Plastic Reconstruction of Upper Extremity Defects in Necrotizing Soft Tissue Infections
Abstract
1. Introduction
2. Local Wound Care
3. Use of Negative Pressure Wound Therapy (NPWT)
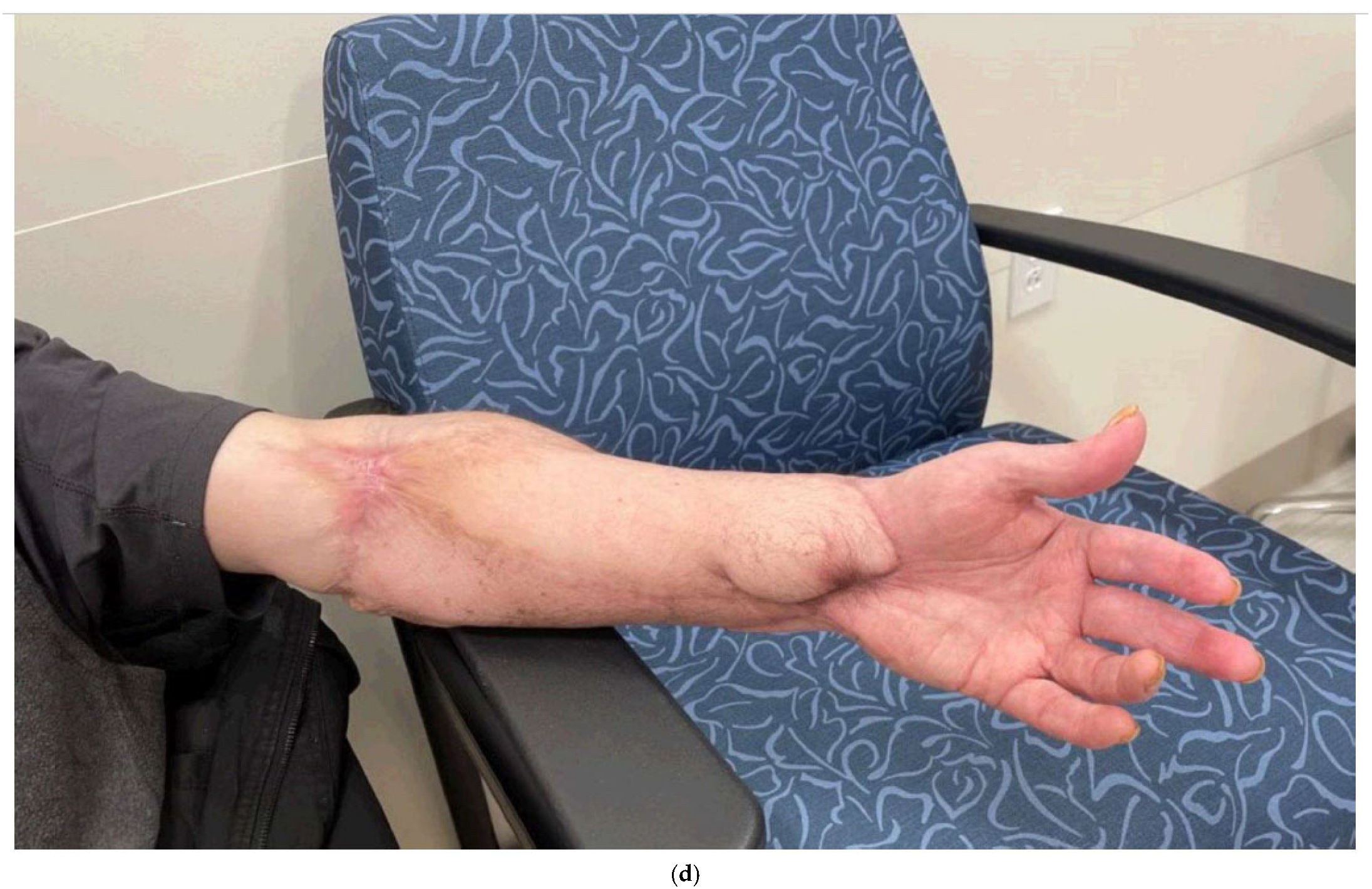
4. Delayed Primary Closure
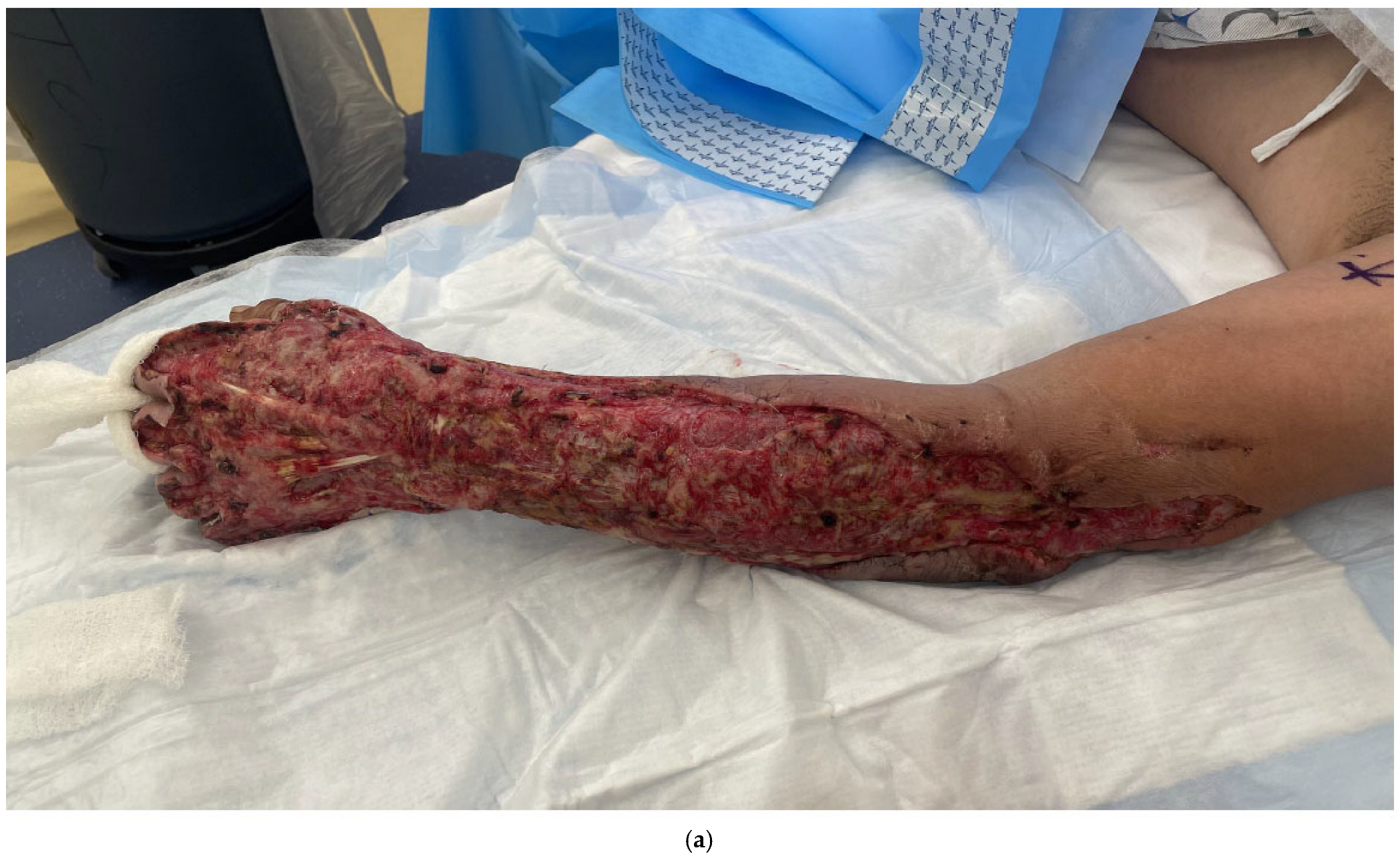
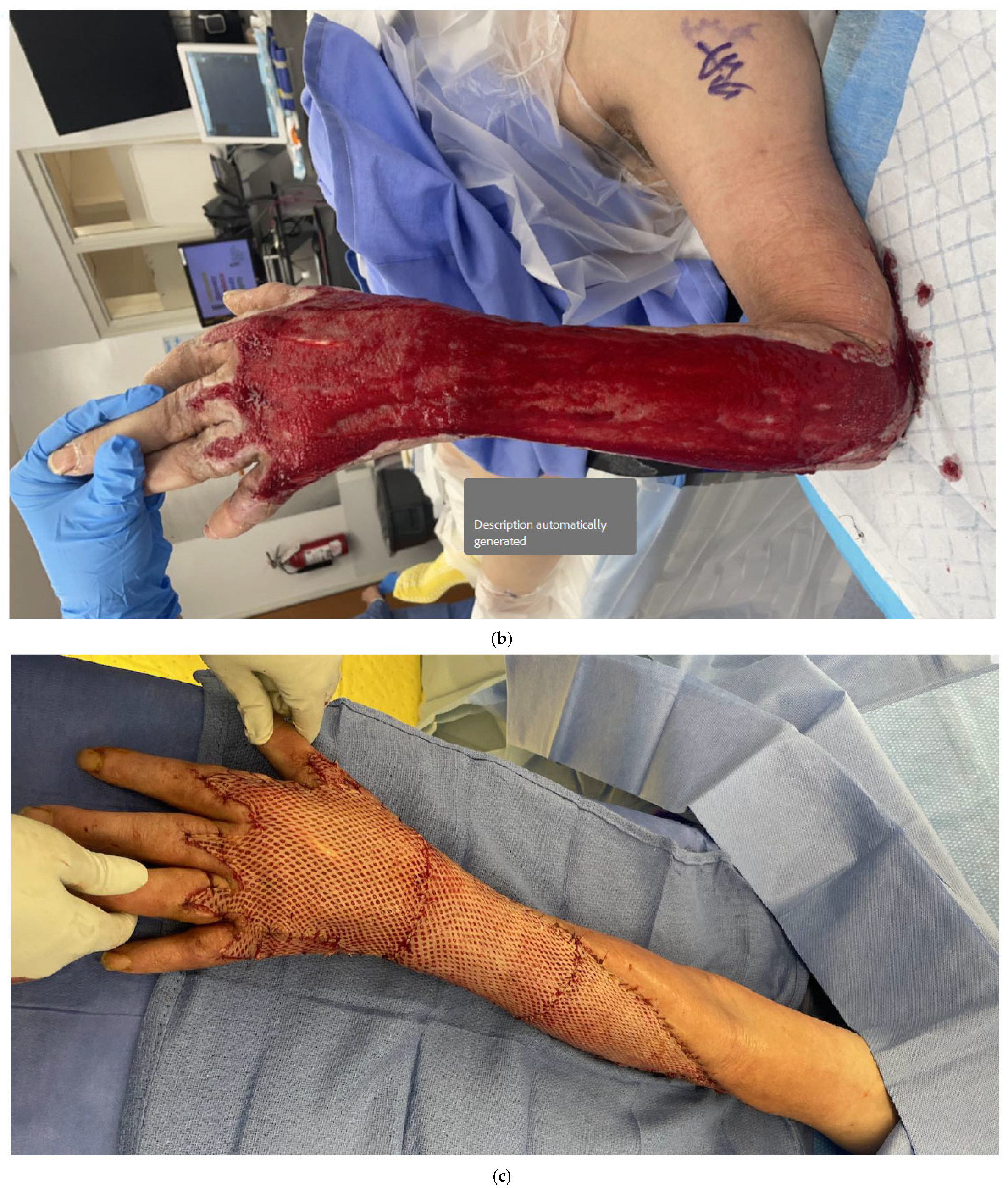
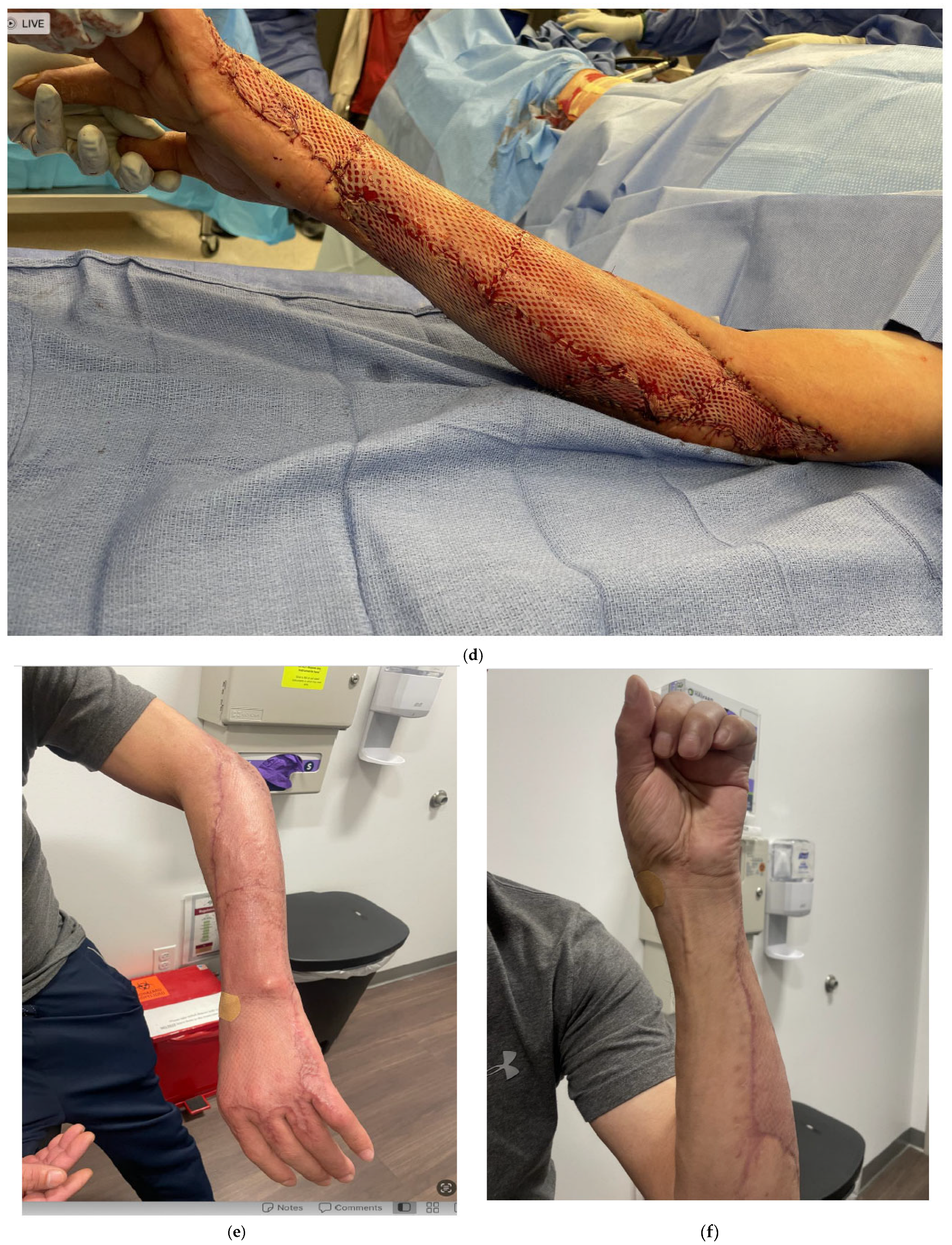
5. Skin Grafting/Dermal Substitutes
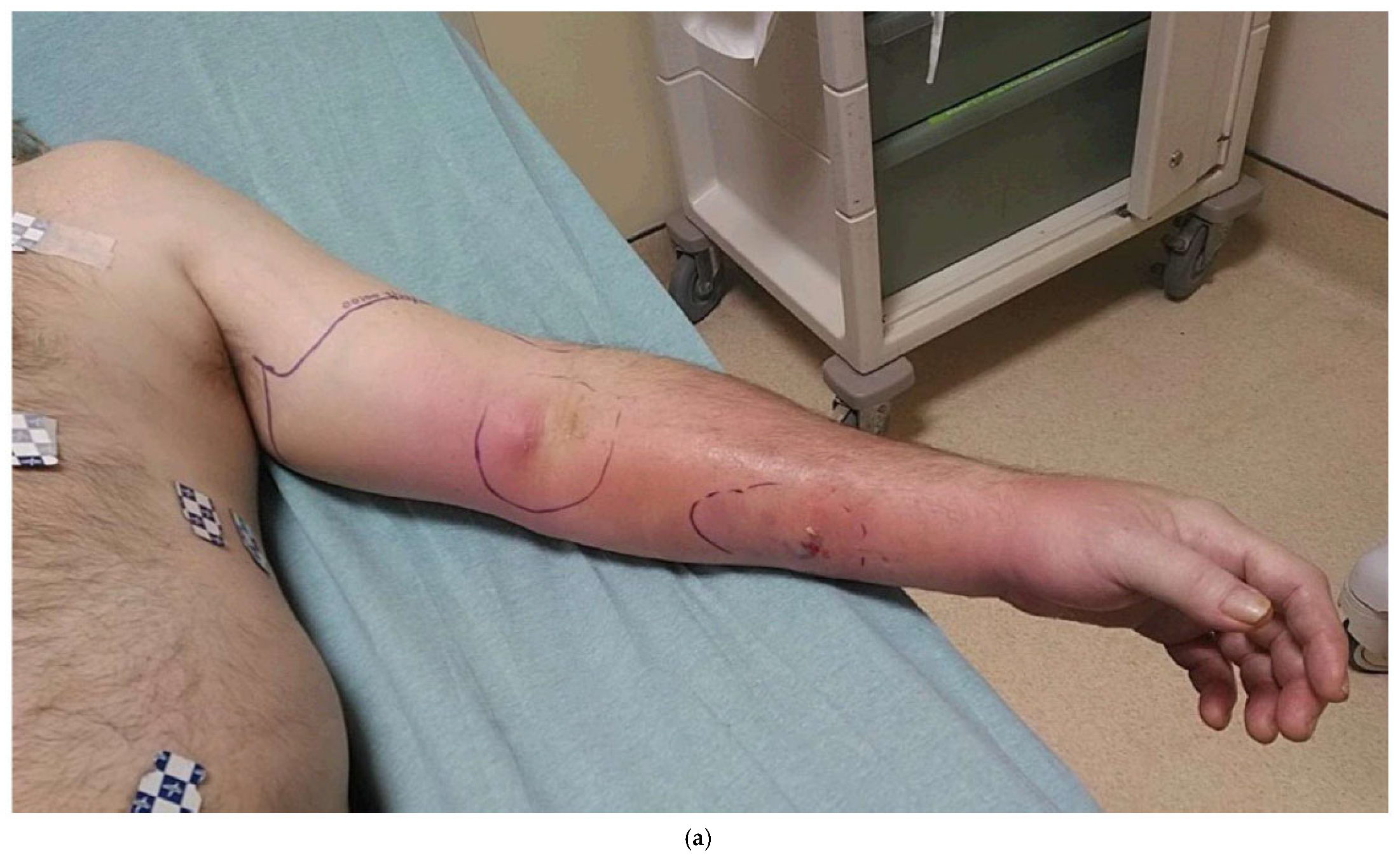
6. Local Regional Flaps
7. Remote Pedicled Flaps
8. Free Tissue Transfers
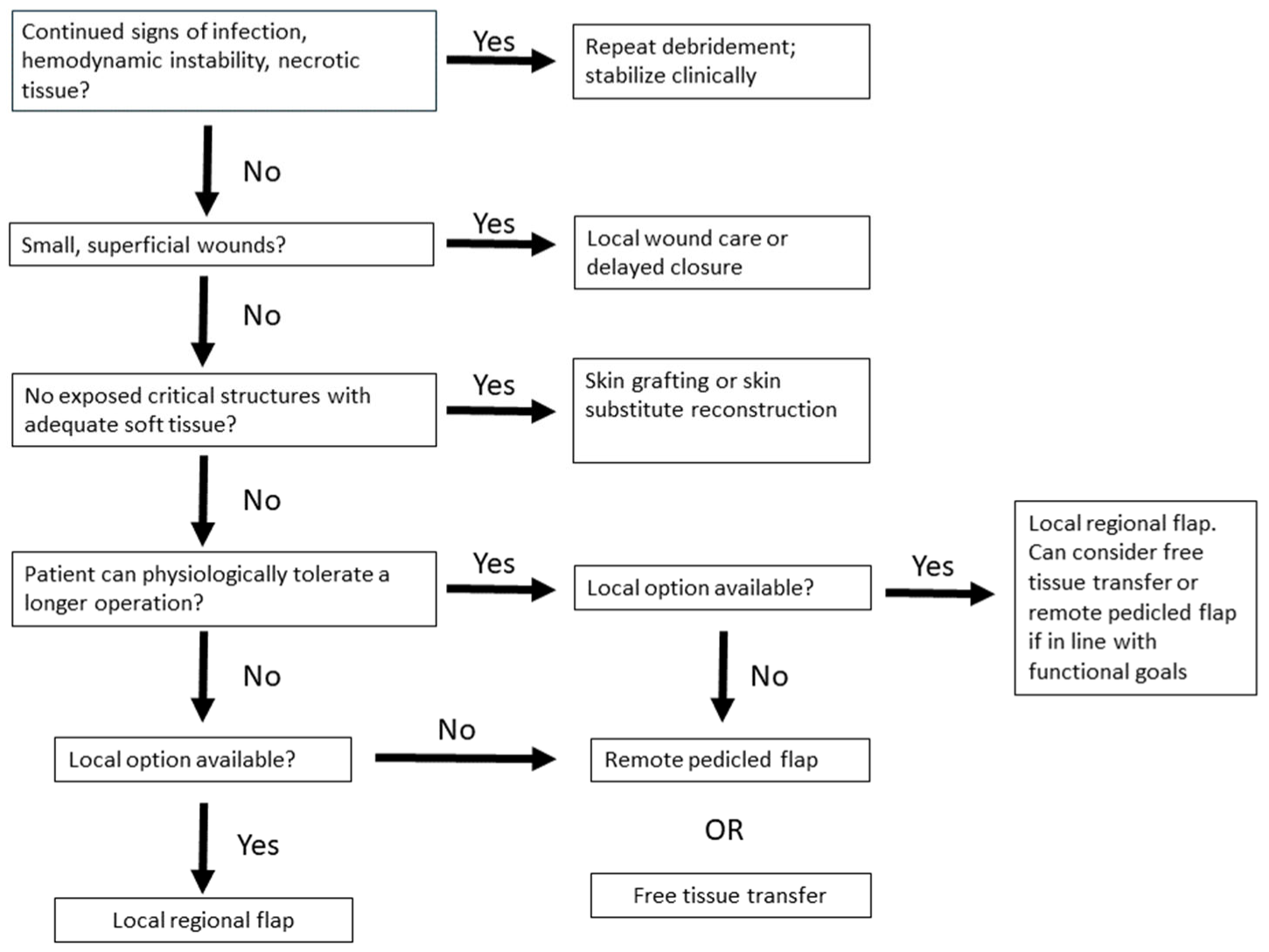
9. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Everett, E.D.; Dellinger, P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, E.L.; Montoya, J.G.; et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin. Infect. Dis. 2005, 41, 1373–1406. [Google Scholar] [CrossRef]
- Wall, D.B.; De Virgilio, C.; Black, S.; Klein, S.R. Objective criteria may assist in distinguishing necrotizing fasciitis from nonnecrotizing soft tissue infection. Am. J. Surg. 2000, 179, 17–20. [Google Scholar] [CrossRef]
