Study Protocol: Investigating the Effects of Transcranial Pulse Stimulation in Parkinson’s Disease
Abstract
1. Introduction
1.1. Transcranial Pulse Stimulation (TPS) in the Context of Neurological and Psychiatric Conditions
1.2. The Present Study
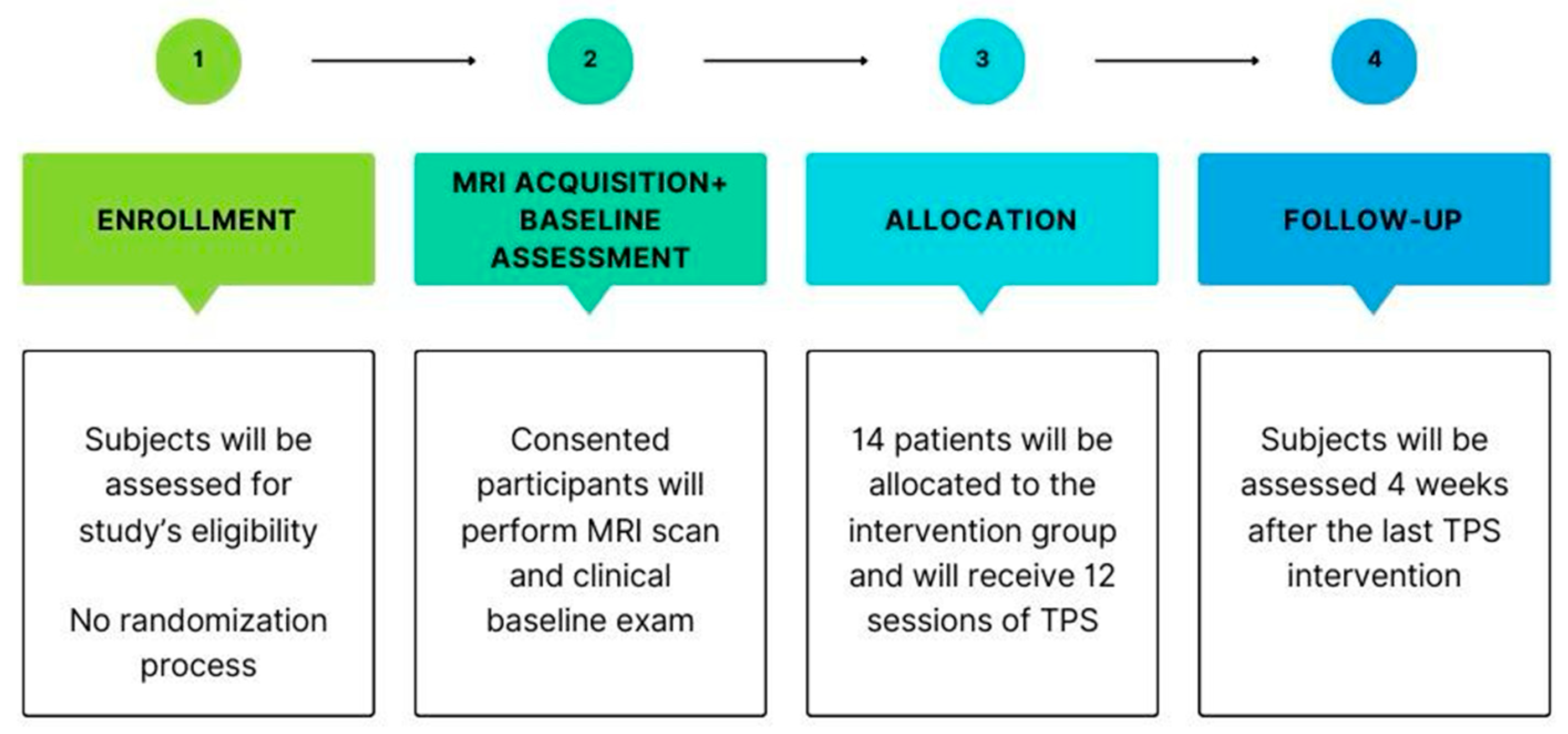
2. Experimental Design
3. Materials and Equipment
3.1. MRI Data Acquisition
3.2. TPS Protocol
4. Detailed Procedure
4.1. Intervention
4.2. Assessments and Outcomes
4.3. Safety
4.4. Data and Statistical Analysis
4.5. Sample Size and Power Calculation
5. Expected Results
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| AE | Adverse Effects |
| AIM | Acceptability of Intervention Measure |
| BBB | Blood–Brain Barrier |
| BDI | Beck Depression Inventory |
| BDNF | Brain-Derived Neurotrophic Factors |
| CAPE-V | Consensus Auditory-Perceptual Evaluation of Voice |
| DBS | Deep Brain Stimulation |
| DLPFC | Dorsolateral Prefrontal Cortex |
| EEG | Electroencephalogram |
| ESWT | Extracorporeal Shock Wave Therapy |
| FIM | Feasibility of Intervention Measures |
| FOGQ | Freezing of Gait Questionnaire |
| M1 | Motor Cortex |
| MRI | Magnetic Resonance Imaging |
| NIBS | Non-Invasive Brain Stimulation |
| NMSS | Non-Motor Symptoms Scale |
| PD | Parkinson’s Disease |
| PDQ-39 | Parkinson’s Disease Questionnaire-39 |
| PDSS-2 | Parkinson’s Disease Sleep Scale |
| PFS-16 | Parkinson’s Disease Fatigue Scale |
| PIGD | Postural Instability And Gait Difficulty |
| PPC | Posterior Parietal Cortex |
| PQAT-RW | Patient’s Qualitative Assessment of Treatment–Real-World |
| QOL | Quality Of Life |
| RBD | Rapid Eye Movement Sleep Behavior Disorder |
| SCOPA-COG | SCales for Outcomes in PArkinson’s disease-COGnition |
| SLT | Speech and Language Therapy |
| tDCS | Transcranial Direct Current Stimulation |
| TMS | Transcranial Magnetic Stimulation |
| TPS | Transcranial Pulse Stimulation |
| TUG | Timed Up and Go |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| VAMS | Visual Analog Mood Scale |
| VAS | Visual Analog Scale |
| VEGF | Vascular Growth Factors |
| VHI-10 | Voice Handicap Index |
References
- Grotewold, N.; Albin, R.L. Update: Descriptive epidemiology of Parkinson disease. Park. Relat. Disord. 2024, 120, 106000. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Marsh, L. Depression and Parkinson’s disease: Current knowledge. Curr. Neurol. Neurosci. Rep. 2013, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Berganzo, K.; Tijero, B.; Gonzalez-Eizaguirre, A.; Somme, J.; Lezcano, E.; Gabilondo, I.; Fernandez, M.; Zarranz, J.J.; Gomez-Esteban, J.C. Motor and non-motor symptoms of Parkinson’s disease and their impact on quality of life and on different clinical subgroups. Neurologia 2016, 31, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Griffin, H.J.; Quinn, N.P.; Jahanshahi, M. Quality of life in Parkinson’s disease: The relative importance of the symptoms. Mov. Disord. 