Organic and Synthetic Substitutes in Tracheal Reconstruction: A Scoping Review (2015–2025)
Abstract
1. Introduction
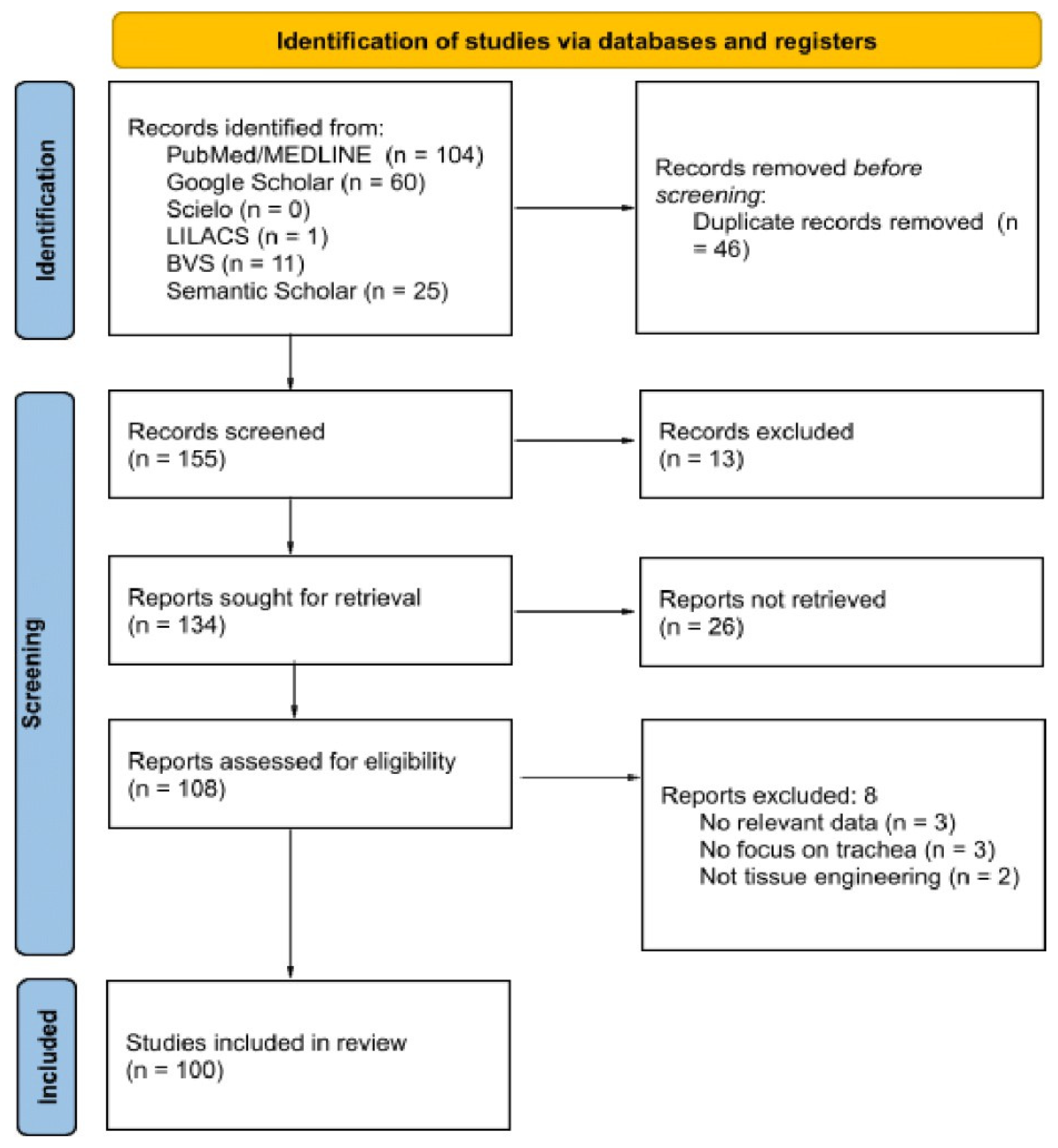
2. Materials and Methods
3. Results
3.1. Studies Evaluating Synthetic and Natural Materials In Vitro
3.2. Studies Evaluating Synthetic and Natural Materials In Vivo
3.3. Characterization and Analysis of Biomaterials
3.4. Biocompatibility Analysis
3.5. Limitations and Gaps
4. Discussion
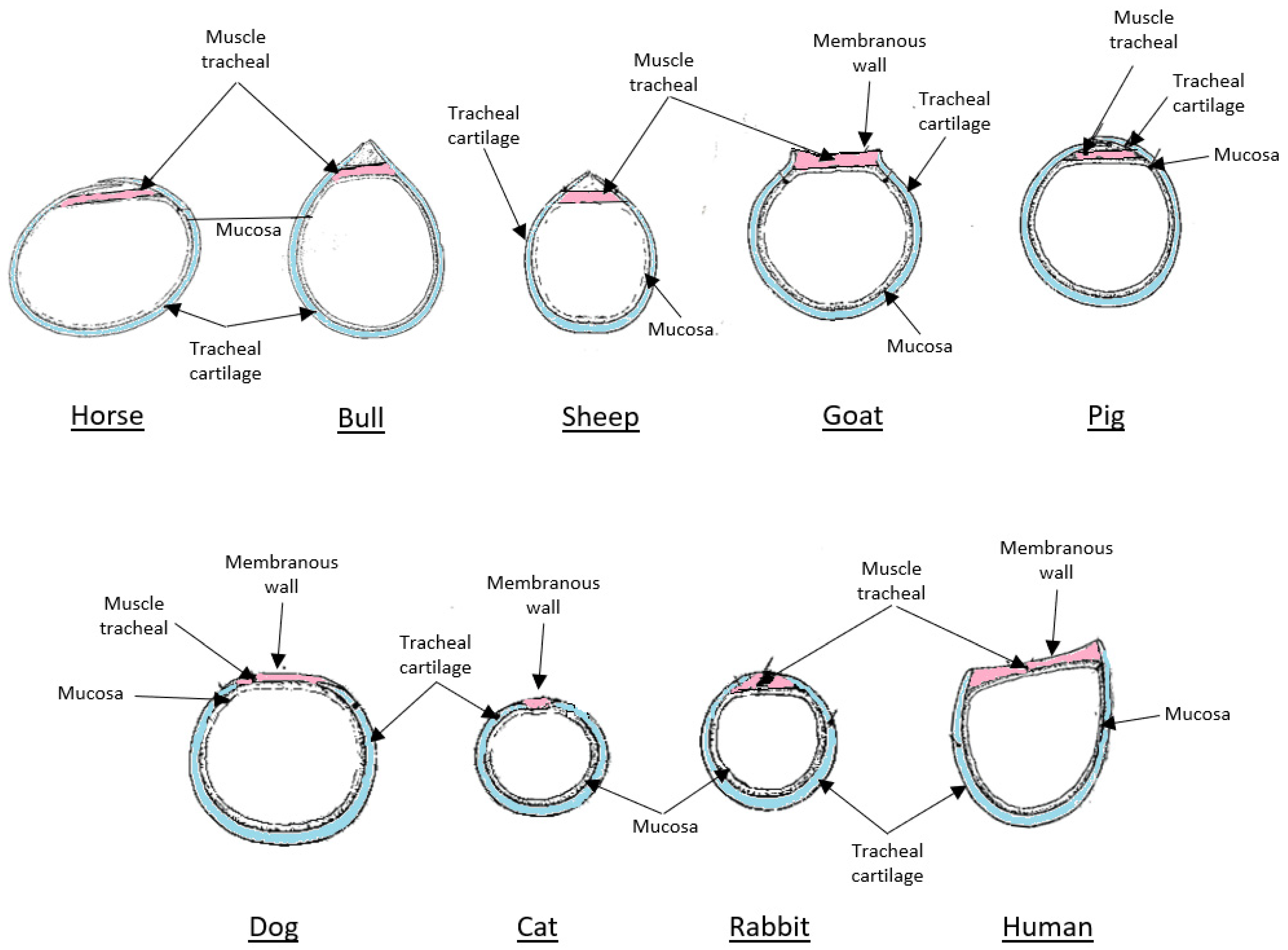
4.1. The Role and Limitations of Animal Models in Tracheal Bioengineering
4.2. Inherent Challenges in Translating Preclinical Findings to Clinical Practice
4.3. Hybrid Approaches in Tracheal Reconstruction
4.4. Advanced Fabrication Using 3D Printing and Bioprinting
4.5. The Role of Decellularized Matrices as a Biological Scaffold
4.6. Bioengineering Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Erich König, H.; Liebich, H.-G. Anatomy of Domestic Animals: Text and Color Atlas, 6th ed.; Grupo A Educação S.A.: Porto Alegre, Brazil, 2016; ISBN 9788582713006. [Google Scholar]
- Bader, A.; Macchiarini, P. Moving towards in Situ Tracheal Regeneration: The Bionic Tissue Engineered Transplantation Approach. J. Cell Mol. Med. 2010, 14, 1877. [Google Scholar] [CrossRef] [PubMed]
- RUN: 3D Printing of Biocompatible Polymers. Available online: https://run.unl.pt/handle/10362/61557 (accessed on 18 March 2025).
