Evaluation of Periodontal Infrabony Defect Topography via CBCT and Comparisons with Direct Intrasurgical Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subject Recruitment
2.2. Collection of Presurgical Data
2.3. Radiographic Examination
- -
- Parallel Periapical Radiographs (PAs)
- -
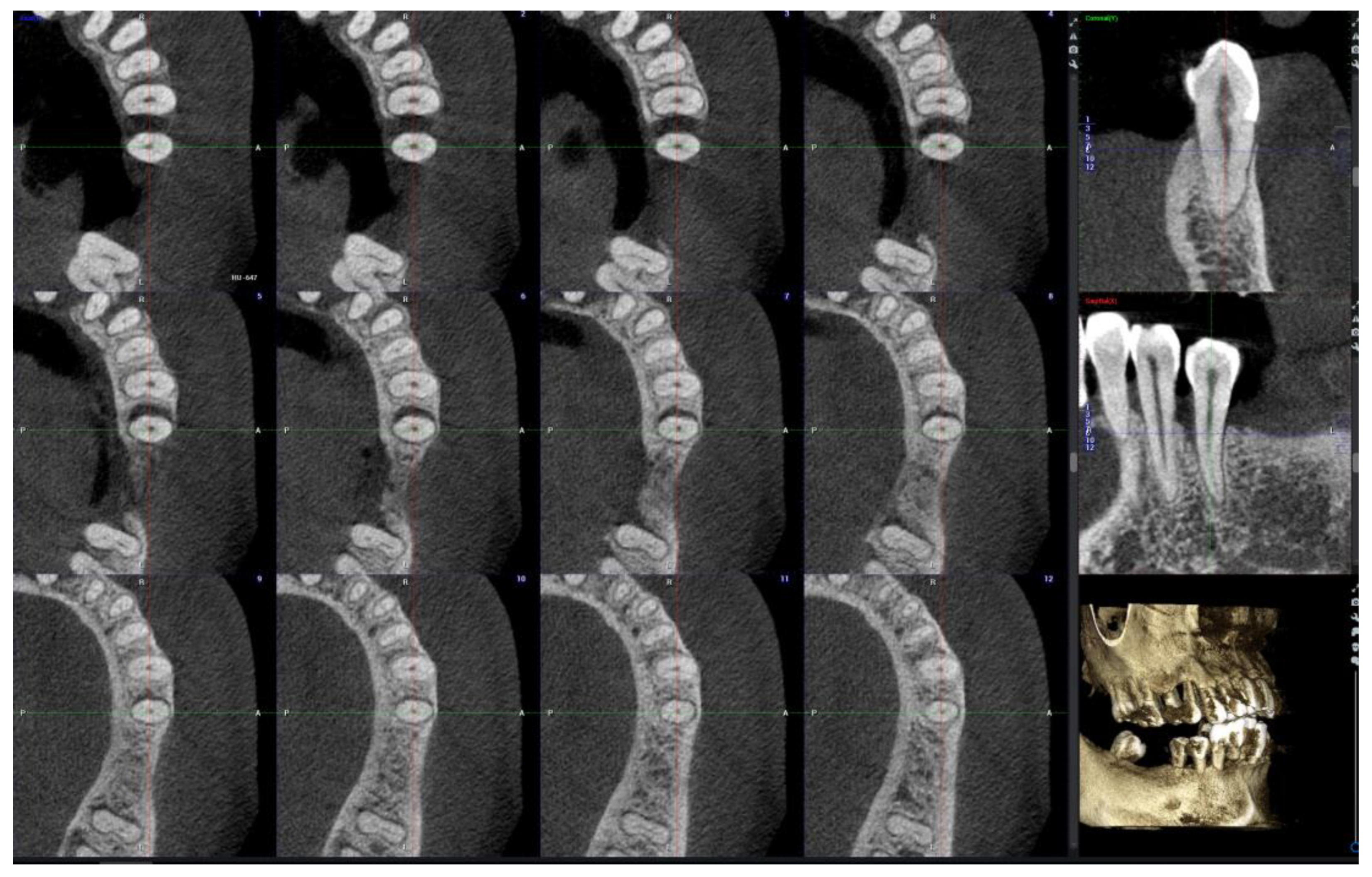
- CBCT
2.4. Measurements
- -
- Intrasurgical Measurements
- -
- Parallel Periapical Radiographs (PAs)
- -
- CBCT
2.5. Sample Size and Statistical Analysis
3. Results
3.1. Demographics
3.2. Intra-Examiner and Inter-Examiner Agreement
3.3. Agreement Between ISs and CBCT or PAs for Linear Measurements
3.4. Agreement Between CBCT and PAs for Angle
3.5. Agreement Between IS and PA or CBCT Observations of Defect Type and Extension
4. Discussion
4.1. Linear Measurements
4.2. Agreement in Defect Type and Extension
4.3. Angle Measurements
4.4. Factors Affecting the Accuracy of PAs and CBCT
4.5. The Use of CBCT in Periodontal Assessment and Treatment Planning
4.6. Limitations of the Current Study
4.6.1. Accuracy of Intrasurgical Measurements
4.6.2. Standardization of Radiographic Examination Protocols
4.6.3. Sample Size
4.6.4. Applicability of the Results
5. Conclusions
- (1)
- No significant differences were found between CBCT and intrasurgical measurements of infrabony defect depth and width, based on an assessment of CBCT axial slices.
- (2)
- CBCT demonstrated high agreement with intrasurgical observations in all defect characteristics, including depth, width, defect type (categorized as predominantly one-wall, two-wall, or three-wall), and defect extension (specified according to the involved root surfaces).
- (3)
- CBCT showed higher accuracy than PAs in measuring defect width when compared to intrasurgical measurement as the gold standard, as well as higher agreement in the determination of defect type and extension than PAs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| n | Percentage | ||
|---|---|---|---|
| Gender | |||
| Male | 9 | 35% | |
| Female | 17 | 65% | |
| CBCT | |||
| Planmeca | 18 | 69% | |
| Morita | 8 | 31% | |
| Voxel Size | |||
| 125 µm | 14 | 54% | |
| 200 µm | 12 | 46% | |
| Tooth Type | |||
| Anterior | 6 | 23% | |
| Premolar | 18 | 69% | |
| Molar | 2 | 8% | |
| Surgery Performed | |||
| Access Flap | 1 | 4% | |
| OFD with osseous | 9 | 35% | |
| Regeneration | 16 | 61% | |
| Flap Design | |||
| M-MIST | 3 | 11% | |
| MIST | 8 | 31% | |
| Extended | 6 | 23% | |
| Resective | 9 | 35% | |
| Defect Type (Predominance) | |||
| One-wall | 6 | 23% | |
| Two-wall | 9 | 35% | |
| Three-wall | 11 | 42% | |