2008, 23, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.M.; Zeighami, Y.; Dagher, A.; Postuma, R.B. Clinical criteria for subtyping Parkinson’s disease: Biomarkers and longitudinal progression. Brain 2017, 140, 1959–1976. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Mehta, A.; Xiao, B.; Ray Chaudhuri, K.; Tan, E.K.; Tan, L.C. Parkinson’s disease subtypes: Approaches and clinical implications. Park. Relat. Disord. 2025, 130, 107208. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, T.; Raschka, T.; Sapienza, S.; Klucken, J.; Glaab, E.; Corvol, J.C.; Falkenburger, B.H.; Frohlich, H. Progression subtypes in Parkinson’s disease identified by a data-driven multi cohort analysis. npj Park. Dis. 2024, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Saba, R.A.; Maia, D.P.; Cardoso, F.E.C.; Borges, V.; LA, F.A.; Ferraz, H.B.; Barbosa, E.R.; Rieder, C.R.M.; da Silva, D.J.; Chien, H.F.; et al. Guidelines for Parkinson’s disease treatment: Consensus from the Movement Disorders Scientific Department of the Brazilian Academy of Neurology—Motor symptoms. Arq. Neuropsiquiatr. 2022, 80, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Church, F.C. Treatment Options for Motor and Non-Motor Symptoms of Parkinson’s Disease. Biomolecules 2021, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Scelzo, E.; Beghi, E.; Rosa, M.; Angrisano, S.; Antonini, A.; Bagella, C.; Bianchi, E.; Caputo, E.; Lena, F.; Lopiano, L.; et al. Deep brain stimulation in Parkinson’s disease: A multicentric, long-term, observational pilot study. J. Neurol. Sci. 2019, 405, 116411. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Shiraishi, T.; Umehara, T.; Omoto, S.; Iguchi, Y. Recent Advances in Drug Therapy for Parkinson’s Disease. Intern. Med. 2023, 62, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, J.; Tan, Y.; Chen, S. Freezing of gait in Parkinson’s disease: Pathophysiology, risk factors and treatments. Transl. Neurodegener. 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Poletti, M.; Bonuccelli, U. Acute and chronic cognitive effects of levodopa and dopamine agonists on patients with Parkinson’s disease: A review. Ther. Adv. Psychopharmacol. 2013, 3, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Valero-Cabre, A.; Pascual-Leone, A. Noninvasive human brain stimulation. Annu. Rev. Biomed. Eng. 2007, 9, 527–565. