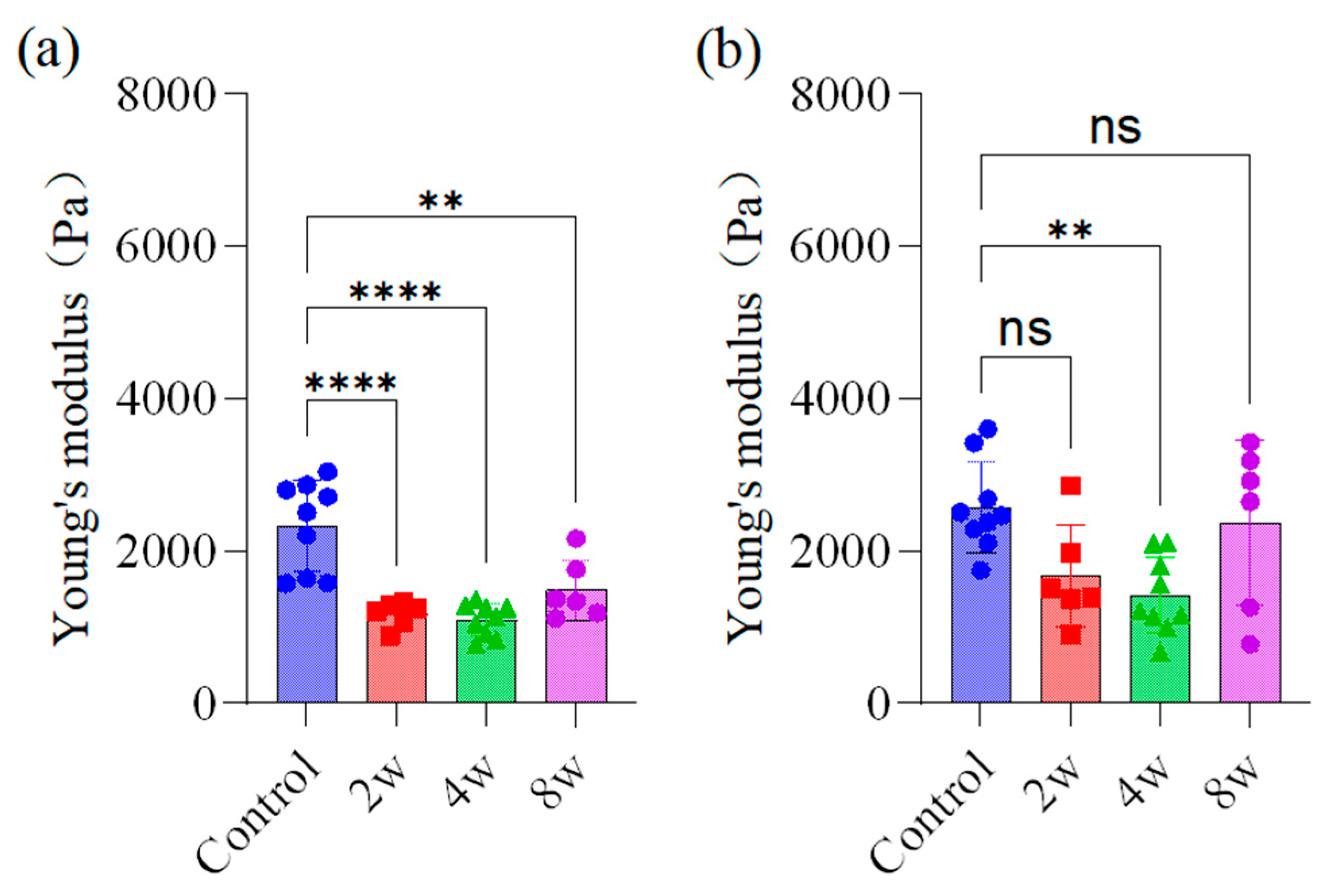
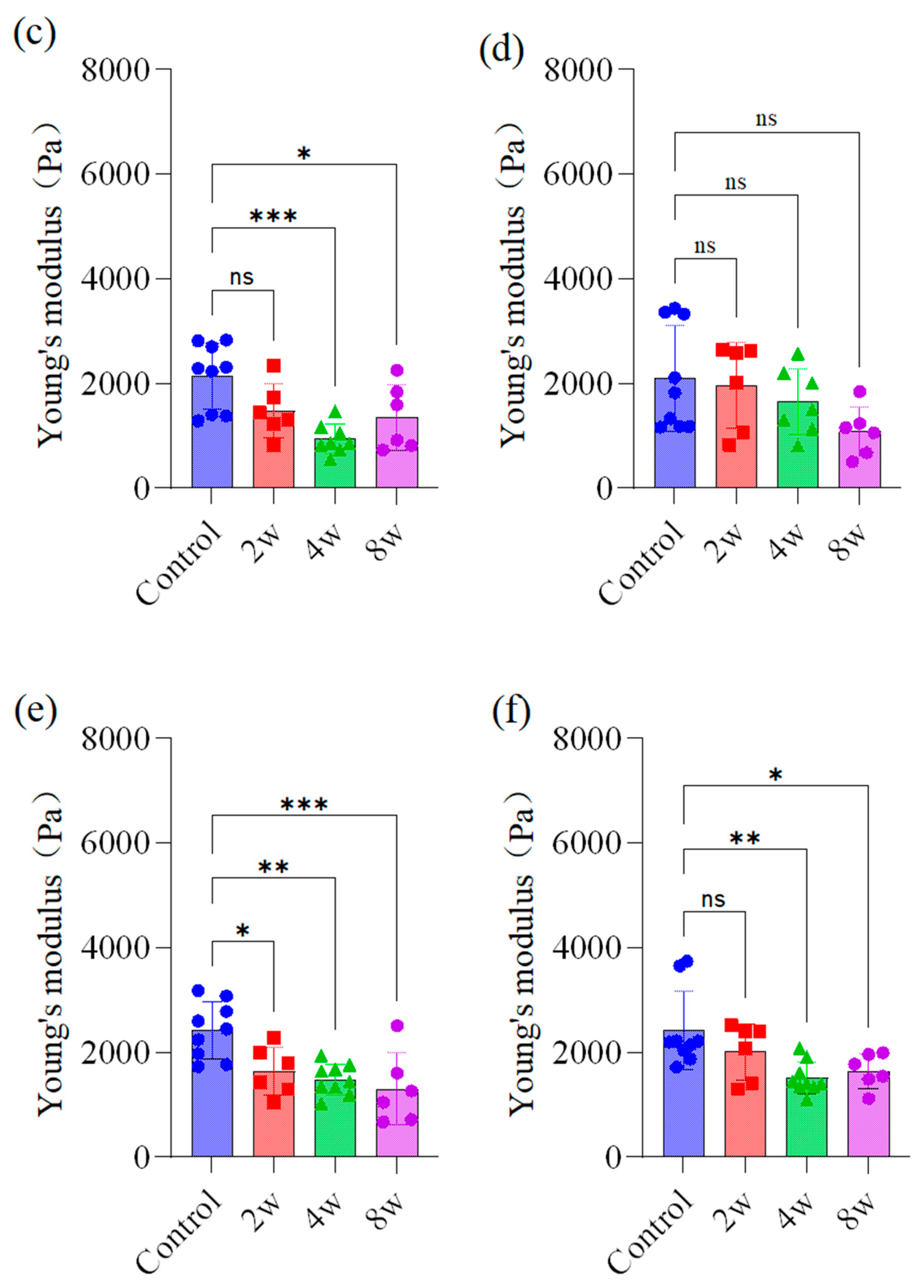
3.2. Mechanical Property Measurement of Different Regions of Rat Brain
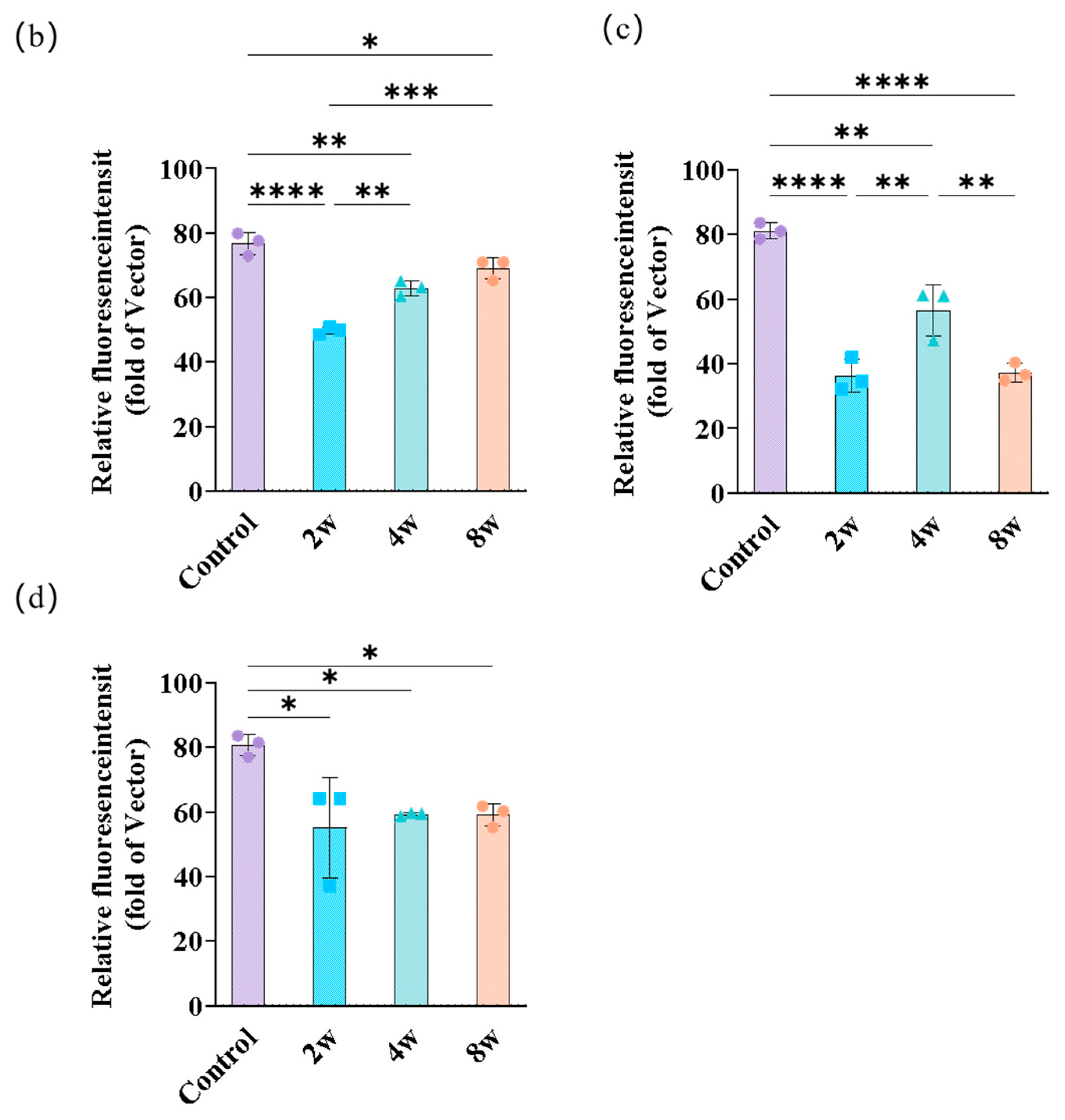
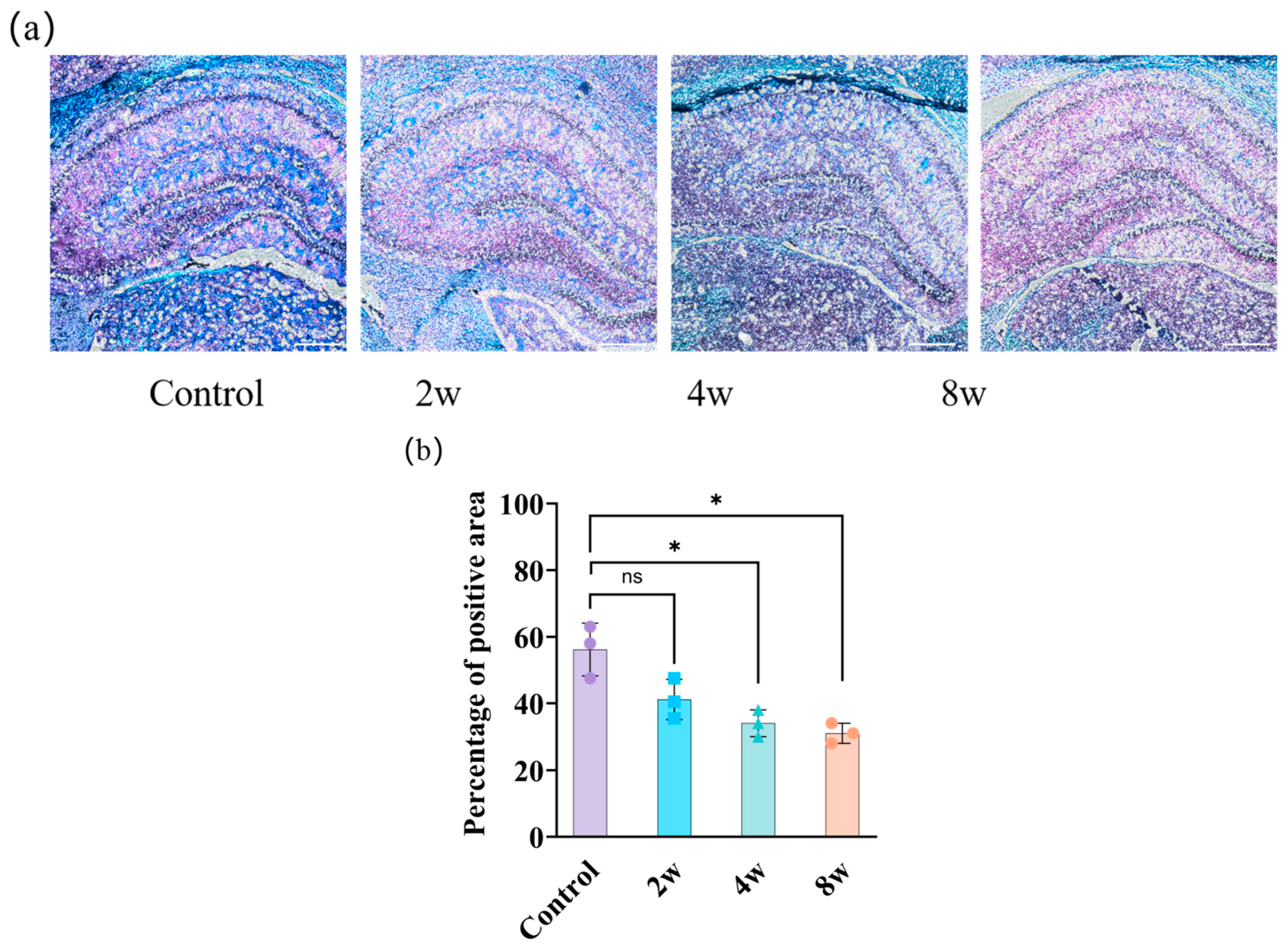
3.3. Morphological Changes of Rat Brain with Different Durations of High IOP
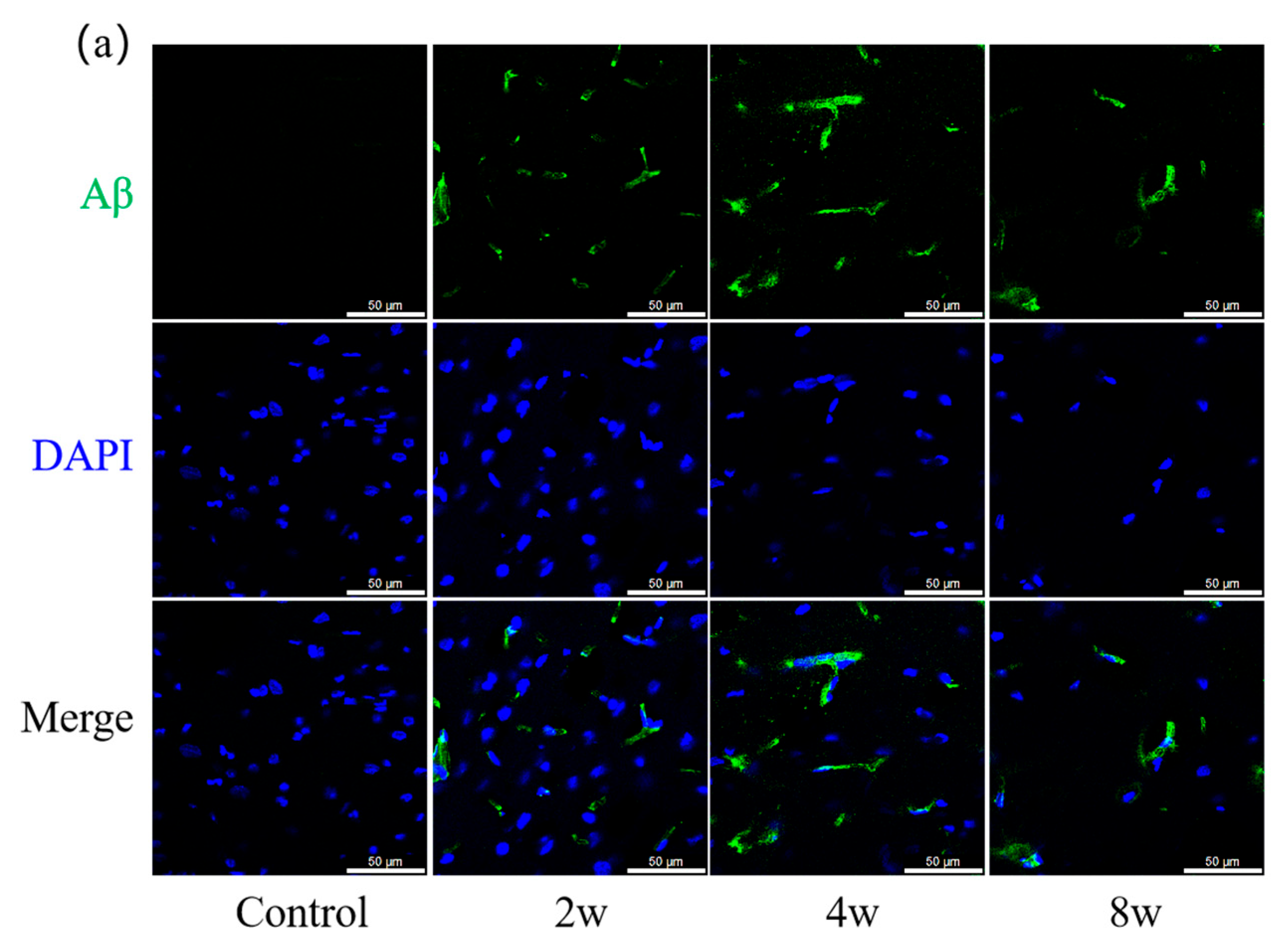
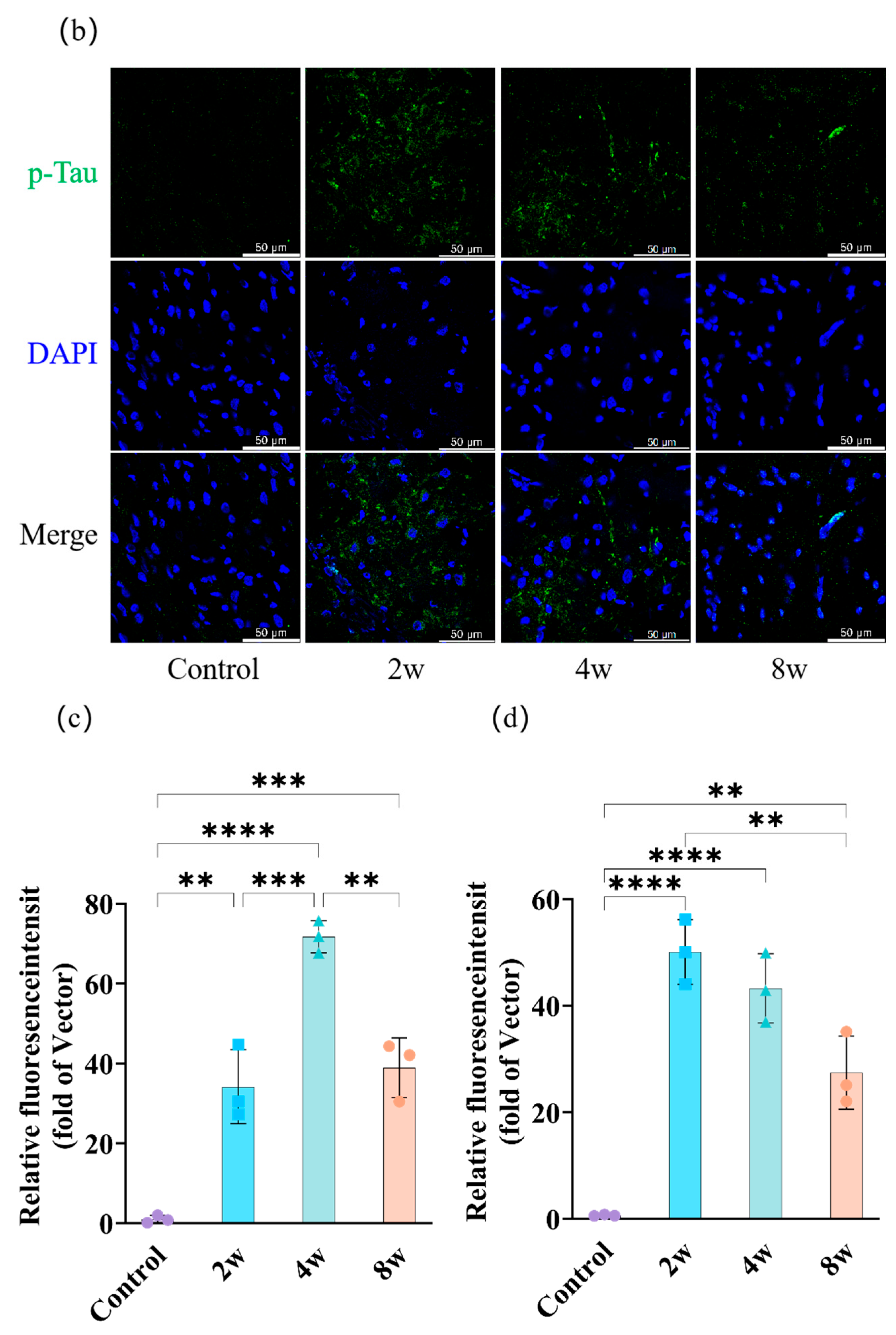
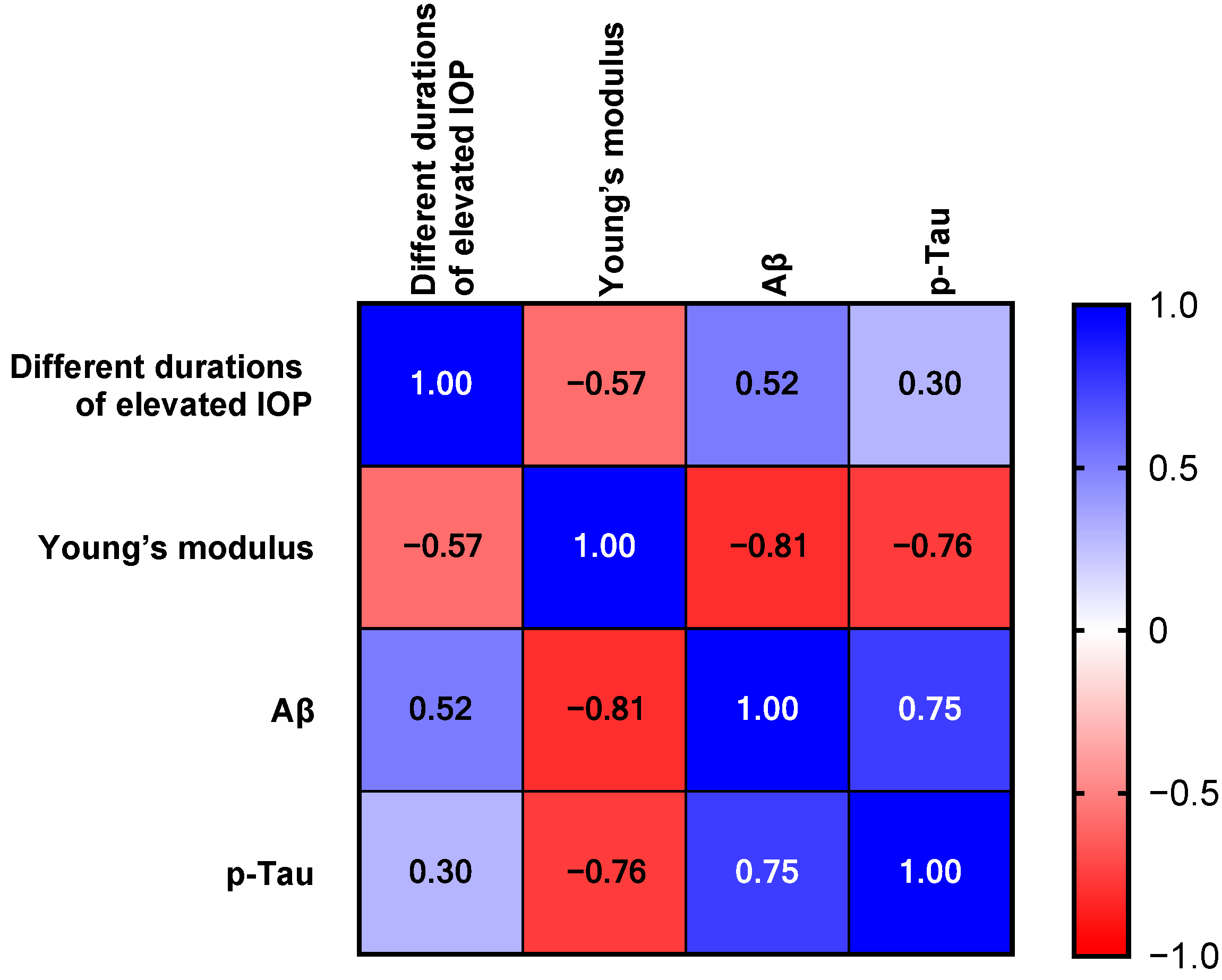
3.4. Aβ and p-Tau of Rat Hippocampus with Different Durations of High IOP
4. Discussion
4.1. IOP in the Rat Model
4.2. Brain Young’s Modulus Variation in Different Regions
4.3. Chronic IOP Changes Brain Young’s Modulus of the Rats
4.4. Young’s Modulus Changes of the Brain with the Changes in F-actin and Myelin
4.5. Chronic High IOP Induces Aβ Accumulation and Tau Phosphorylation in Hippocampus Region
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IOP | intraocular pressure |
| 5-Fu | 5-Fluorouracil |
| LGN | lateral geniculate nucleus |
| OL | occipital lobe |
| Aβ | β-amyloid |
| p-Tau | phosphorylated tau protein |
| AD | Alzheimer’s disease |
| AFM | atomic force microscope |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| LFB | Luxol Fast Blue |
| OCT | Optimal Cutting Temperature |
| PBS | phosphate-buffered saline |
References
- Yuecel, Y.; Gupta, N. Glaucoma of the brain: A disease model for the study of transsynaptic neural degeneration. Prog. Brain Res. 2008, 173, 465–478. [Google Scholar] [CrossRef]
- Gupta, N.; Yücel, Y.H. Glaucoma as a neurodegenerative disease. Curr. Opin. Ophthalmol. 2007, 18, 110–114. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Rong, R.; Zeng, Z.; Xia, X.; Ji, D. Transneuronal degeneration in the brain during glaucoma. Front. Aging Neurosci. 2021, 13, 643685. [Google Scholar] [CrossRef] [PubMed]
- Harwerth, R.S.; Smith, E.L.; DeSantis, L. Experimental glaucoma: Perimetric field defects and intraocular pressure. J. Glaucoma 1997, 6, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Garaci, F.G.; Bolacchi, F.; Cerulli, A.; Melis, M.; Spano, A.; Cedrone, C.; Floris, R.; Simonetti, G.; Nucci, C. Optic nerve and optic radiation neurodegeneration in patients with glaucoma: In vivo analysis with 3-T diffusion-tensor MR imaging. Radiology 2009, 252, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Parra, C.; Bang, J.W.; Fieremans, E.; Wollstein, G.; Schuman, J.S.; Chan, K.C. Diffusion kurtosis imaging reveals optic tract damage that correlates with clinical severity in glaucoma. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2020, 2020, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Kruper, J.; Richie-Halford, A.; Benson, N.C.; Caffarra, S.; Owen, J.; Wu, Y.; Egan, C.; Lee, A.Y.; Lee, C.S.; Yeatman, J.D.; et al. Convolutional neural network-based classification of glaucoma using optic radiation tissue properties. Commun. Med. 2024, 4, 72. [Google Scholar] [CrossRef] [PubMed]
- Frezzotti, P.; Giorgio, A.; Motolese, I.; De Leucio, A.; Iester, M.; Motolese, E.; Federico, A.; De Stefano, N. Structural and functional brain changes beyond visual system in patients with advanced glaucoma. PLoS ONE 2014, 9, e105931. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Zhou, J.; Qiu, J.; Yan, T.; Xie, Y.; Li, L.; Lu, W. Brain morphological alterations of cerebral cortex and subcortical nuclei in high-tension glaucoma brain and its associations with intraocular pressure. Neuroradiology 2020, 62, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Yan, T.; Zhou, J.; Xie, Y.; Qiu, J.; Wang, Y.; Lu, W. Elevated intraocular pressure moderated brain morphometry in high-tension glaucoma: A structural MRI study. Clin. Neuroradiol. 2024, 34, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, C.; Zhao, H. Mechanical properties of brain tissue based on microstructure. J. Mech. Behav. Biomed. Mater. 2022, 126, 104924. [Google Scholar] [CrossRef] [PubMed]
- Delgorio, P.L.; Hiscox, L.; Daugherty, A.M.; Sanjana, F.; McIlvain, G.; Pohlig, R.T.; McGarry, M.D.J.; Martens, C.R.; Schwarb, H.; Johnson, C.L. Structure-function dissociations of human hippocampal subfield stiffness and memory performance. J. Neurosci. 2022, 42, 7957–7968. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Murphy, M.C.; Hojo, E.; Li, F.; Roberts, N. Magnetic resonance elastography in the study of neurodegenerative diseases. J. Magn. Reson. Imaging 2024, 59, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, Z.; Li, L.; Li, X.; Xia, Q.; Zhang, H.; Duan, Q.; Zhao, Y. High intraocular pressure produces learning and memory impairments in rats. Brain Res. 2017, 1675, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Budday, S.; Ovaert, T.C.; Holzapfel, G.A.; Steinmann, P.; Kuhl, E. Fifty shades of brain: A review on the mechanical testing and modeling of brain tissue. Arch. Computat Methods Eng. 2020, 27, 1187–1230. [Google Scholar] [CrossRef]