| Defect Extension | |||
| Single surface | 14 | 54% | |
| Multiple surfaces | 12 | 46% |
| n | Minimum | Maximum | Mean | S.D. | |
|---|---|---|---|---|---|
| IS | 26 | ||||
| Depth | 1.0 | 8.00 | 4.11 | 1.54 | |
| Width | 1.5 | 6.0 | 2.96 | 1.14 | |
| CBCT | 26 | ||||
| Depth | 1.2 | 7.2 | 4.01 | 1.26 | |
| Width | 1.4 | 5.4 | 2.90 | 0.91 | |
| Angle | 18.1 | 48.0 | 32.16 | 8.03 | |
| PA | 26 | ||||
| Depth | 1.9 | 7.1 | 4.27 | 1.37 | |
| Width | 1.1 | 4.7 | 2.60 | 0.86 | |
| Angle | 18.2 | 50.4 | 31.06 | 9.30 |
| Examiner 1 | Examiner 2 | Inter-Examiner | ||||
|---|---|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | ICC | 95% CI | |
| CBCT | ||||||
| Depth | 1.000 | --- | 1.000 | --- | 0.922 | 0.876–0.964 |
| Width | 0.972 | 0.938–0.987 | 0.960 | 0.914–0.982 | 0.905 | 0.802–0.956 |
| Angle | 0.926 | 0.843–0.966 | 0.918 | 0.826–0.963 | 0.915 | 0.821–0.961 |
| PA | ||||||
| Depth | 0.990 | 0.977–0.995 | 0.987 | 0.972–0.994 | 0.925 | 0.841–0.966 |
| Width | 0.980 | 0.951–0.991 | 0.965 | 0.924–0.984 | 0.917 | 0.904–0.981 |
| Angle | 0.940 | 0.865–0.973 | 0.959 | 0.911–0.981 | 0.889 | 0.770–0.949 |
| Cohen’s Kappa | % of Total Agreement | |||
|---|---|---|---|---|
| CBCT | PA | CBCT | PA | |
| Defect Type | 0.876 | 0.631 | 92.3 | 76.9 |
| Defect Extension | 0.951 | 0.745 | 96.2 | 84.6 |
References
- Goldman, H.M.; Cohen, D.W. The infrabony pocket: Classification and treatment. J. Periodontol. 1958, 29, 272–291. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Tonetti, M.S. Diagnosis and epidemiology of periodontal osseous lesions. Periodontol. 2000 2000, 22, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Wennstrom, J.L. The angular bony defect as indicator of further alveolar bone loss. J. Clin. Periodontol. 1991, 18, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Tonetti, M.S. Clinical concepts for regenerative therapy in intrabony defects. Periodontol. 2000 2015, 68, 282–307. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Gennai, S.; Cei, S.; Cairo, F.; Baggiani, A.; Miccoli, M.; Gabriele, M.; Tonetti, M. Clinical performance of access flap surgery in the treatment of the intrabony defect. A systematic review and meta-analysis of randomized clinical trials. J. Clin. Periodontol. 2012, 39, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Zybutz, M.; Rapoport, D.; Laurell, L.; Persson, G.R. Comparisons of clinical and radiographic measurements of inter-proximal vertical defects before and 1 year after surgical treatments. J. Clin. Periodontol. 