- Chen, X.; Zhou, C.W.; Fu, Y.Y.; Li, Y.Z.; Chen, L.; Zhang, Q.W.; Chen, Y.F. Global, Regional, and National Burden of Chronic Respiratory Diseases and Associated Risk Factors, 1990-2019: Results from the Global Burden of Disease Study 2019. Front. Med. 2023, 10, 1066804. [Google Scholar] [CrossRef]
- Fossum, T.W.; MacPhail, C. Small Animal Surgery, 5th ed.; Fossum, T.W., Cho, J., Dewey, W.C., Hayashi, K., Huntingford, J.L., MacPhail, C., Quandt, J.E., Radlinsky, M.G., Schulz, K.S., Willard, M.D., et al., Eds.; Elsevier: Rio de Janeiro, Brazil, 2021; Volume 1, ISBN 9788595150119. [Google Scholar]
- Mendes, H.D.A.L.C.; Costa, G.P.; Rocha, D.D.E.O.A.C.; Silva, D.P.C.; Souza, J.H.B. Colapso De Traqueia Em Um Canino Da Raça Spitz Alemão. Rev. Multidiscip. Saúde 2025, 6. [Google Scholar] [CrossRef]
- Jiwangga, D.; Mahyudin, F.; Mastutik, G.; Juliana; Meitavany, E.N. Current Strategies for Tracheal Decellularization: A Systematic Review. Int. J. Biomater. 2024, 2024, 3355239. [Google Scholar] [CrossRef]
- Sindhi, K.; Pingili, R.B.; Beldar, V.; Bhattacharya, S.; Rahaman, J.; Mukherjee, D. The Role of Biomaterials-BasedScaffolds in Advancing Skin Tissue Construct. J. Tissue Viability 2025, 34, 100858. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A Guide to the Composition and Functions of the Extracellular Matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Neishabouri, A.; Soltani Khaboushan, A.; Daghigh, F.; Kajbafzadeh, A.M.; Majidi Zolbin, M. Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef] [PubMed]
- Batioglu-Karaaltin, A.; Ovali, E.; Karaaltin, M.V.; Yener, M.; Yılmaz, M.; Eyüpoğlu, F.; Yılmaz, Y.Z.; Bozkurt, E.R.; Demir, N.; Konuk, E.; et al. Decellularization of Trachea With Combined Techniques for Tissue-Engineered Trachea Transplantation. Clin. Exp. Otorhinolaryngol. 2018, 12, 86. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated Methodological Guidance for the Conduct of Scoping Reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Lange, P.; Shah, H.; Birchall, M.; Sibbons, P.; Ansari, T. Characterization of a Biologically Derived Rabbit Tracheal Scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2126–2135. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.B.; Correia, A.T.; Alves, B.P.; Da Silva, R.S.; Martins, J.K.; Pêgo-Fernandes, P.M.; Xavier, N.S.; Dolhnikoff, M.; Cardoso, P.F.G. Evaluation of a Physical-Chemical Protocol for Porcine Tracheal Decellularization. Transplant. Proc. 2019, 51, 1611–1613. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, S.; Iwai, R.; Nakayama, Y.; Moriwaki, T.; Okuyama, H. Successful Tracheal Regeneration Using Biofabricated Autologous Analogues without Artificial Supports. Sci. Rep. 2022, 12, 20279. [Google Scholar] [CrossRef]
- Johnson, C.; Sheshadri, P.; Ketchum, J.M.; Narayanan, L.K.; Weinberger, P.M.; Shirwaiker, R.A. In Vitro Characterization of Design and Compressive Properties of 3D-Biofabricated/Decellularized Hybrid Grafts for Tracheal Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2016, 59, 572–585. [Google Scholar] [CrossRef]
- Mahoney, C.; Conklin, D.; Waterman, J.; Sankar, J.; Bhattarai, N. Electrospun Nanofibers of Poly (ε-Caprolactone)/Depolymerized Chitosan for Respiratory Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2016, 27, 611–625. [Google Scholar] [CrossRef]
- Wu, L.; Magaz, A.; Huo, S.; Darbyshire, A.; Loizidou, M.; Emberton, M.; Birchall, M.; Song, W. Human Airway-like Multilayered Tissue on 3D-TIPS Printed Thermoresponsive Elastomer/Collagen Hybrid Scaffolds. Acta Biomater. 2020, 113, 177–195. [Google Scholar] [CrossRef]
- Miar, S.; Dion, G.R.; Montelongo, S.; Ong, J.L.; Bizios, R.; Guda, T. Development of a Bioinspired, Self-Adhering, and Drug-Eluting Laryngotracheal Patch. Laryngoscope 2021, 131, 1958–1966. [Google Scholar] [CrossRef]
- Ravindra, A.; D’Angelo, W.; Zhang, L.; Reing, J.; Johnson, S.; Myerburg, M.; Badylak, S.F. Human Bronchial Epithelial Cell Growth on Homologous Versus Heterologous Tissue Extracellular Matrix. J. Surg. Res. 2021, 263, 215–223. [Google Scholar] [CrossRef]
- Hong, P.; Bezuhly, M.; Graham, M.E.; Gratzer, P.F. Efficient Decellularization of Rabbit Trachea to Generate a Tissue Engineering Scaffold Biomatrix. Int. J. Pediatr. Otorhinolaryngol. 2018, 112, 67–74. [Google Scholar] [CrossRef]