- Streitberger, K.-J.; Sack, I.; Krefting, D.; Pfueller, C.; Braun, J.; Paul, F.; Wuerfel, J. Brain viscoelasticity alteration in chronic-progressive multiple sclerosis. PLoS ONE 2012, 7, e29888. [Google Scholar] [CrossRef] [PubMed]
- Wuerfel, J.; Paul, F.; Beierbach, B.; Hamhaber, U.; Klatt, D.; Papazoglou, S.; Zipp, F.; Martus, P.; Braun, J.; Sack, I. MR-elastography reveals degradation of tissue integrity in multiple sclerosis. Neuroimage 2010, 49, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.C.; Jones, D.T.; Jack, C.R.; Glaser, K.J.; Senjem, M.L.; Manduca, A.; Felmlee, J.P.; Carter, R.E.; Ehman, R.L.; Huston, J. Regional brain stiffness changes across the Alzheimer’s disease spectrum. Neuroimage Clin. 2016, 10, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Gerischer, L.M.; Fehlner, A.; Köbe, T.; Prehn, K.; Antonenko, D.; Grittner, U.; Braun, J.; Sack, I.; Flöel, A. Combining viscoelasticity, diffusivity and volume of the hippocampus for the diagnosis of Alzheimer’s disease based on magnetic resonance imaging. Neuroimage Clin. 2018, 18, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Schregel, K.; Wuerfel née Tysiak, E.; Garteiser, P.; Gemeinhardt, I.; Prozorovski, T.; Aktas, O.; Merz, H.; Petersen, D.; Wuerfel, J.; Sinkus, R. Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance elastography. Proc. Natl. Acad. Sci. USA 2012, 109, 6650–6655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Weickenmeier, J. Brain stiffness follows cuprizone-induced variations in local myelin content. Acta Biomater. 2023, 170, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Telzer, E.H. Magnetic resonance elastography for examining developmental changes in the mechanical properties of the brain. Dev. Cogn. Neurosci. 2018, 33, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, H.; Wang, G.; Han, X.; Zhang, S. Compressive properties and constitutive modeling of different regions of 8-week-old pediatric porcine brain under large strain and wide strain rates. J. Mech. Behav. Biomed. Mater. 