2000, 27, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Fontenele, R.C.; Lahoud, P.; Shujaat, S.; Bornstein, M.M. Radiographic diagnosis of periodontal diseases—Current evidence versus innovations. Periodontol. 2000 2024, 95, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Misch, K.A.; Yi, E.S.; Sarment, D.P. Accuracy of cone beam computed tomography for periodontal defect measurements. J. Periodontol. 2006, 77, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, R.A.; Bucker, A.; Diedrich, P.R. Assessment of alveolar bone loss with high resolution computed tomography. J. Periodontal Res. 1995, 30, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Schmidt, J.C.; Rinne, C.A.; Mendes, S.; Dula, K.; Sculean, A. Cone beam computed tomography (CBCT) for diagnosis and treatment planning in periodontology: Systematic review update. Clin. Oral Investig. 2020, 24, 2943–2958. [Google Scholar] [CrossRef] [PubMed]
- Haas, L.F.; Zimmermann, G.S.; De Luca Canto, G.; Flores-Mir, C.; Correa, M. Precision of cone beam CT to assess periodontal bone defects: A systematic review and meta-analysis. Dentomaxillofac. Radiol. 2018, 47, 20170084. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.G.G.; Cortes, A.R.G.; Arita, E.S.; Georgetti, M.A.P. Comparison of conventional imaging techniques and CBCT for periodontal evaluation: A systematic review. Imaging Sci. Dent. 2018, 48, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nikolic-Jakoba, N.; Spin-Neto, R.; Wenzel, A. Cone-Beam Computed Tomography for Detection of Intrabony and Furcation Defects: A Systematic Review Based on a Hierarchical Model for Diagnostic Efficacy. J. Periodontol. 2016, 87, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.A.M.; Noor, E.; Yusof, M.Y.P.M. The accuracy of linear measurements in cone beam computed tomography for assessing intrabony and furcation defects: A systematic review and meta-analysis. J. Oral Res. 2019, 8, 527–539. [Google Scholar] [CrossRef][Green Version]
- Grimard, B.A.; Hoidal, M.J.; Mills, M.P.; Mellonig, J.T.; Nummikoski, P.V.; Mealey, B.L. Comparison of clinical, periapical radiograph, and cone-beam volume tomography measurement techniques for assessing bone level changes following regenerative periodontal therapy. J. Periodontol. 