High-Speed Full-Color Polarized Light Imaging of Collagen Using a Polarization Camera
Abstract
1. Introduction
2. Materials and Methods
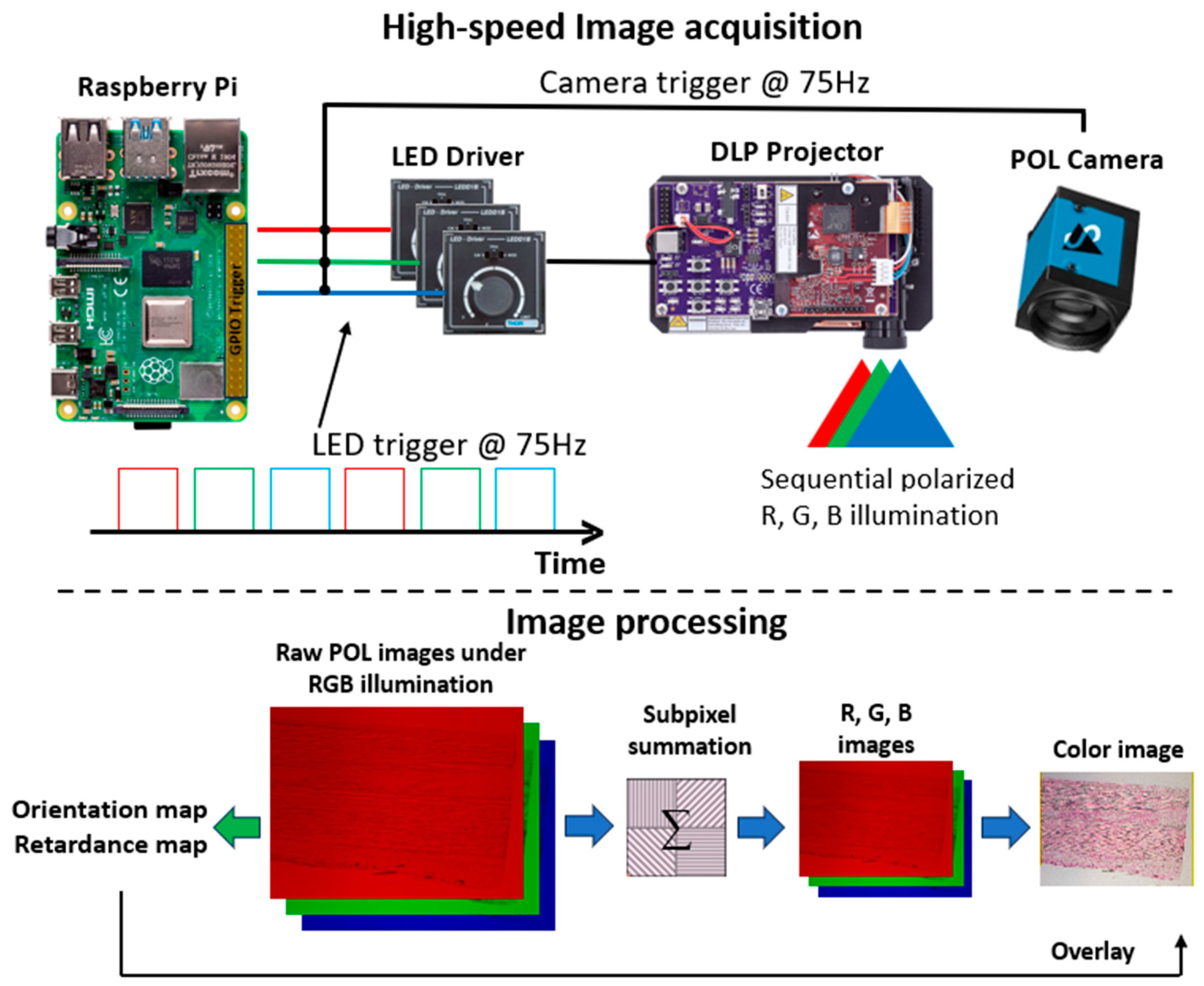
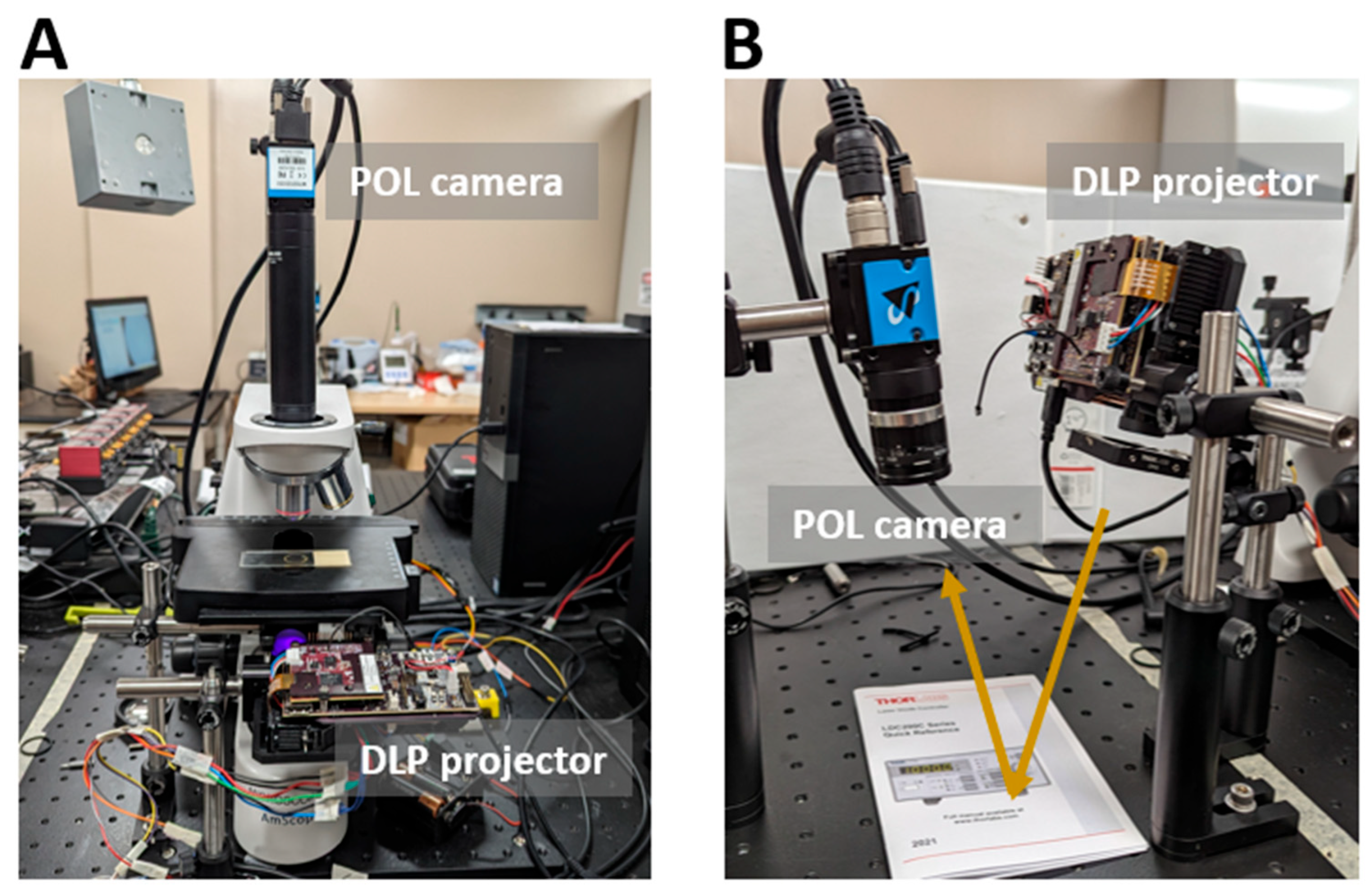
2.1. Imaging System Development
2.2. Quantitative Analysis and Visualization Pipeline
2.3. Sample Preparation and Imaging
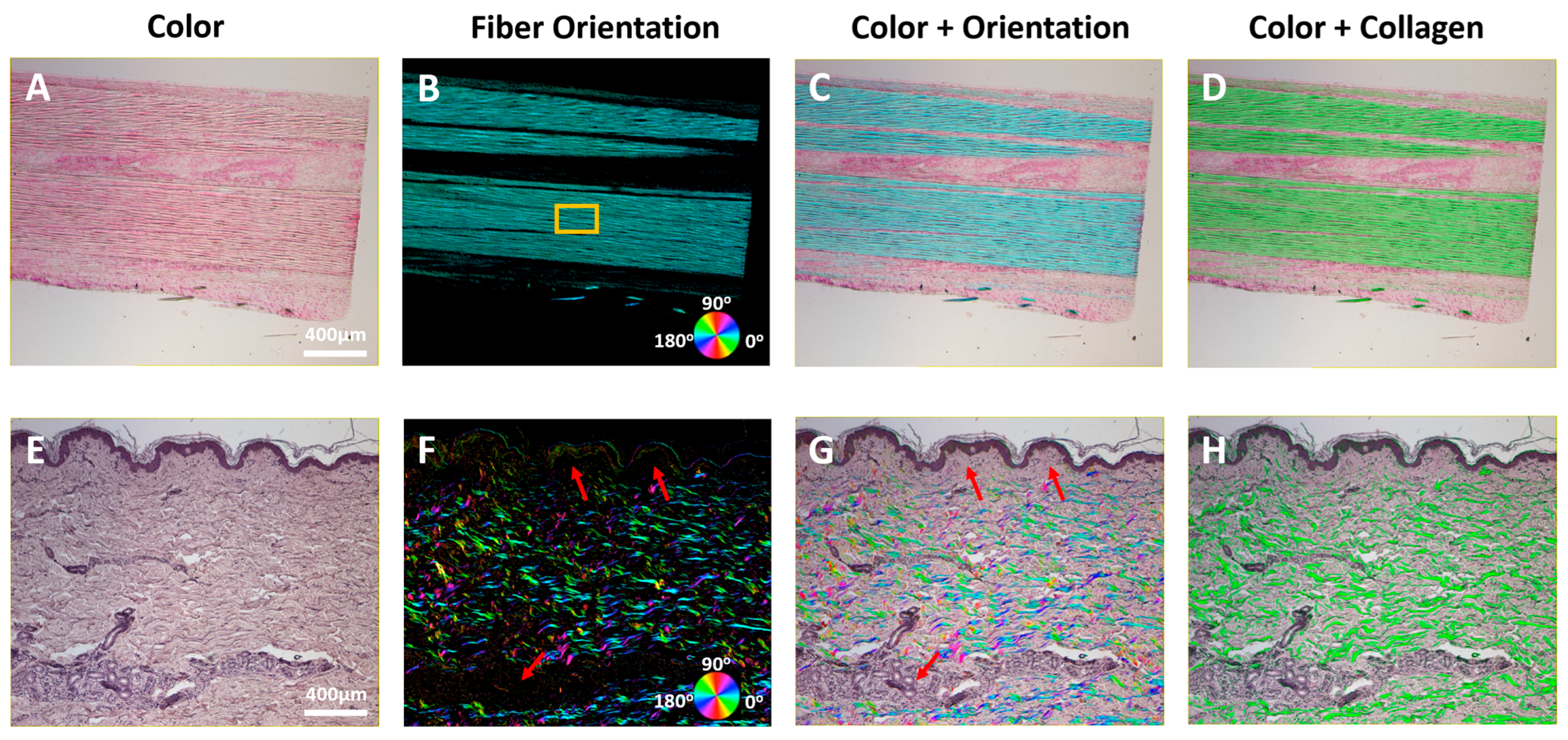
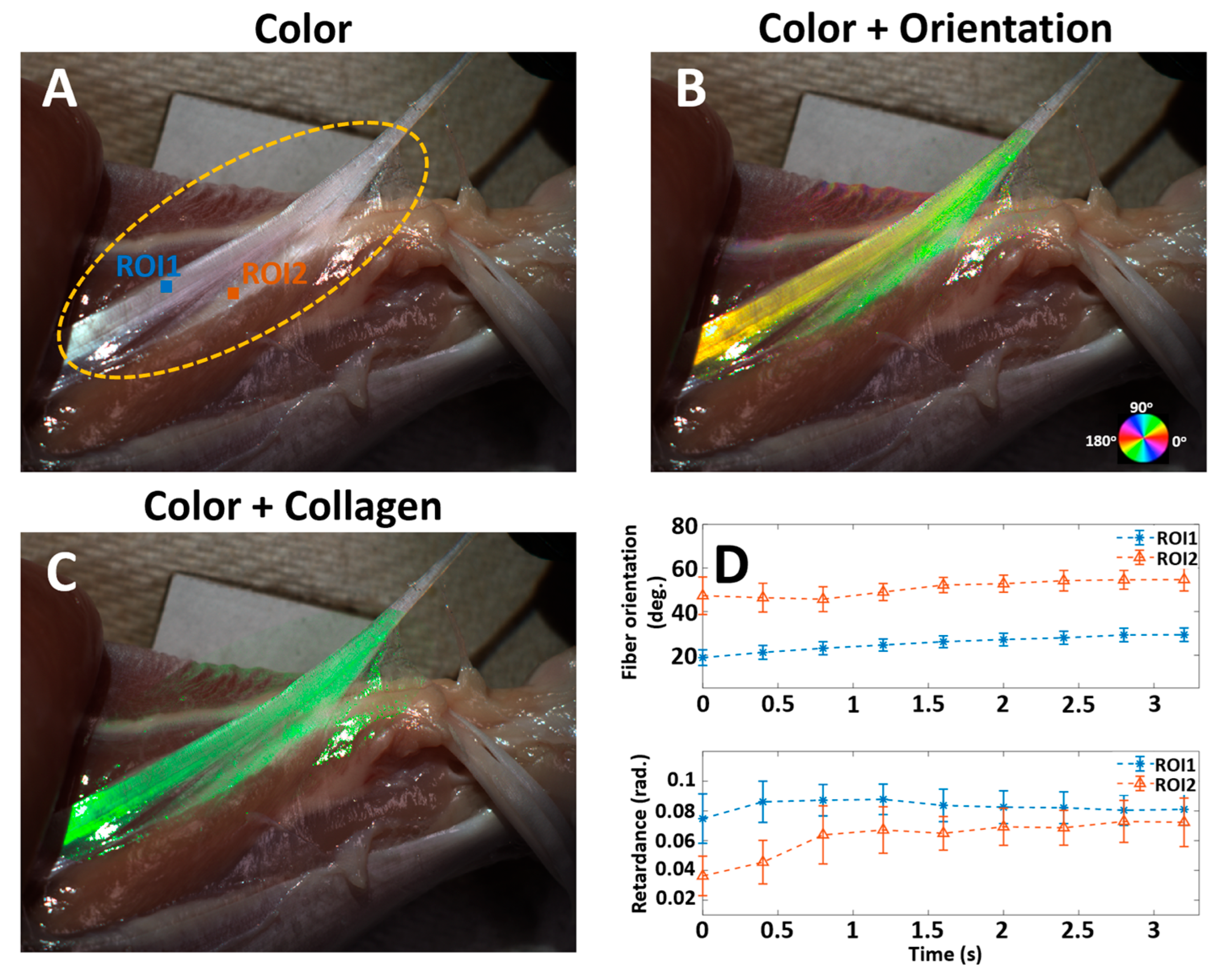
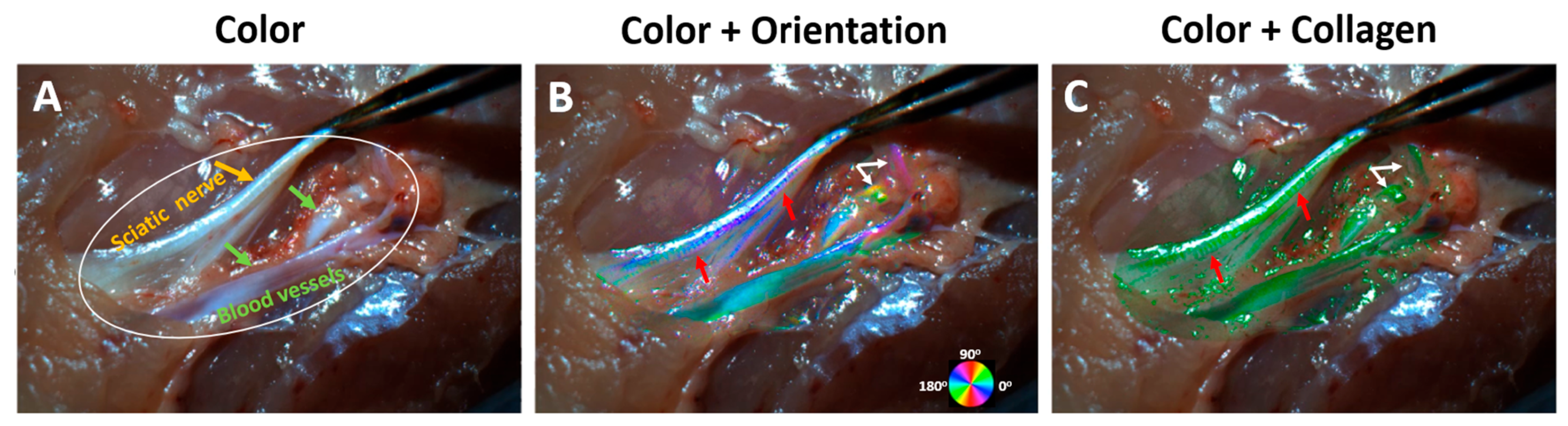
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franchi, M.; Trirè, A.; Quaranta, M.; Orsini, E.; Ottani, V. Collagen structure of tendon relates to function. Sci. World J. 2007, 7, 404–420. [Google Scholar] [CrossRef]
- Tedder, M.E.; Liao, J.; Weed, B.; Stabler, C.; Zhang, H.; Simionescu, A.; Simionescu, D.T. Stabilized collagen scaffolds for heart valve tissue engineering. Tissue Eng. Part A 2009, 15, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthetic Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Meek, K.M.; Boote, C. The organization of collagen in the corneal stroma. Exp. Eye Res. 2004, 78, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P. Collagen: Structure and mechanics, an introduction. In Collagen: Structure and Mechanics; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–13. [Google Scholar]