2019, 89, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Rashid, B.; Destrade, M.; Gilchrist, M.D. Mechanical characterization of brain tissue in compression at dynamic strain rates. J. Mech. Behav. Biomed. Mater. 2012, 10, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Chinzei, K. Mechanical properties of brain tissue in tension. J. Biomech. 2002, 35, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Rashid, B.; Destrade, M.; Gilchrist, M.D. Mechanical characterization of brain tissue in simple shear at dynamic strain rates. J. Mech. Behav. Biomed. Mater. 2013, 28, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Hrapko, M.; Van Dommelen, J.A.W.; Peters, G.W.M.; Wismans, J.S.H.M. The mechanical behaviour of brain tissue: Large strain response and constitutive modelling. Biorheology 2006, 43, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Budday, S.; Sommer, G.; Birkl, C.; Langkammer, C.; Haybaeck, J.; Kohnert, J.; Bauer, M.; Paulsen, F.; Steinmann, P.; Kuhl, E.; et al. Mechanical characterization of human brain tissue. Acta Biomater. 2017, 48, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.L.; Tweten, D.J.; Badachhape, A.A.; Reiter, A.J.; Okamoto, R.J.; Garbow, J.R.; Bayly, P.V. Measurement of anisotropic mechanical properties in porcine brain white matter ex vivo using magnetic resonance elastography. J. Mech. Behav. Biomed. Mater. 2018, 79, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Weickenmeier, J.; Kurt, M.; Ozkaya, E.; Wintermark, M.; Pauly, K.B.; Kuhl, E. Magnetic resonance elastography of the brain: A comparison between pigs and humans. J. Mech. Behav. Biomed. Mater. 2018, 77, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Liu, D.; Xu, L.; Su, C.; Li, G.Y.; Qian, L.X.; Cao, Y. In vivo and ex vivo elastic properties of brain tissues measured with ultrasound elastography. J. Mech. Behav. Biomed. Mater. 2018, 83, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Herthum, H.; Dempsey, S.C.H.; Samani, A.; Schrank, F.; Shahryari, M.; Warmuth, C.; Tzschatzsch, H.; Braun, J.; Sack, I. Superviscous properties of the in vivo brain at large scales. Acta Biomater. 2021, 121, 393–404. [Google Scholar] [CrossRef] [PubMed]