- Danino, A.M. Necrotizing dermohypodermitis or fasciitis? Plast. Reconstr. Surg. 2008, 122, 669. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bryant, A.E. Necrotizing Soft-Tissue Infections’. N. Engl. J. Med. 2017, 377, 2253–2265. [Google Scholar] [CrossRef]
- Sunderland, I.R.; Friedrich, J.B. Predictors of Mortality and Limb Loss in Necrotizing Soft Tissue Infections of the Upper Extremity. J. Hand Surg. 2009, 34, 1900–1901. [Google Scholar] [CrossRef]
- La Padula, S.; Pensato, R.; Zaffiro, A.; Hermeziu, O.; D’andrea, F.; Pizza, C.; Meningaud, J.P.; Hersant, B. Necrotizing Fasciitis of the Upper Limb: Optimizing Management to Reduce Complications. J. Clin. Med. 2022, 11, 2182. [Google Scholar] [CrossRef]
- Angoules, A.G.; Kontakis, G.; Drakoulakis, E.; Vrentzos, G.; Granick, M.S.; Giannoudis, P.V. Necrotising fasciitis of upper and lower limb: A systematic review. Injury 2007, 38 (Suppl. 5), S18–S25. [Google Scholar] [CrossRef]
- Gonzalez, M.H.; Kay, T.; Weinzweig, N.; Brown, A.; Pulvirenti, J. Necrotizing fasciitis of the upper extremity. J. Hand Surg. 1996, 21, 689–692. [Google Scholar] [CrossRef]
- Hankins, C.L.; Southern, S. Factors that affect the clinical course of group A β-haemolytic streptococcal infections of the hand and upper extremity: A retrospective study. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2008, 42, 153–157. [Google Scholar] [CrossRef]
- Singh, G.; Bharpoda, P.; Reddy, R. Necrotizing Fasciitis: A Study of 48 Cases. Indian J. Surg. 2013, 77 (Suppl. 2), 345. [Google Scholar] [CrossRef]
- Yeung, Y.; Ho, S.; Yen, C.; Ho, P.; Tse, W.; Lau, Y.; Choi, K.; Choi, S.; Lam, M.; Cheng, S.; et al. Factors affecting mortality in Hong Kong patients with upper limb necrotising fasciitis. Hong Kong Med. J. 2011, 17, 96104. [Google Scholar]
- Bilolikar, V.; Rengifo, S.; Desai, K.; Hozack, B. Necrotizing Fasciitis of the Upper Extremity: A Systematic Review of the Literature. SurgiColl 2023, 1, 2023. [Google Scholar] [CrossRef]