- de Wit, R.J.J.; van Dis, D.J.; Bertrand, M.E.; Tiemessen, D.; Siddiqi, S.; Oosterwijk, E.; Verhagen, A.F.T.M. Scaffold-Based Tissue Engineering: Supercritical Carbon Dioxide as an Alternative Method for Decellularization and Sterilization of Dense Materials. Acta Biomater. 2023, 155, 323–332. [Google Scholar] [CrossRef]
- Sun, W.; Yang, Y.; Wang, L.; Tang, H.; Zhang, L.; She, Y.; Xiao, X.; Hu, X.; Feng, Q.; Chen, C. Utilization of an Acellular Cartilage Matrix-Based Photocrosslinking Hydrogel for Tracheal Cartilage Regeneration and Circumferential Tracheal Repair. Adv. Funct. Mater. 2022, 32, 2201257. [Google Scholar] [CrossRef]
- Den Hondt, M.; Vanaudenaerde, B.; Verbeken, E.; Vranckx, J.J. Requirements for Successful Trachea Transplantation: A Study in the Rabbit Model. Plast. Reconstr. Surg. 2018, 141, 845e–856e. [Google Scholar] [CrossRef]
- Wang, J.; Kong, L.; Gafur, A.; Peng, X.; Kristi, N.; Xu, J.; Ma, X.; Wang, N.; Humphry, R.; Durkan, C.; et al. Photooxidation Crosslinking to Recover Residual Stress in Decellularized Blood Vessel. Regen. Biomater. 2021, 8, rbaa058. [Google Scholar] [CrossRef]
- Dang, L.H.; Tseng, Y.; Tseng, H.; Hung, S.-H. Partial Decellularization for Segmental Tracheal Scaffold Tissue Engineering: A Preliminary Study in Rabbits. Biomolecules 2021, 11, 866. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Gomez, D.M.; García-López, S.J.; Tamay-de-Dios, L.; Sánchez-Sánchez, R.; Villalba-Caloca, J.; Sotres-Vega, A.; Del Prado-Audelo, M.L.; Gómez-Lizárraga, K.K.; Garciadiego-Cázares, D.; Piña-Barba, M.C. Fast Cyclical-Decellularized Trachea as a Natural 3D Scaffold for Organ Engineering. Mater. Sci. Eng. C 2019, 105, 110142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Jung, H.; Ajiteru, O.; Lee, O.J.; Kim, S.H.; Park, H.S.; Park, C.H. Hybrid 3D Bioprinting for AdvancedTissue-Engineered Trachea: Merging Fused Deposition Modeling (FDM) and Top-down Digital Light Processing (DLP). Biofabrication 2024, 17, 015026. [Google Scholar] [CrossRef]
- Nakaegawa, Y.; Nakamura, R.; Tada, Y.; Nomoto, Y.; Imaizumi, M.; Suzuki, R.; Nakamura, T.; Omori, K. Effect of Structural Differences in Collagen Sponge Scaffolds on Tracheal Epithelium Regeneration. Ann. Otol. Rhinol. Laryngol. 2016, 125, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Moradi, L.; Shadmehr, M.B.; Bonakdar, S.; Droodinia, A.; Safshekan, F. In-Vivo Characterization of a 3D Hybrid Scaffold Based on PCL/Decellularized Aorta for Tracheal Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 74–83. [Google Scholar] [CrossRef]
- Hamilton, N.J.I.; Hynds, R.E.; Gowers, K.H.C.; Tait, A.; Butler, C.R.; Hopper, C.; Burns, A.J.; Birchall, M.A.; Lowdell, M.; Janes, S.M. Using a Three-Dimensional Collagen Matrix to Deliver Respiratory Progenitor Cells to Decellularized Trachea In Vivo. Tissue Eng. Part. C Methods 2019, 25, 93–102. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, J.H.; Son, S.J.; Han, M.; Kim, G.; Kang, S.S.; Choi, S.H.; Cho, D.W. Evaluation of Immunosuppressive Therapy Use for Tracheal Transplantation with Trachea-Mimetic Bellows Scaffolds in a Rabbit Model. Biomed. Res. Int. 2017, 2017, 5205476. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Shen, Z.; Xia, T.; Pan, Z.; Dan, Y.; Li, J.; Shi, H. Hydrogel Modification of 3D Printing Hybrid Tracheal Scaffold to Construct an Orthotopic Transplantation. Am. J. Transl. Res. 2022, 14, 2910–2925. [Google Scholar]
- Shan, Y.; Wang, Y.; Li, J.; Shi, H.; Fan, Y.; Yang, J.; Ren, W.; Yu, X. Biomechanical Properties and Cellular Biocompatibility of 3D Printed Tracheal Graft. Bioprocess. Biosyst. Eng. 2017, 40, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Jin, D.; Wang, Q.; Gao, M.; Zhang, J.; Zhang, H.; Bai, J.; Feng, B.; Chen, M.; Huang, Y.; et al. Tissue-Engineered Trachea from a 3D-Printed Scaffold Enhances Whole-Segment Tracheal Repair in a Goat Model. J. Tissue Eng. Regen. Med. 2019, 13, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, J.Y.; Nam, I.-C.; Ahn, M.; Lee, J.Y.; Choi, S.H.; Kim, S.W.; Cho, D.-W. A Rational Tissue Engineering Strategy Based on Three-Dimensional (3D) Printing for Extensive Circumferential Tracheal Reconstruction. Biomaterials 2018, 185, 276–283. [Google Scholar] [CrossRef]