- Jan, N.-J.; Grimm, J.L.; Tran, H.; Lathrop, K.L.; Wollstein, G.; Bilonick, R.A.; Ishikawa, H.; Kagemann, L.; Schuman, J.S.; Sigal, I.A. Polarization microscopy for characterizing fiber orientation of ocular tissues. Biomed. Opt. Express 2015, 6, 4705–4718. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jan, N.J.; Brazile, B.; Voorhees, A.; Lathrop, K.L.; Sigal, I.A. Polarized light microscopy for 3-dimensional mapping of collagen fiber architecture in ocular tissues. J. Biophotonics 2018, 11, e201700356. [Google Scholar] [CrossRef]
- Bromage, T.G.; Goldman, H.M.; McFarlin, S.C.; Warshaw, J.; Boyde, A.; Riggs, C.M. Circularly polarized light standards for investigations of collagen fiber orientation in bone. Anat. Rec. Part B New Anat. Off. Publ. Am. Assoc. Anat. 2003, 274, 157–168. [Google Scholar] [CrossRef]
- Rieppo, J.; Hallikainen, J.; Jurvelin, J.S.; Kiviranta, I.; Helminen, H.J.; Hyttinen, M.M. Practical considerations in the use of polarized light microscopy in the analysis of the collagen network in articular cartilage. Microsc. Res. Tech. 2008, 71, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Pircher, M.; Götzinger, E.; Leitgeb, R.; Sattmann, H.; Findl, O.; Hitzenberger, C.K. Imaging