- Wagstaff, M.J.; Salna, I.M.; Caplash, Y.; Greenwood, J.E. Biodegradable Temporising Matrix (BTM) for the reconstruction of defects following serial debridement for necrotising fasciitis: A case series. Burn. Open 2019, 3, 12–30. [Google Scholar] [CrossRef]
- Crowe, C.S.; Yu, J.L.; Yesantharao, P.; Keys, K.; Kennedy, S.A. Predictors of Mortality and Amputation in Patients With Necrotizing Soft Tissue Infections of the Upper Extremity. J. Hand Surg. 2022, 47, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Ditsios, K.; Chitas, K.; Christidis, P.; Charatsis, K.; Katsimentzas, T.; Papadopoulos, P. Necrotizing Fasciitis of the Upper Extremity—A Review. Orthop. Rev. 2022, 14, 35320. [Google Scholar] [CrossRef] [PubMed]
- Nawijn, F.; Verhiel, S.H.; Lunn, K.N.; Eberlin, K.R.; Hietbrink, F.; Chen, N.C. Factors Associated with Mortality and Amputation Caused by Necrotizing Soft Tissue Infections of the Upper Extremity: A Retrospective Cohort Study. World J. Surg. 2020, 44, 730–740. [Google Scholar] [CrossRef]
- Argenta, L.; Morykwas, M. Vacuum-assisted closure: A new method for wound control and treatment: Clinical experience. Ann. Plast. Surg. 1997, 38, 56376. [Google Scholar] [CrossRef]
- Kim, Y.H.; Ha, J.H.; Kim, J.T.; Kim, S.W. Managing necrotising fasciitis to reduce mortality and increase limb salvage. J. Wound Care 2018, 27, S20–S27. [Google Scholar] [CrossRef]
- Corona, P.S.; Erimeiku, F.; Reverté-Vinaixa, M.M.; Soldado, F.; Amat, C.; Carrera, L. Necrotising fasciitis of the extremities: Implementation of new management technologies. Injury 2016, 47, S66–S71. [Google Scholar] [CrossRef]
- Kim, P.J.; Attinger, C.E.; Constantine, T.; Crist, B.D.; Faust, E.; Hirche, C.R.; Lavery, L.A.; Messina, V.J.; Ohura, N.; Punch, L.J.; et al. Negative pressure wound therapy with instillation: International consensus guidelines update. Int. Wound J. 2020, 17, 174–186. [Google Scholar] [CrossRef]
- Anghel, E.L.; Kim, P.J. Negative-pressure wound therapy: A comprehensive review of the evidence. Plast. Reconstr. Surg. 2016, 138, 129s–137s. [Google Scholar] [CrossRef] [PubMed]