- Shai, S.-E.; Lai, Y.-L.; Hung, Y.-W.; Hsieh, C.-W.; Su, K.-C.; Wang, C.-H.; Chao, T.-H.; Chiu, Y.-T.; Wu, C.-C.; Hung, S.-C. Lessons Learned from Various 3D-Printed Tracheal Grafts in an Extensive Porcine Model for De Novo Tracheal Regeneration. Ann. Thorac. Surg. 2025, in press. [Google Scholar] [CrossRef]
- Jung, S.Y.; Tran, A.N.-T.; Kim, H.Y.; Choi, E.; Lee, S.J.; Kim, H.S. Development of Acellular Respiratory Mucosal Matrix Using Porcine Tracheal Mucosa. Tissue Eng. Regen. Med. 2020, 17, 433–443. [Google Scholar] [CrossRef]
- Wu, W.; Jia, S.; Chen, W.; Liu, X.; Zhang, S. Fast Degrading Elastomer Stented Fascia Remodels into Tough and Vascularized Construct for Tracheal Regeneration. Mater. Sci. Eng. C 2019, 101, 1–14. [Google Scholar] [CrossRef]
- Rehmani, S.S.; Al-Ayoubi, A.M.; Ayub, A.; Barsky, M.; Lewis, E.; Flores, R.; Lebovics, R.; Bhora, F.Y. Three-Dimensional-Printed Bioengineered Tracheal Grafts: Preclinical Results and Potential for Human Use. Ann. Thorac. Surg. 2017, 104, 998–1004. [Google Scholar] [CrossRef]
- Ott, L.M.; Vu, C.H.; Farris, A.L.; Fox, K.D.; Galbraith, R.A.; Weiss, M.L.; Weatherly, R.A.; Detamore, M.S. Functional Reconstruction of Tracheal Defects by Protein-Loaded, Cell-Seeded, Fibrous Constructs in Rabbits. Tissue Eng. Part A 2015, 21, 2390–2403. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, H.R.; Yu, S.J.; Han, M.-E.; Lee, D.Y.; Kim, S.Y.; Ahn, H.-J.; Han, M.-J.; Lee, T.-I.; Kim, T.-S.; et al. Hydrogel-Laden Paper Scaffold System for Origami-Based Tissue Engineering. Proc. Natl. Acad. Sci. USA 2015, 112, 15426–15431. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, J.H.; Cho, D.-W. Comparison of Tracheal Reconstruction with Allograft, Fresh Xenograft and Artificial Trachea Scaffold in a Rabbit Model. J. Artif. Organs 2018, 21, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, Y.; Li, Y.; Gong, X.; Wei, M.; Zhang, W.; Zhang, X.; Xu, Y. Nanofibrous Wharton’s Jelly Scaffold in Combination with Adipose-Derived Stem Cells for Cartilage Engineering. Mater. Des. 2020, 186, 108216. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, J.S.; Jung, H.; Kim, D.Y.; Kim, S.W.; Sultan, M.T.; Park, C.H. An Omentum-Cultured 3D-Printed Artificial Trachea: In Vivo Bioreactor. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1131–S1140. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Bai, B.; Li, K.; Wei, X.; Wang, Y.; Jia, Z.; Ma, Y.; Zhang, Y.; Ji, G.; Zhou, G.; et al. Epithelialized Chimeric Repairing Patch Supports in Situ Healing of Tracheal Fistula. Sci. Adv. 2025, 11, eadt1320. [Google Scholar] [CrossRef]
- Lu, Y.; Shan, Y.; Zhu, J.; Shen, Z.; Chen, W.; Chen, H.; Shi, H. Enhancing Epithelial Regeneration with Gelatin Methacryloyl Hydrogel Loaded with Extracellular Vesicles Derived from Adipose Mesenchymal Stem Cells for Decellularized Tracheal Patching. Int. J. Biol. Macromol. 2025, 284, 137927. [Google Scholar] [CrossRef]
- Umeda, S.; Nakayama, Y.; Terazawa, T.; Iwai, R.; Hiwatashi, S.; Nakahata, K.; Takama, Y.; Okuyama, H. Long-Term Outcomes of Patch Tracheoplasty Using Collagenous Tissue Membranes (Biosheets) Produced by in-Body Tissue Architecture in a Beagle Model. Surg. Today 2019, 49, 958–964. [Google Scholar] [CrossRef]
- Kim, S.H.; Seo, Y.B.; Yeon, Y.K.; Lee, Y.J.; Park, H.S.; Sultan, M.T.; Lee, J.M.; Lee, J.S.; Lee, O.J.; Hong, H.; et al. 4D-Bioprinted Silk Hydrogels for Tissue Engineering. Biomaterials 2020, 260, 120281. [Google Scholar] [CrossRef]
- Rein, A.; da Costa, M.C.; Montanhin, G.; Fernandes, G.; Leite, M.D.A.; Carra, G.J.U.; Vasconcelos, R.d.O.; Dias, L.G.G.G.; Rocha, T.A.S.d.S.; Moraes, P.C. 3D Implant of Copolyamide Associated with Thermoplastic Elastomer (PCTPE) for Tracheal Repair in Rabbits (Oryctolagus cuniculus): Preliminary Study. Ciência Anim. Bras. 2024, 25, 76225E. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Zheng, C.; Zhao, C.; Shi, H.; Pan, S.; Zhang, W. Biocompatibility and Cellular Compatibility of Decellularized Tracheal Matrix Derived from Rabbits. Int. J. Artif. Organs 2019, 42, 500–507. [Google Scholar] [CrossRef]
- She, Y.; Fan, Z.; Wang, L.; Li, Y.; Sun, W.; Tang, H.; Zhang, L.; Wu, L.; Zheng, H.; Chen, C. 3D Printed Biomimetic PCL Scaffold as Framework Interspersed With Collagen for Long Segment Tracheal Replacement. Front. Cell Dev. Biol. 2021, 9, 629796. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Lin, X.; Wang, S.; Zhang, X.; Pang, Y.; Li, S.; Yu, T.; Fan, S.; Zhao, F. An Injectable Nucleus Pulposus Cell-Modified Decellularized Scaffold: Biocompatible Material for Prevention of Disc Degeneration. Oncotarget 2017, 8, 40276–40288. [Google Scholar] [CrossRef] [PubMed]