- van Dommelen, J.A.W.; van der Sande, T.P.J.; Hrapko, M.; Peters, G.W.M. Mechanical properties of brain tissue by indentation: Interregional variation. J. Mech. Behav. Biomed. Mater. 2010, 3, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, M.M.; Brendel, M.B.; Melendez-Vasquez, C.V. Acute and chronic demyelinated CNS lesions exhibit opposite elastic properties. Sci. Rep. 2019, 9, 999. [Google Scholar] [CrossRef] [PubMed]
- Budday, S.; Nay, R.; de Rooij, R.; Steinmann, P.; Wyrobek, T.; Ovaert, T.C.; Kuhl, E. Mechanical properties of gray and white matter brain tissue by indentation. J. Mech. Behav. Biomed. Mater. 2015, 46, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Monavarfeshani, A.; Sabbagh, U.; Fox, M.A. Not a one-trick pony: Diverse connectivity and functions of the rodent lateral geniculate complex. Vis. Neurosci. 2017, 34, E012. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.L.; Ganaraja, B.; Murlimanju, B.V.; Joy, T.; Krishnamurthy, A.; Agrawal, A. Hippocampus and its involvement in Alzheimer’s disease: A review. 3 Biotech 2022, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, Z.; Chen, H.; Sheng, X.; Qin, R.; Shao, P.; Yang, Z.; Yao, W.; Zhao, H.; Xu, Y.; et al. Applying retinal vascular structures characteristics coupling with cortical visual system in Alzheimer’s disease spectrum patients. Brain Sci. 2023, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Li, T.; Li, L.; Qian, X.; Liu, Z. A feasible method for independently evaluating the mechanical properties of glial LC and RGC axons by combining atomic force microscopy measurement with image segmentation. J. Mech. Behav. Biomed. Mater. 2022, 126, 105041. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Ren, J.; Qian, X. Study on the polarization of astrocytes in the optic nerve head of rats under high intraocular pressure: In vitro. Bioengineering 2025, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Ma, L.; Liu, L.; Li, L.; Qian, X. Preliminary study on the blockade of axonal transport by activated astrocytes in optic nerve head under chronic ocular hypertension. J. Mech. Med. Biol. 2019, 19, 1940040. [Google Scholar] [CrossRef]
- Baro, V.J.; Bonnevie, E.D.; Lai, X.; Price, C.; Burris, D.L.; Wang, L. Functional characterization of normal and degraded bovine meniscus: Rate-dependent indentation and friction studies. Bone 2012, 51, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Mittag, T.W.; Danias, J.; Pohorenec, G.; Yuan, H.M.; Burakgazi, E.; Chalmers-Redman, R.; Podos, S.M.; Tatton, W.G. Retinal damage after 3 to 4 months of elevated intraocular pressure in a rat glaucoma model. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3451–3459. [Google Scholar]