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Simon, D.K.; Wu, A.; Pascual-Leone, A. Non-invasive brain stimulation for Parkinson’s disease: A systematic review and meta-analysis of the literature. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.D.; Fregni, F.; Simon, D.K.; Deblieck, C.; Pascual-Leone, A. Noninvasive brain stimulation for Parkinson’s disease and dystonia. Neurotherapeutics 2008, 5, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Boggio, P.S.; Ferrucci, R.; Rigonatti, S.P.; Covre, P.; Nitsche, M.; Pascual-Leone, A.; Fregni, F. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J. Neurol. Sci. 2006, 249, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cantello, R.; Tarletti, R.; Civardi, C. Transcranial magnetic stimulation and Parkinson’s disease. Brain Res. Rev. 2002, 38, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Zhu, X.; Xiao, W.; Gan, C.; Luo, Y.; Jiang, M.; Liu, H.; Chen, X. Comparative efficacy of transcranial magnetic stimulation on different targets in Parkinson’s disease: A Bayesian network meta-analysis. Front. Aging Neurosci. 2022, 14, 1073310. [Google Scholar] [CrossRef] [PubMed]
- Mi, T.M.; Garg, S.; Ba, F.; Liu, A.P.; Wu, T.; Gao, L.L.; Dan, X.J.; Chan, P.; McKeown, M.J. High-frequency rTMS over the supplementary motor area improves freezing of gait in Parkinson’s disease: A randomized controlled trial. Park. Relat. Disord. 2019, 68, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Cosentino, G.; Brighina, F.; Pozzi, N.G.; Sandrini, G.; Fierro, B.; Savettieri, G.; D’Amelio, M.; Pacchetti, C. Transcranial direct current stimulation for treatment of freezing of gait: A cross-over study. Mov. Disord. 2014, 29, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Matt, E.; Kaindl, L.; Tenk, S.; Egger, A.; Kolarova, T.; Karahasanovic, N.; Amini, A.; Arslan, A.; Saricicek, K.; Weber, A.; et al. First evidence of long-term effects of transcranial pulse stimulation (TPS) on the human brain. J. Transl. Med. 2022, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Beisteiner, R.; Lozano, A.M. Transcranial Ultrasound Innovations Ready for Broad Clinical Application. Adv. Sci. 2020, 7, 2002026. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ning, H.; Reed-Maldonado, A.B.; Zhou, J.; Ruan, Y.; Zhou, T.; Wang, H.S.; Oh, B.S.; Banie, L.; Lin, G.; et al. Low-Intensity Extracorporeal Shock Wave Therapy Enhances Brain-Derived Neurotrophic Factor Expression through PERK/ATF4 Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kang, N.; Yu, X.; Ma, Y.; Pang, X. Radial Extracorporeal Shock Wave Therapy Enhances the Proliferation and Differentiation of Neural Stem Cells by Notch, PI3K/AKT, and Wnt/beta-catenin Signaling. Sci. Rep. 2017, 7, 15321. [Google Scholar] [CrossRef] [PubMed]