- Best, C.A.; Pepper, V.K.; Ohst, D.; Bodnyk, K.; Heuer, E.; Onwuka, E.A.; King, N.; Strouse, R.; Grischkan, J.; Breuer, C.K.; et al. Designing a Tissue-Engineered Tracheal Scaffold for Preclinical Evaluation. Int. J. Pediatr. Otorhinolaryngol. 2018, 104, 155–160. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, F.; Shen, Y.; Ding, Q.; Wang, Y.; Liang, L.; Dai, W.; Chen, Y. Temporal Control in Shell-Core Structured Nanofilm for Tracheal Cartilage Regeneration: Synergistic Optimization of Anti-Inflammation and Chondrogenesis. Regen. Biomater. 2024, 11, rbae040. [Google Scholar] [CrossRef]
- Gao, B.; Jing, H.; Gao, M.; Wang, S.; Fu, W.; Zhang, X.; He, X.; Zheng, J. Long-Segmental Tracheal Reconstruction in Rabbits with Pedicled Tissue-Engineered Trachea Based on a 3D-Printed Scaffold. Acta Biomater. 2019, 97, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Chen, Y.; Wu, Y.; Zang, M.; Largo, R.D.; Chang, E.I.; Schaverien, M.V.; Yu, P.; Zhang, Q. Pre-Epithelialized Cryopreserved Tracheal Allograft for Neo-Trachea Flap Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1196521. [Google Scholar] [CrossRef]
- de Wit, R.J.J.; Tiemessen, D.; Oosterwijk, E.; Verhagen, A.F.T.M. Functional Outcome of Cell Seeded Tracheal Scaffold after Mechanical Stress in Vitro. Biomater. Adv. 2025, 167, 214088. [Google Scholar] [CrossRef]
- Den Hondt, M.; Vanaudenaerde, B.M.; Maughan, E.F.; Butler, C.R.; Crowley, C.; Verbeken, E.K.; Verleden, S.E.; Vranckx, J.J. An Optimized Non-Destructive Protocol for Testing Mechanical Properties in Decellularized Rabbit Trachea. Acta Biomater. 2017, 60, 291–301. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, J.S.; Eom, M.R.; Jo, H.H.; Kwon, I.K.; Kwon, S.K.; Park, S.A. Dexamethasone Loaded Bilayered 3D Tubular Scaffold Reduces Restenosis at the Anastomotic Site of Tracheal Replacement: In Vitro and in Vivo Assessments. Nanoscale 2020, 12, 4846–4858. [Google Scholar] [CrossRef]
- Kreber, L.; Liu, L.; Dharmadhikari, S.; Tan, Z.H.; Chan, C.; Huddle, J.; Hussein, Z.; Shontz, K.; Breuer, C.K.; Johnson, J.; et al. Assessing the Biocompatibility and Regeneration of Electrospun-Nanofiber Composite Tracheal Grafts. Laryngoscope 2024, 134, 1155–1162. [Google Scholar] [CrossRef]
- Sun, F.; Jiang, Y.; Xu, Y.; Shi, H.; Zhang, S.; Liu, X.; Pan, S.; Ye, G.; Zhang, W.; Zhang, F.; et al. Genipin Cross-Linked Decellularized Tracheal Tubular Matrix for Tracheal Tissue Engineering Applications. Sci. Rep. 2016, 6, 24429. [Google Scholar] [CrossRef]
- Gao, E.; Wang, P.; Chen, F.; Xu, Y.; Wang, Q.; Chen, H.; Jiang, G.; Zhou, G.; Li, D.; Liu, Y.; et al. Skin-Derived Epithelial Lining Facilitates Orthotopic Tracheal Transplantation by Protecting the Tracheal Cartilage and Inhibiting Granulation Hyperplasia. Biomater. Adv. 2022, 139, 213037. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Z.; Wang, X.; Ni, Y.; Liu, Y.; Liu, T.; Wang, W.; Xing, H.; Sun, Y.; Wang, J.; et al. PLGA-PTMC-Cultured Bone Mesenchymal Stem Cell Scaffold Enhances Cartilage Regeneration in Tissue-Engineered Tracheal Transplantation. Artif. Organs 2017, 41, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Den Hondt, M.; Vanaudenaerde, B.M.; Verbeken, E.K.; Vranckx, J.J. Tracheal Tissue-Engineering: In-Vivo Biocompatibility of Mechanically-Stripped Allogenic Rabbit Trachea with Autologous Epithelial Covering. Acta Chir. Belg. 2016, 116, 164–174. [Google Scholar] [CrossRef]
- Fayzullin, A.; Vladimirov, G.; Kuryanova, A.; Gafarova, E.; Tkachev, S.; Kosheleva, N.; Istranova, E.; Istranov, L.; Efremov, Y.; Novikov, I.; et al. A Defined Road to Tracheal Reconstruction: Laser Structuring and Cell Support for Rapid Clinic Translation. Stem Cell Res. Ther. 2022, 13, 317. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Mammana, M.; Zambello, G.; Contran, M.; Parnigotto, P.P.; Macchi, V.; Conconi, M.T.; Rea, F.; De Caro, R.; et al. Preclinical and Clinical Orthotopic Transplantation of Decellularized/Engineered Tracheal Scaffolds: A Systematic Literature Review. J. Tissue Eng. 2023, 14, 20417314231151824. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, D.; Yin, Z.; He, A.; Lin, M.; Jiang, G.; Song, X.; Hu, X.; Liu, Y.; Wang, J.; et al. Tissue-Engineered Trachea Regeneration Using Decellularized Trachea Matrix Treated with Laser Micropore Technique. Acta Biomater. 2017, 58, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-W.; Lee, K.-W.; Park, J.-H.; Lee, J.; Jung, C.-R.; Yu, J.; Kim, H.-Y.; Kim, D.-H. 3D Bioprinted Artificial Trachea with Epithelial Cells and Chondrogenic-Differentiated Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 1624. [Google Scholar] [CrossRef]