- Zhong, L. A modified chronic ocular hypertension rat model for retinal ganglion cell neuroprotection. Front. Med. 2013, 7, 367. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hu, F.; Wang, M.; Gao, F.; Xu, P.; Xing, C.; Sun, X.; Zhang, S.; Wu, J. Comparative analysis of retinal ganglion cell damage in three glaucomatous rat models. Exp. Eye Res. 2018, 172, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, A.L.; Guo, L.; Cordeiro, M.F.; Salt, T.E. Electroretinogram and visual-evoked potential assessment of retinal and central visual function in a rat ocular hypertension model of glaucoma. Curr. Eye Res. 2014, 39, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Sappington, R.M.; Carlson, B.J.; Crish, S.D.; Calkins, D.J. The microbead occlusion model: A paradigm for induced ocular hypertension in rats and mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, L.; Liu, Z. Time course changes of the mechanical properties of the iris pigment epithelium in a rat chronic ocular hypertension model. BioMed Res. Int. 2018, 2018, 4862309. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.J.; Kabir, I.E.; Doyley, M.M.; Faiyaz, A.; Uddin, M.N.; Flores, G.; Schifitto, G. Brain elastography in aging relates to fluid/solid trendlines. Phys. Med. Biol. 2024, 69, 115037. [Google Scholar] [CrossRef] [PubMed]
- MacManus, D.B.; Pierrat, B.; Murphy, J.G.; Gilchrist, M.D. Region and species dependent mechanical properties of adolescent and young adult brain tissue. Sci. Rep. 2017, 7, 13729. [Google Scholar] [CrossRef] [PubMed]
- Elkin, B.S.; Azeloglu, E.U.; Costa, K.D.; Morrison, B. Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J. Neurotrauma 2007, 24, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.J.; Chen, H.; Hubbard, W.C.; Kaufman, P.L. Experimental glaucoma and cell size, density, and number in the primate lateral geniculate nucleus. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1370–1379. [Google Scholar]