2009, 80, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, Y.; Kubota, T.; Nohno, K.; Nezu, A.; Morozumi, T.; Yoshie, H. Clinical and CBCT evaluation of combined periodontal regenerative therapies using enamel matrix derivative and deproteinized bovine bone mineral with or without collagen membrane. Int. J. Periodontics Restor. Dent. 2018, 38, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Palkovics, D.; Molnar, B.; Pinter, C.; Gera, I.; Windisch, P. Utilizing a novel radiographic image segmentation method for the assessment of periodontal healing following regenerative surgical treatment. Quintessence Int. 2022, 53, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Mengel, R.; Candir, M.; Shiratori, K.; Flores-de-Jacoby, L. Digital volume tomography in the diagnosis of periodontal defects: An in vitro study on native pig and human mandibles. J. Periodontol. 2005, 76, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Braun, X.; Ritter, L.; Jervoe-Storm, P.M.; Frentzen, M. Diagnostic accuracy of CBCT for periodontal lesions. Clin. Oral Investig. 2014, 18, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Bayat, S.; Talaeipour, A.R.; Sarlati, F. Detection of simulated periodontal defects using cone-beam CT and digital intraoral radiography. Dentomaxillofac. Radiol. 2016, 45, 20160030. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, B.; Jacobs, R.; Yang, J. Diagnostic validity (or acuity) of 2D CCD versus 3D CBCT-images for assessing periodontal breakdown. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, B.; Jacobs, R.; Yang, J. Topographic assessment of periodontal craters and furcation involvements by using 2D digital images versus 3D cone beam CT: An in-vitro Study. Chin. J. Dent. Res. 2007, 10, 21. [Google Scholar]
- Vandenberghe, B.; Jacobs, R.; Yang, J. Detection of periodontal bone loss using digital intraoral and cone beam computed tomography images: An in vitro assessment of bony and/or infrabony defects. Dentomaxillofac. Radiol. 2008, 37, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Mol, A.; Balasundaram, A. In vitro cone beam computed tomography imaging of periodontal bone. Dentomaxillofac. Radiol. 2008, 37, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Noujeim, M.; Prihoda, T.; Langlais, R.; Nummikoski, P. Evaluation of high-resolution cone beam computed tomography in the detection of simulated interradicular bone lesions. Dentomaxillofac. Radiol. 2009, 38, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Fleiner, J.; Hannig, C.; Schulze, D.; Stricker, A.; Jacobs, R. Digital method for quantification of circumferential periodontal bone level using cone beam CT. Clin. Oral Investig. 2013, 17, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Ruetters, M.; Hagenfeld, D.; ElSayed, N.; Zimmermann, N.; Gehrig, H.; Kim, T.-S. Ex vivo comparison of CBCT and digital periapical radiographs for the quantitative assessment of periodontal defects. Clin. Oral Investig. 2020, 24, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.C.; Palomo, L.; Griffith, R.; Hans, M.G. Accuracy and reliability of cone-beam computed tomography for measuring alveolar bone height and detecting bony dehiscences and fenestrations. Am. J. Orthod Dentofac. Orthop. 2010, 137, S109–S119. [Google Scholar] [CrossRef] [PubMed]