- Karakatsani, M.E.; Nozdriukhin, D.; Tiemann, S.; Yoshihara, H.A.I.; Storz, R.; Belau, M.; Ni, R.; Razansky, D.; Dean-Ben, X.L. Multimodal imaging of murine cerebrovascular dynamics induced by transcranial pulse stimulation. Alzheimer’s Dement. 2025, 21, e14511. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.K.H.; Cheung, T.; Ngan, S.T.J.; Tong, K.; Lui, W.Y.V.; Chan, W.C.; Wong, C.S.M.; Cheng, C.P.W. Transcranial pulse stimulation in the treatment of mild neurocognitive disorders. Ann. Clin. Transl. Neurol. 2023, 10, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.K.; Fong, T.K.; Cheung, T.; Ngan, S.J.; Lui, W.V.; Chan, W.C.; Wong, C.S.; Wong, T.K.; Cheng, C.P. Enhanced Cognition and Modulation of Brain Connectivity in Mild Neurocognitive Disorder: The Promise of Transcranial Pulse Stimulation. Biomedicines 2024, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Shinzato, G.T.; Assone, T.; Sandler, P.C.; Pacheco-Barrios, K.; Fregni, F.; Radanovic, M.; Forlenza, O.V.; Battistella, L.R. Non-invasive sound wave brain stimulation with Transcranial Pulse Stimulation (TPS) improves neuropsychiatric symptoms in Alzheimer’s disease. Brain Stimul. 2024, 17, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Cont, C.; Stute, N.; Galli, A.; Schulte, C.; Logmin, K.; Trenado, C.; Wojtecki, L. Retrospective real-world pilot data on transcranial pulse stimulation in mild to severe Alzheimer’s patients. Front. Neurol. 2022, 13, 948204. [Google Scholar] [CrossRef] [PubMed]
- Beisteiner, R.; Hallett, M.; Lozano, A.M. Ultrasound Neuromodulation as a New Brain Therapy. Adv. Sci. 2023, 10, e2205634. [Google Scholar] [CrossRef] [PubMed]
- Beisteiner, R.; Matt, E.; Fan, C.; Baldysiak, H.; Schonfeld, M.; Philippi Novak, T.; Amini, A.; Aslan, T.; Reinecke, R.; Lehrner, J.; et al. Transcranial Pulse Stimulation with Ultrasound in Alzheimer’s Disease-A New Navigated Focal Brain Therapy. Adv. Sci. 2020, 7, 1902583. [Google Scholar] [CrossRef] [PubMed]
- Dorl, G.; Matt, E.; Beisteiner, R. Functional Specificity of TPS Brain Stimulation Effects in Patients with Alzheimer’s Disease: A Follow-up fMRI Analysis. Neurol. Ther. 2022, 11, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Matt, E.; Dorl, G.; Beisteiner, R. Transcranial pulse stimulation (TPS) improves depression in AD patients on state-of-the-art treatment. Alzheimer’s Dement. 2022, 8, e12245. [Google Scholar] [CrossRef] [PubMed]
- Popescu, T.; Pernet, C.; Beisteiner, R. Transcranial ultrasound pulse stimulation reduces cortical atrophy in Alzheimer’s patients: A follow-up study. Alzheimer’s Dement. 2021, 7, e12121. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; You, J.; Ma, H.; Zhou, M.; Huang, C. Transcranial pulse stimulation in Alzheimer’s disease. CNS Neurosci. Ther. 2024, 30, e14372. [Google Scholar] [CrossRef] [PubMed]