of polarization properties of human retina in vivo with phase resolved transversal PS-OCT. Opt. Express 2004, 12, 5940–5951. [Google Scholar] [CrossRef]
- Giattina, S.D.; Courtney, B.K.; Herz, P.R.; Harman, M.; Shortkroff, S.; Stamper, D.L.; Liu, B.; Fujimoto, J.G.; Brezinski, M.E. Assessment of coronary plaque collagen with polarization sensitive optical coherence tomography (PS-OCT). Int. J. Cardiol. 2006, 107, 400–409. [Google Scholar] [CrossRef]
- Mansfield, J.C.; Mandalia, V.; Toms, A.; Winlove, C.P.; Brasselet, S. Collagen reorganization in cartilage under strain probed by polarization sensitive second harmonic generation microscopy. J. R. Soc. Interface 2019, 16, 20180611. [Google Scholar] [CrossRef] [PubMed]
- Stoller, P.; Reiser, K.M.; Celliers, P.M.; Rubenchik, A.M. Polarization-modulated second harmonic generation in collagen. Biophys. J. 2002, 82, 3330–3342. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Broch, A.; Mudge, S.; Kim, K.; Namgoong, J.-M.; Oh, E.; Kim, P. Real-time, label-free, intraoperative visualization of peripheral nerves and micro-vasculatures using multimodal optical imaging techniques. Biomed. Opt. Express 2018, 9, 1097–1110. [Google Scholar]