- Abdinian, M.; Yaghini, J.; Jazi, L. Comparison of intraoral digital radiography and cone-beam computed tomography in the measurement of periodontal bone defects. Dent. Med. Probl. 2020, 57, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Banodkar, A.B.; Gaikwad, R.P.; Gunjikar, T.U.; Lobo, T.A. Evaluation of accuracy of cone beam computed tomography for measurement of periodontal defects: A clinical study. J. Indian Soc. Periodontol. 2015, 19, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Nikolic-Jakoba, N.; Barac, M.; Jankovic, S.; Aleksic, Z.; Spin-Neto, R.; Wenzel, A. Effect of section thickness on cone beam computed tomography-based measurements of intrabony defects compared with clinical measurements. J. Periodontol. 2021, 92, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Raichur, P.S.; Setty, S.B.; Thakur, S.L.; Naikmasur, V.G. Comparison of radiovisiography and digital volume tomography to direct surgical measurements in the detection of infrabony defects. J. Clin. Exp. Dent. 2012, 4, e43–e47. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, P.; Lamba, A.K.; Grewal, H.; Faraz, F.; Tandon, S.; Yadav, N. Evaluation of two-dimensional and three-dimensional radiography with direct surgical assessment of periodontal osseous defects: A clinical study. Indian J. Dent. Res. 2014, 25, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Haghgoo, J.M.; Shokri, A.; Khodadoustan, A.; Khoshhal, M.; Rabienejad, N.; Farhadian, M. Comparison the accuracy of the cone-beam computed tomography with digital direct intraoral radiography, in assessment of periodontal osseous lesions. Avicenna J. Dent. Res. 2014, 6, 58–63. [Google Scholar] [CrossRef]
- Li, F.; Jia, P.Y.; Ouyang, X.Y. Comparison of Measurements on Cone Beam Computed Tomography for Periodontal Intrabony Defect with Intra-surgical Measurements. Chin. J. Dent. Res. 2015, 18, 171–176. [Google Scholar] [PubMed]
- Yang, J.; Li, X.; Duan, D.; Bai, L.; Zhao, L.; Xu, Y. Cone-beam computed tomography performance in measuring periodontal bone loss. J. Oral Sci. 2019, 61, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Suphanantachat, S.; Tantikul, K.; Tamsailom, S.; Kosalagood, P.; Nisapakultorn, K.; Tavedhikul, K. Comparison of clinical values between cone beam computed tomography and conventional intraoral radiography in periodontal and infrabony defect assessment. Dentomaxillofac. Radiol. 2017, 46, 20160461. [Google Scholar] [CrossRef] [PubMed]