- Lohse-Busch, H.; Reime, U.; Falland, R. Symptomatic treatment of unresponsive wakefulness syndrome with transcranially focused extracorporeal shock waves. NeuroRehabilitation 2014, 35, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.; Ho, Y.S.; Fong, K.H.; Lam, Y.T.J.; Li, M.H.; Tse, A.C.; Li, C.T.; Cheng, C.P.; Beisteiner, R. Evaluating the Safety and Efficacy of Transcranial Pulse Stimulation on Autism Spectrum Disorder: A Double-Blinded, Randomized, Sham-Controlled Trial Protocol. Int. J. Environ. Res. Public Health 2022, 19, 15614. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.; Li, T.M.H.; Lam, J.Y.T.; Fong, K.H.; Chiu, L.Y.; Ho, Y.S.; Tse, A.C.; Li, C.T.; Cheng, C.P.; Beisteiner, R. Effects of transcranial pulse stimulation on autism spectrum disorder: A double-blind, randomized, sham-controlled trial. Brain Commun. 2023, 5, fcad226. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.; Yee, B.K.; Chau, B.; Lam, J.Y.T.; Fong, K.H.; Lo, H.; Li, T.M.H.; Li, A.M.; Sun, L.; Beisteiner, R.; et al. Efficacy and safety of transcranial pulse stimulation in young adolescents with attention-deficit/hyperactivity disorder: A pilot, randomized, double-blind, sham-controlled trial. Front. Neurol. 2024, 15, 1364270. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.; Chau, B.; Fong, K.H.; Lam, J.Y.T.; Lo, H.; Li, M.H.; Li, A.; Beisteiner, R.; Lei, S.; Yee, B.K.; et al. Evaluating the efficacy and safety of transcranial pulse stimulation on adolescents with attention deficit hyperactivity disorder: Study protocol of a pilot randomized, double-blind, sham-controlled trial. Front. Neurol. 2023, 14, 1076086. [Google Scholar] [CrossRef]
- Cheung, T.; Li, T.M.H.; Ho, Y.S.; Kranz, G.; Fong, K.N.K.; Leung, S.F.; Lam, S.C.; Yeung, W.F.; Lam, J.Y.T.; Fong, K.H.; et al. Effects of Transcranial Pulse Stimulation (TPS) on Adults with Symptoms of Depression-A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 2333. [Google Scholar] [CrossRef] [PubMed]
- Osou, S.; Radjenovic, S.; Bender, L.; Gaal, M.; Zettl, A.; Dorl, G.; Matt, E.; Beisteiner, R. Novel ultrasound neuromodulation therapy with transcranial pulse stimulation (TPS) in Parkinson’s disease: A first retrospective analysis. J. Neurol. 2024, 271, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Manganotti, P.; Liccari, M.; Maria Isabella Lombardo, T.; Della Toffola, J.; Cenacchi, V.; Catalan, M.; Busan, P. Effect of a single session of transcranial pulse stimulation (TPS) on resting tremor in patients with Parkinson’s disease. Brain Res. 2025, 1850, 149405. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Poewe, W.; Litvan, I.; Lewis, S.; Lang, A.E.; Halliday, G.; Goetz, C.G.; Chan, P.; Slow, E.; Seppi, K.; et al. Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2018, 33, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Lohse-Busch, H.; Marlinghaus, E.; Reime, U.; Mowis, U. Focused low-energy extracorporeal shock waves with distally symmetric polyneuropathy (DSPNP): A pilot study. NeuroRehabilitation 2014, 35, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Lohse-Busch, H. Transcranial pulse stimulation (TPS) with focused extracorporeal shock waves. A new promising non invasive symptomatic treatment of Parkinson’s disease. Casuistics Feasibility Study 2021. preprint. [Google Scholar] [CrossRef]
- Brognara, L.; Cauli, O. Mechanical Plantar Foot Stimulation in Parkinson’s Disease: A Scoping Review. Diseases 2020, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Rodriguez-Blazquez, C.; Alvarez-Sanchez, M.; Arakaki, T.; Bergareche-Yarza, A.; Chade, A.; Garretto, N.; Gershanik, O.; Kurtis, M.M.; Martinez-Castrillo, J.C.; et al. Expanded and independent validation of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). J. Neurol. 2013, 260, 228–236. [Google Scholar] [CrossRef] [PubMed]
- van Wamelen, D.J.; Martinez-Martin, P.; Weintraub, D.; Schrag, A.; Antonini, A.; Falup-Pecurariu, C.; Odin, P.; Ray Chaudhuri, K. The Non-Motor Symptoms Scale in Parkinson’s disease: Validation and use. Acta Neurol. Scand. 2021, 143, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Isella, V.; Mapelli, C.; Morielli, N.; Siri, C.; De Gaspari, D.; Pezzoli, G.; Antonini, A.; Poletti, M.; Bonuccelli, U.; Picchi, L.; et al. Diagnosis of possible mild cognitive impairment in Parkinson’s disease: Validity of the SCOPA-Cog. Park. Relat. Disord. 2013, 19, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N.; Tal, J.; Azulay, T.; Rascol, O.; Brooks, D.J.; Melamed, E.; Oertel, W.; Poewe, W.H.; Stocchi, F.; Tolosa, E. Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov. Disord. 2009, 24, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Steffen, T.M.; Hacker, T.A.; Mollinger, L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys. Ther. 2002, 82, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Trenkwalder, C.; Kohnen, R.; Hogl, B.; Metta, V.; Sixel-Doring, F.; Frauscher, B.; Hulsmann, J.; Martinez-Martin, P.; Chaudhuri, K.R. Parkinson’s disease sleep scale--validation of the revised version PDSS-2. Mov. Disord. 2011, 26, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.G.; Dittner, A.; Findley, L.; Wessely, S.C. The Parkinson fatigue scale. Park. Relat. Disord. 