- Liu, Y.; Dong, Y.; Si, L.; Meng, R.; Dong, Y.; Ma, H. Comparison between image texture and polarization features in histopathology. Biomed. Opt. Express 2021, 12, 1593–1608. [Google Scholar] [PubMed]
- Ning, B.; Kim, W.W.; Katz, I.; Park, C.H.; Sandler, A.D.; Cha, J. Improved nerve visualization in head and neck surgery using mueller polarimetric imaging: Preclinical feasibility study in a swine model. Lasers Surg. Med. 2021, 53, 1427–1434. [Google Scholar] [CrossRef]
- Shribak, M. Polychromatic polarization microscope: Bringing colors to a colorless world. Sci. Rep. 2015, 5, 17340. [Google Scholar] [CrossRef]
- Yang, B.; Lee, P.Y.; Hua, Y.; Brazile, B.; Waxman, S.; Ji, F.; Zhu, Z.; Sigal, I.A. Instant polarized light microscopy for imaging collagen microarchitecture and dynamics. J. Biophotonics 2021, 14, e202000326. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-Y.; Schilpp, H.; Naylor, N.; Watkins, S.C.; Yang, B.; Sigal, I.A. Instant polarized light microscopy pi (IPOLπ) for quantitative imaging of collagen architecture and dynamics in ocular tissues. Opt. Lasers Eng. 2023, 166, 107594. [Google Scholar] [CrossRef]