- Eickholz, P.; Horr, T.; Klein, F.; Hassfeld, S.; Kim, T.-S. Radiographic parameters for prognosis of periodontal healing of infrabony defects: Two different definitions of defect depth. J. Periodontol. 2004, 75, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Bjorn, H.; Halling, A.; Thyberg, H. Radiographic assessment of marginal bone loss. Odontol Revy. 1969, 20, 165–179. [Google Scholar] [PubMed]
- Steffensen, B.; Webert, H.P. Relationship between the radiographic periodontal defect angle and healing after treatment. J. Periodontol. 1989, 60, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Tsitoura, E.; Tucker, R.; Suvan, J.; Laurell, L.; Cortellini, P.; Tonetti, M. Baseline radiographic defect angle of the intrabony defect as a prognostic indicator in regenerative periodontal surgery with enamel matrix derivative. J. Clin. Periodontol. 2004, 31, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Pometti, D.; Chen, T.-T.; Tu, Y.-K. Minimally invasive non-surgical approach for the treatment of periodontal intrabony defects: A retrospective analysis. J. Clin. Periodontol. 2015, 42, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Adurty, C.; Tejaswi, K.S.; Shivani, C.R.N.; Navya, D.; Gopinath, C.; Dhulipalla, R. Accuracy of digital intraoral periapical radiography and cone-beam computed tomography in the measurement of intrabony defects: A comparative study. J. Indian Soc. Periodontol. 2021, 25, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Sultan, D.; Arena, C.; Pelekos, G.; Lin, G.H.; Tonetti, M. Periodontal infrabony defects: Systematic review of healing by defect morphology following regenerative surgery. J. Clin. Periodontol. 2021, 48, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, J.B.; Davies-Ludlow, L.E.; Brooks, S.L.; Howerton, W.B. Dosimetry of 3 CBCT devices for oral and maxillofacial radiology: CB Mercuray, NewTom 3G and i-CAT. Dentomaxillofac. Radiol. 2006, 35, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Oenning, A.C.; Jacobs, R.; Salmon, B.; Group, D.R. ALADAIP, beyond ALARA and towards personalized optimization for paediatric cone-beam CT. Int. J. Paediatr. Dent. 2021, 31, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Smith, T.; Kortam, S.; Kim, D.-G.; Tee, B.C.; Fields, H. Effect of bone thickness on alveolar bone-height measurements from cone-beam computed tomography images. Am. J. Orthod. Dentofac. Orthop. 2011, 139, e117–e127. [Google Scholar] [CrossRef] [PubMed]