- Rupert, P.; Ochoa, R.A.; Punch, L.; Van Epps, J.; Gordon-Burroughs, S.; Martinez, S. The Use of NPWT-i Technology in Complex Surgical Wounds. Cureus 2016, 8, e920. [Google Scholar] [CrossRef] [PubMed]
- Mattison, G.; Leis, A.R.; Gupta, S.C. Single-specialty management and reconstruction of necrotizing fasciitis of the upper extremities: Clinical and economic benefits from a case series. Ann. Plast. Surg. 2014, 72 (Suppl. 1), 2182. [Google Scholar] [CrossRef]
- Schwartz, J.M.; Taleghani, E.R.; Natal-Albelo, E.J.; Chhabra, A.B.; Freilich, A.M. Successfully Treated Upper-Extremity Necrotizing Fasciitis Caused by Photobacterium damselae. J. Hand Surg. Glob. Online 2023, 5, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Prantl, L.; Schreml, S.; Schwarze, H.; Eisenmann-Klein, M.; Nerlich, M.; Angele, P.; Jung, M.; Füchtmeier, B. A safe and simple technique using the distal pedicled reversed upper arm flap to cover large elbow defects. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 546–551. [Google Scholar] [CrossRef]
- Na, Y.; Shin, D.; Choi, H.; Kim, J.; Lee, M. Reconstruction of Upper Extremity Using Pedicled Latissimus Dorsi Myocutaneous Island Flap and Skin Graft after Necrotizing Fasciitis: A Case Report. J. Wound Manag. Res. 2023, 19, 80–85. [Google Scholar] [CrossRef]
- Wagner, R.D.; Carr, L.; Netscher, D.T. Current indications for abdominal-based flaps in hand and forearm reconstruction. Injury 2020, 51, 2916–2921. [Google Scholar] [CrossRef]
- Frank, J.; Barker, J.H.; Marzi, I. Necrotizing fasciitis of the extremities. Eur. J. Trauma. Emerg. Surg. 2008, 34, 229–236. [Google Scholar] [CrossRef]
- Balakrishnan, C.; Erella, V.S.; Vandemark, S.; Mussman, J. Groin flap for salvage of upper extremity following necrotizing fasciitis: A case report. Can. J. Plast. Surg. 2005, 13, 153. [Google Scholar] [CrossRef]
- Gawaziuk, J.P.; Liu, T.; Sigurdson, L.; Buchel, E.; Hayakawa, T.E.J.; Shiga, S.; Logsetty, S. Free tissue transfer for necrotizing fasciitis reconstruction: A case series. Burns 2017, 43, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Schecter, W.; Meyer, A.; Schecter, G.; Giuliano, A.; Newmeyer, W.; Kilgore, E. Necrotizing fasciitis of the upper extremity. J. Hand Surg. 1982, 7, 15–20. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takamura, K.M.; Yoo, J.J. Plastic Reconstruction of Upper Extremity Defects in Necrotizing Soft Tissue Infections. Bioengineering 2025, 12, 718. https://doi.org/10.3390/bioengineering12070718
Takamura KM, Yoo JJ. Plastic Reconstruction of Upper Extremity Defects in Necrotizing Soft Tissue Infections. Bioengineering. 2025; 12(7):718. https://doi.org/10.3390/bioengineering12070718
Chicago/Turabian StyleTakamura, Karren M., and Jason J. Yoo. 2025. "Plastic Reconstruction of Upper Extremity Defects in Necrotizing Soft Tissue Infections" Bioengineering 12, no. 7: 718. https://doi.org/10.3390/bioengineering12070718
APA StyleTakamura, K. M., & Yoo, J. J. (2025). Plastic Reconstruction of Upper Extremity Defects in Necrotizing Soft Tissue Infections. Bioengineering, 12(7), 718. https://doi.org/10.3390/bioengineering12070718