2005, 11, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.R.; Hirsch, E.S.; Anderson, K.; Bush, A.L.; Goldstein, S.R.; Grill, S.; Lehmann, S.; Little, J.T.; Margolis, R.L.; Palanci, J.; et al. A comparison of nine scales to detect depression in Parkinson disease: Which scale to use? Neurology 2012, 78, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.A. Assessment of Mood States in Neurodegenerative Disease: Methodological Issues and Diagnostic Recommendations. Semin. Clin. Neuropsychiatry 1996, 1, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Lechuga, M.J.; Izquierdo-Dominguez, A.; Chiesa-Estomba, C.; Calvo-Henriquez, C.; Villarreal, I.M.; Cuesta-Chasco, G.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I. Chemosensory dysfunction in COVID-19 out-patients. Eur. Arch. Otorhinolaryngol. 2021, 278, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.A.; Lee, A.S.; Osborne, J.; Zullo, T.; Murry, T. Development and validation of the voice handicap index-10. Laryngoscope 2004, 114, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Zraick, R.I.; Kempster, G.B.; Connor, N.P.; Thibeault, S.; Klaben, B.K.; Bursac, Z.; Thrush, C.R.; Glaze, L.E. Establishing validity of the Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V). Am. J. Speech Lang. Pathol. 2011, 20, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Sauder, C.; Bretl, M.; Eadie, T. Predicting Voice Disorder Status from Smoothed Measures of Cepstral Peak Prominence Using Praat and Analysis of Dysphonia in Speech and Voice (ADSV). J. Voice 2017, 31, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Schonenberg, A.; Prell, T. Measuring quality of life with the Parkinson’s Disease Questionnaire-39 in people with cognitive impairment. PLoS ONE 2022, 17, e0266140. [Google Scholar] [CrossRef] [PubMed]
- Weiner, B.J.; Lewis, C.C.; Stanick, C.; Powell, B.J.; Dorsey, C.N.; Clary, A.S.; Boynton, M.H.; Halko, H. Psychometric assessment of three newly developed implementation outcome measures. Implement. Sci. 2017, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- de Climens, A.R.; Findley, A.; Bury, D.P.; Brady, K.J.S.; Reaney, M.; Gater, A. Development and Content Validation of the Patient’s Qualitative Assessment of Treatment—Real-World (PQAT-RW): An Instrument to Evaluate Benefits and Disadvantages of Treatments in Real-World Settings. Patient Relat. Outcome Meas. 2024, 15, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Guo, Z.; Peng, H.; Xing, G.; Chen, H.; McClure, M.A.; He, B.; He, L.; Du, F.; Xiong, L.; et al. Repetitive transcranial magnetic stimulation therapy for motor recovery in Parkinson’s disease: A Meta-analysis. Brain Behav. 2018, 8, e01132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Liu, Z.; Rao, J.; Wang, J.; Wang, P.; Gong, X.; Wen, Y. Transcranial Direct Current Stimulation for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2021, 13, 746797. [Google Scholar] [CrossRef] [PubMed]