- el Zoheiry, H.S.; Abou-Khalaf, A.; Farid, M.M. Assessment of periodontal defects using cone beam computed tomography: An in-vitro study. Egypt. J. Oral Maxillofac. Surg. 2011, 2, 27–33. [Google Scholar] [CrossRef]
- Anter, E.; Zayet, M.K.; El-Dessouky, S.H. Accuracy and precision of cone beam computed tomography in periodontal defects measurement (systematic review). J. Indian Soc. Periodontol. 2016, 20, 235–243. [Google Scholar] [CrossRef] [PubMed]

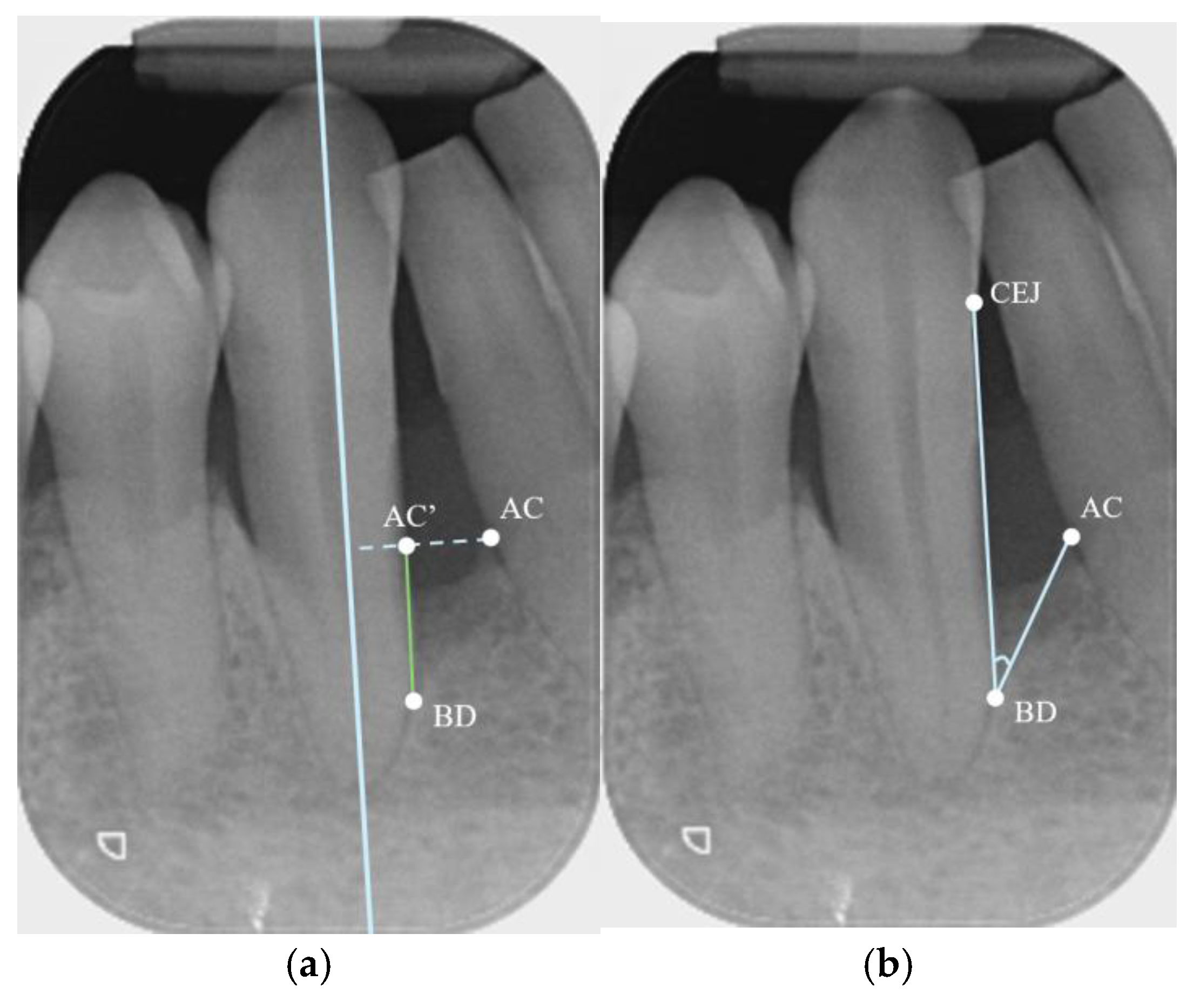

| Depth | Width | |||
|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | |
| IS–CBCT | 0.938 | 0.869–0.972 | 0.923 | 0.838–0.965 |
| IS–PA | 0.790 | 0.588–0.900 | 0.704 | 0.410–0.860 |
| Mean Difference ± SD | 95% CI | p-Value | ||
|---|---|---|---|---|
| CBCT | ||||
| Depth (mm) | −0.11 ± 0.49 | −0.31–0.09 | 0.274 | |
| Width (mm) | −0.07 ± 0.41 | −0.23–0.01 | 0.420 | |
| PA | ||||
| Depth (mm) | 0.15 ± 0.95 | −0.23–0.53 | 0.427 | |
| Width (mm) | −0.36 ± 0.73 | −0.65–−0.06 | 0.019 * |
| IS–CBCT | IS–PA | |||
|---|---|---|---|---|
| Cohen’s Kappa | % of Total Agreement | Cohen’s Kappa | % of Total Agreement | |
| Defect Type | 0.819 | 88 (n = 23) | 0.214 | 46 * (n = 12) |
| Defect Extension | 0.855 | 88 (n = 23) | 0.381 | 54 † (n = 14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.S.N.; Sung, N.D.; Fok, M.R.; Tarce, M.; Tavedhikul, K.; Pelekos, G. Evaluation of Periodontal Infrabony Defect Topography via CBCT and Comparisons with Direct Intrasurgical Measurements. Bioengineering 2025, 12, 780. https://doi.org/10.3390/bioengineering12070780
Chen TSN, Sung ND, Fok MR, Tarce M, Tavedhikul K, Pelekos G. Evaluation of Periodontal Infrabony Defect Topography via CBCT and Comparisons with Direct Intrasurgical Measurements. Bioengineering. 2025; 12(7):780. https://doi.org/10.3390/bioengineering12070780
Chicago/Turabian StyleChen, Tiffany See Nok, Nicholas David Sung, Melissa Rachel Fok, Mihai Tarce, Kanoknadda Tavedhikul, and Georgios Pelekos. 2025. "Evaluation of Periodontal Infrabony Defect Topography via CBCT and Comparisons with Direct Intrasurgical Measurements" Bioengineering 12, no. 7: 780. https://doi.org/10.3390/bioengineering12070780
APA StyleChen, T. S. N., Sung, N. D., Fok, M. R., Tarce, M., Tavedhikul, K., & Pelekos, G. (2025). Evaluation of Periodontal Infrabony Defect Topography via CBCT and Comparisons with Direct Intrasurgical Measurements. Bioengineering, 12(7), 780. https://doi.org/10.3390/bioengineering12070780