- Keikhosravi, A.; Shribak, M.; Conklin, M.W.; Liu, Y.; Li, B.; Loeffler, A.; Levenson, R.M.; Eliceiri, K.W. Real-time polarization microscopy of fibrillar collagen in histopathology. Sci. Rep. 2021, 11, 19063. [Google Scholar]
- Rebhan, D.; Rosenberger, M.; Notni, G. Principle investigations on polarization image sensors. In Proceedings of the Photonics and Education in Measurement Science 2019, Jena, Germany, 17–19 September 2019; pp. 50–54. [Google Scholar]
- Ingram, G.; Lee, P.-Y.; Bernarding, B.; Sigal, I.A.; Yang, B. Real-time structured polarized light imaging of dynamics of thick collagenous tissues. In Proceedings of the Emerging Digital Micromirror Device Based Systems and Applications XIII, Online, 6–11 March 2021; pp. 16–22. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Chong, A.K.; Le, L.A.T.; Lahiri, A.; Yusoff, K.; Yip, G.W.; Pan, F.; Teo, W.; Liao, J.C.; Lim, J.X. Surgical anatomy and exercises using the chicken thigh sciatic nerve for microsurgery training. J. Hand Microsurg. 2023, 15, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Ushiki, T.; Ide, C. Three-dimensional organization of the collagen fibrils in the rat sciatic nerve as revealed by transmission-and scanning electron microscopy. Cell Tissue Res. 1990, 260, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Niland, S. The extracellular matrix of