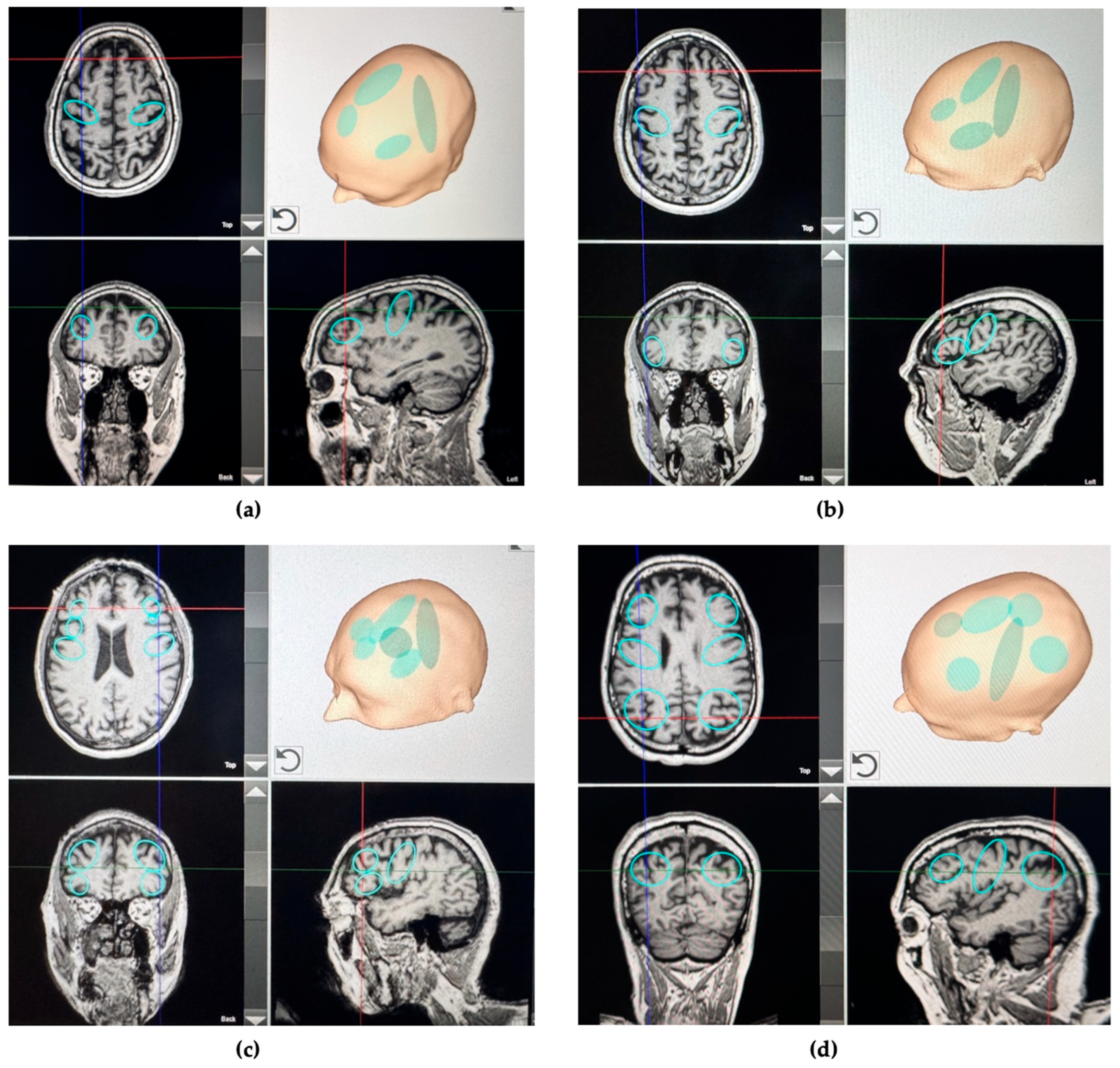
| Symptoms | Clinical Feature | Test Definition | Brain Region of Interest |
|---|---|---|---|
| Motor impairment | Moderate to severe rigidity, freezing of gait, postural instability, tremor, and bradykinesia | UPDRS-III score > 33 | Bilateral and entire strip of the primary motor cortex (M1) |
| Cognitive impairment | Mild memory and executive function alterations | SCOPA-COG ≤ 24 | Bilateral dorsolateral prefrontal cortex (DLPFC) |
| Psychiatric disorder | Depression | BDI-II score ≥ 14 | Bilateral dorsolateral prefrontal cortex (DLPFC) |
| Sleep disorder | Moderate to severe sleep disturbance | PDSS-2 score ≥ 18 | Bilateral posterior parietal cortex (PPC) |
| Fatigue | Moderate to severe fatigue | PFS-16 binary score of >8 | Bilateral dorsolateral prefrontal cortex (DLPFC) |
| Voice/speech dysfunction | Moderate to severe dysarthria and poor voice control | VHI-10 score ≥ 11 | Bilateral inferior frontal cortex extending on the left to Broca’s area |
| Taste and smell disorders | Moderate or severe olfactory and gustatory dysfunction | VAS for taste or smell ≥ 4 | Bilateral orbitofrontal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianlorenço, A.C.; Camargo, L.; Fernandes, E.B.; Pichardo, E.; Yeh, H.J.; Hazer-Rau, D.; Storz, R.; Fregni, F. Study Protocol: Investigating the Effects of Transcranial Pulse Stimulation in Parkinson’s Disease. Bioengineering 2025, 12, 773. https://doi.org/10.3390/bioengineering12070773
Gianlorenço AC, Camargo L, Fernandes EB, Pichardo E, Yeh HJ, Hazer-Rau D, Storz R, Fregni F. Study Protocol: Investigating the Effects of Transcranial Pulse Stimulation in Parkinson’s Disease. Bioengineering. 2025; 12(7):773. https://doi.org/10.3390/bioengineering12070773
Chicago/Turabian StyleGianlorenço, Anna Carolyna, Lucas Camargo, Elayne Borges Fernandes, Elly Pichardo, Huan Jui Yeh, Dilana Hazer-Rau, Rafael Storz, and Felipe Fregni. 2025. "Study Protocol: Investigating the Effects of Transcranial Pulse Stimulation in Parkinson’s Disease" Bioengineering 12, no. 7: 773. https://doi.org/10.3390/bioengineering12070773
APA StyleGianlorenço, A. C., Camargo, L., Fernandes, E. B., Pichardo, E., Yeh, H. J., Hazer-Rau, D., Storz, R., & Fregni, F. (2025). Study Protocol: Investigating the Effects of Transcranial Pulse Stimulation in Parkinson’s Disease. Bioengineering, 12(7), 773. https://doi.org/10.3390/bioengineering12070773