- Chaturvedi, N.; Hedley-Whyte, E.T.; Dreyer, E.B. Lateral geniculate nucleus in glaucoma. Am. J. Ophthalmol. 1993, 116, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Greenberg, G.; de Tilly, L.N.; Gray, B.; Polemidiotis, M.; Yucel, Y.H. Atrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imaging. Br. J. Ophthalmol. 2009, 93, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xie, B.; Yin, X.; Liang, M.; Evans, A.C.; Wang, J.; Dai, C. Reduced cortical thickness in primary open-angle glaucoma and its relationship to the retinal nerve fiber layer thickness. PLoS ONE 2013, 8, e73208. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, M.; Dyda, W.; Rejdak, R.; Grieb, P. Magnetic resonance in studies of glaucoma. Med. Sci. Monitor. 2011, 17, RA227–RA232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, J.; Liu, X.; Shang, F.; Deng, Y.; Ye, J.; Wang, Y.; Yan, J.; Chen, H.; Yu, M.; et al. Multi-domain interaction mediated strength-building in human α-actinin dimers unveiled by direct single-molecule quantification. Nat. Commun. 2024, 15, 6151. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.M.; Johnson, K.M.; Krueger, E.W.; Razidlo, G.L.; McNiven, M.A. Distinct forms of the actin cross-linking protein α-actinin support macropinosome internalization and trafficking. Mol. Biol. Cell 2021, 32, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Le, S.; Hu, X.; Yao, M.; Chen, H.; Yu, M.; Xu, X.; Nakazawa, N.; Margadant, F.M.; Sheetz, M.P.; Yan, J. Mechanotransmission and mechanosensing of human alpha-Actinin 1. Cell Rep. 2017, 21, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.V.F.; Loures, C.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.D.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liao, H.; Chen, H.; Deng, S.; Jia, Y.; Deng, C.; Lin, J.; Ge, J.; Zhuo, Y. Elevated intraocular pressure induces amyloid-β deposition and tauopathy in the lateral geniculate nucleus in a monkey model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5434. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Jay, T.R.; Miller, C.M.; Cheng, P.J.; Graham, L.C.; Bemiller, S.; Broihier, M.L.; Xu, G.; Margevicius, D.; Karlo, J.C.; Sousa, G.L.; et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 2015, 212, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Hsu, S.M.; Shie, F.S.; Shiao, Y.J.; Chao, L.J.; Chen, H.W.; Yao, H.H.; Chien, M.A.; Lin, C.C.; Tsay, H.J. Reduced mitochondria membrane potential and lysosomal acidification are associated with decreased oligomeric Aβ degradation induced by hyperglycemia: A study of mixed glia cultures. PLoS ONE 2022, 17, e0260966. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.; Lucas, J.J.; Avila, J. GSK3 and tau: Two convergence points in Alzheimer’s disease. J. Alzheimers Dis. 2013, 33 (Suppl. S1), S141–S144. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Martín, M.; Jurado-Arjona, J.; Fuster-Matanzo, A.; Hernández, F.; Rábano, A.; Ávila, J. Peripherally triggered and GSK-3β-driven brain inflammation differentially skew adult hippocampal neurogenesis, behavioral pattern separation and microglial activation in response to ibuprofen. Transl. Psychiatry 2014, 4, e463. [Google Scholar] [CrossRef] [PubMed]
- Nunbhakdi-Craig, V.; Schuechner, S.; Sontag, J.M.; Montgomery, L.; Pallas, D.C.; Juno, C.; Mudrak, I.; Ogris, E.; Sontag, E. Expression of protein phosphatase 2A mutants and silencing of the regulatory B alpha subunit induce a selective loss of acetylated and detyrosinated microtubules. J. Neurochem. 2007, 101, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Tavner, A.C.R.; Roy, T.D.; Hor, K.W.W.; Majimbi, M.; Joldes, G.R.; Wittek, A.; Bunt, S.; Miller, K. On the appropriateness of modelling brain parenchyma as a biphasic continuum. J. Mech. Behav. Biomed. Mater. 2016, 61, 511–518. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Zhang, K.; Ma, Z.; Qian, X. Change in Mechanical Property of Rat Brain Suffering from Chronic High Intraocular Pressure. Bioengineering 2025, 12, 787. https://doi.org/10.3390/bioengineering12080787
Zeng Y, Zhang K, Ma Z, Qian X. Change in Mechanical Property of Rat Brain Suffering from Chronic High Intraocular Pressure. Bioengineering. 2025; 12(8):787. https://doi.org/10.3390/bioengineering12080787
Chicago/Turabian StyleZeng, Yukai, Kunya Zhang, Zhengyuan Ma, and Xiuqing Qian. 2025. "Change in Mechanical Property of Rat Brain Suffering from Chronic High Intraocular Pressure" Bioengineering 12, no. 8: 787. https://doi.org/10.3390/bioengineering12080787
APA StyleZeng, Y., Zhang, K., Ma, Z., & Qian, X. (2025). Change in Mechanical Property of Rat Brain Suffering from Chronic High Intraocular Pressure. Bioengineering, 12(8), 787. https://doi.org/10.3390/bioengineering12080787