blood vessels. Curr. Pharm. Des. 2009, 15, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Fertala, J.; Rivlin, M.; Wang, M.L.; Beredjiklian, P.K.; Steplewski, A.; Fertala, A. Collagen-rich deposit formation in the sciatic nerve after injury and surgical repair: A study of collagen-producing cells in a rabbit model. Brain Behav. 2020, 10, e01802. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Nayyar, N.; Sanchez, B. High-Speed Full-Color Polarized Light Imaging of Collagen Using a Polarization Camera. Bioengineering 2025, 12, 720. https://doi.org/10.3390/bioengineering12070720
Yang B, Nayyar N, Sanchez B. High-Speed Full-Color Polarized Light Imaging of Collagen Using a Polarization Camera. Bioengineering. 2025; 12(7):720. https://doi.org/10.3390/bioengineering12070720
Chicago/Turabian StyleYang, Bin, Neil Nayyar, and Billy Sanchez. 2025. "High-Speed Full-Color Polarized Light Imaging of Collagen Using a Polarization Camera" Bioengineering 12, no. 7: 720. https://doi.org/10.3390/bioengineering12070720
APA StyleYang, B., Nayyar, N., & Sanchez, B. (2025). High-Speed Full-Color Polarized Light Imaging of Collagen Using a Polarization Camera. Bioengineering, 12(7), 720. https://doi.org/10.3390/bioengineering12070720