- Jung, S.Y.; Lee, S.J.; Kim, H.Y.; Park, H.S.; Wang, Z.; Kim, H.J.; Yoo, J.J.; Chung, S.M.; Kim, H.S. 3D Printed Polyurethane Prosthesis for Partial Tracheal Reconstruction: A Pilot Animal Study. Biofabrication 2016, 8, 045015. [Google Scholar] [CrossRef]
- Satake, R.; Komura, M.; Komura, H.; Kodaka, T.; Terawaki, K.; Ikebukuro, K.; Komuro, H.; Yonekawa, H.; Hoshi, K.; Takato, T.; et al. Patch Tracheoplasty in Body Tissue Engineering Using Collagenous Connective Tissue Membranes (Biosheets). J. Pediatr. Surg. 2016, 51, 244–248. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, W.; Yin Pan, Z.; Pan, S.; Zhang, S.Q.; Hao Wang, Z.; Gu, S.; Shi, H. In Vivo Transplantation of Stem Cells with a Genipin Linked Scaffold for Tracheal Construction. J. Biomater. Appl. 2019, 34, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Chang, J.W.; Jang, W.S.; Seo, Y.J.; Kang, M.-L.; Sung, H.-J.; Kim, D.H.; Kim, J.M.; Park, J.H.; Ban, M.J.; et al. Tracheal Reconstruction with a Free Vascularized Myofascial Flap: Preclinical Investigation in a Porcine Model to Human Clinical Application. Sci. Rep. 2017, 7, 10022. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Hillinger, S.; van Blitterswijk, C.A.; Weder, W. Intra-Scaffold Continuous Medium Flow Combines Chondrocyte Seeding and Culture Systems for Tissue Engineered Trachea Construction. Interact. Cardiovasc. Thorac. Surg. 2009, 8, 27–30. [Google Scholar] [CrossRef]
- Yin, H.; Wang, J.; Gu, Z.; Feng, W.; Gao, M.; Wu, Y.; Zheng, H.; He, X.; Mo, X. Evaluation of the Potential of Kartogenin Encapsulated Poly(L-Lactic Acid-Co-Caprolactone)/Collagen Nanofibers for Tracheal Cartilage Regeneration. J. Biomater. Appl. 2017, 32, 331–341. [Google Scholar] [CrossRef]
- Kim, I.G.; Park, S.A.; Lee, S.-H.; Choi, J.S.; Cho, H.; Lee, S.J.; Kwon, Y.-W.; Kwon, S.K. Transplantation of a 3D-Printed Tracheal Graft Combined with IPS Cell-Derived MSCs and Chondrocytes. Sci. Rep. 2020, 10, 4326. [Google Scholar] [CrossRef]
- Jing, H.; Gao, B.; Gao, M.; Yin, H.; Mo, X.; Zhang, X.; Luo, K.; Feng, B.; Fu, W.; Wang, J.; et al. Restoring Tracheal Defects in a Rabbit Model with Tissue Engineered Patches Based on TGF-Β3-Encapsulating Electrospun Poly(l-Lactic Acid-Co-ε-Caprolactone)/Collagen Scaffolds. Artif. Cells Nanomed. Biotechnol. 2025, 46, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, H.; Dong, W.; Bai, J.; Gao, B.; Xia, D.; Feng, B.; Chen, M.; He, X.; Yin, M.; et al. Tissue-Engineered Trachea from a 3D-Printed Scaffold Enhances Whole-Segment Tracheal Repair. Sci. Rep. 2017, 7, 5246. [Google Scholar] [CrossRef]
- Maughan, E.F.; Butler, C.R.; Crowley, C.; Teoh, G.Z.; den Hondt, M.; Hamilton, N.J.; Hynds, R.E.; Lange, P.; Ansari, T.; Urbani, L.; et al. A Comparison of Tracheal Scaffold Strategies for Pediatric Transplantation in a Rabbit Model. Laryngoscope 2017, 127, E449–E457. [Google Scholar] [CrossRef]
- Galvez Alegria, C.; Gundogdu, G.; Yang, X.; Costa, K.; Mauney, J.R. Evaluation of Acellular Bilayer Silk Fibroin Grafts for Onlay Tracheoplasty in a Rat Defect Model. Otolaryngol. Head. Neck Surg. 2019, 160, 310–319. [Google Scholar] [CrossRef]
- Pepper, V.K.; Best, C.; Buckley, K.; Schwartz, C.M.; Onwuka, E.A.; King, N.; White, A.; Dharmadhikari, S.; Reynolds, S.D.; Johnson, J.; et al. Factors Influencing Poor Outcomes in Synthetic Tissue-Engineered Tracheal Replacement. Otolaryngol.-Head Neck Surg. 2019, 161, 458–467. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Tian, L.; He, X.; Gao, Q.; Wu, T.; Ramakrishna, S.; Zheng, J.; Mo, X. Evaluation of the Potential of RhTGF-Β3 Encapsulated P(LLA-CL)/Collagen Nanofibers for Tracheal Cartilage Regeneration Using Mesenchymal Stem Cells Derived from Wharton’s Jelly of Human Umbilical Cord. Mater. Sci. Eng. C 2017, 70, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Batioglu-Karaaltin, A.; Karaaltin, M.V.; Ovali, E.; Yigit, O.; Kongur, M.; Inan, O.; Bozkurt, E.; Cansiz, H. In Vivo Tissue-Engineered Allogenic Trachea Transplantation in Rabbits: A Preliminary Report. Stem Cell Rev. Rep. 2015, 11, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, D.; Yin, Z.; Luo, X.; Liu, W.; Zhang, W.; Zhang, Z.; Cao, Y.; Liu, Y.; Zhou, G. Prolonged in Vitro Precultivation Alleviates Post-Implantation Inflammation and Promotes Stable Subcutaneous Cartilage Formation in a Goat Model. Biomed. Mater. 2016, 12, 015006. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, S.; Nakayama, Y.; Umeda, S.; Takama, Y.; Terazawa, T.; Okuyama, H. Tracheal Replacement Using an In-Body Tissue-Engineered Collagenous Tube “BIOTUBE” with a Biodegradable Stent in a Beagle Model: A Preliminary Report on a New Technique. Eur. J. Pediatr. Surg. 2018, 29, 090–096. [Google Scholar]
- Xie, X.; Miao, B.; Yao, J.; Chen, Z. Silk Fibroin-Hydroxyapatite Scaffolds Promote the Proliferation of Adipose-Derived Mesenchymal Stem Cells by Activating the ERK Signal. J. Biomater. Appl. 2023, 37, 1767–1775. [Google Scholar] [CrossRef]
- Hung, S.-H.; Su, C.-H.; Lin, S.-E.; Tseng, H. Preliminary Experiences in Trachea Scaffold Tissue Engineering with Segmental Organ Decellularization. Laryngoscope 2016, 126, 2520–2527. [Google Scholar] [CrossRef]
- Torsello, M.; Salvati, A.; Borro, L.; Meucci, D.; Tropiano, M.L.; Cialente, F.; Secinaro, A.; Del Fattore, A.; Emiliana, C.M.; Francalanci, P.; et al. 3D Bioprinting in Airway Reconstructive Surgery: A Pilot Study. Int. J. Pediatr. Otorhinolaryngol. 2022, 161, 111253. [Google Scholar] [CrossRef]
- Tan, Z.H.; Dharmadhikari, S.; Liu, L.; Wolter, G.; Shontz, K.M.; Reynolds, S.D.; Johnson, J.; Breuer, C.K.; Chiang, T. Tracheal Macrophages During Regeneration and Repair of Long-Segment Airway Defects. Laryngoscope 2022, 132, 737–746. [Google Scholar] [CrossRef]
- Griffin, C.; Choong, W.Y.; Teh, W.; Buxton, A.J.; Bolton, P.S. Head and Cervical Spine Posture in Behaving Rats: Implications for Modeling Human Conditions Involving the Head and Cervical Spine. Anat. Rec. 2015, 298, 455–462. [Google Scholar] [CrossRef]
- Ten Hallers, E.J.O.; Marres, H.A.M.; Rakhorst, G.; Jansen, J.A.; Sommers, M.G.; Van Der Houwen, E.B.; Schutte, H.K.; Van Kooten, T.G.; Van Loon, J.P.; Verkerke, G.J. The Saanen Goat as an Animal Model for Post-Laryngectomy Research: Practical Implications. Lab. Anim. 2007, 41, 270–284. [Google Scholar] [CrossRef]
- Barone, R. (Ed.) Anatomie Comparée Des Mammifères Domestiques Tome 3 Splanchnologie I—Robert Barone, 4th ed.; BARONNE: Paris, France, 1996; Volume 3, ISBN 2711490122. [Google Scholar]
- Safshekan, F.; Tafazzoli-Shadpour, M.; Abdouss, M.; Shadmehr, M.B. Mechanical Characterization and Constitutive Modeling of Human Trachea: Age and Gender Dependency. Materials 2016, 9, 456. [Google Scholar] [CrossRef]
- Samat, A.A.; Hamid, Z.A.A.; Yahaya, B.H. Tissue Engineering for Tracheal Replacement: Strategies and Challenges. In Advances in Mesenchymal Stem Cells and Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Frejo, L.; Goldstein, T.; Swami, P.; Patel, N.A.; Grande, D.A.; Zeltsman, D.; Smith, L.P. A Two-Stage in Vivo Approach for Implanting a 3D Printed Tissue-Engineered Tracheal Replacement Graft: A Proof of Concept. Int. J. Pediatr. Otorhinolaryngol. 2022, 155, 111066. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, J.; Chen, Y.; Lin, W.; Tang, H.; Fan, Z.; Wang, L.; She, Y.; Jin, F.; Zhang, L.; et al. Scaffold-Free tracheal Engineering via a Modular Strategy Based on Cartilage and Epithelium Sheets. Adv. Healthc. Mater. 2023, 12, e2202022. [Google Scholar] [CrossRef] [PubMed]
- Khalid, U.; Uchikov, P.; Hristov, B.; Kraev, K.; Koleva-Ivanova, M.; Kraeva, M.; Batashki, A.; Taneva, D.; Doykov, M.; Uchikov, A. Surgical Innovations in Tracheal Reconstruction: A Review on Synthetic Material Fabrication. Medicina 2024, 60, 40. [Google Scholar] [CrossRef] [PubMed]
- Shai, S.E.; Lai, Y.L.; Hung, Y.W.; Hsieh, C.W.; Su, K.C.; Wang, C.H.; Chao, T.H.; Chiu, Y.T.; Wu, C.C.; Hung, S.C. Long-Term Survival and Regeneration Following Transplantation of 3D-Printed Biodegradable PCL Tracheal Grafts in Large-Scale Porcine Models. Bioengineering 2024, 11, 832. [Google Scholar] [CrossRef]
- Skardal, A.; Murphy, S.V.; Devarasetty, M.; Mead, I.; Kang, H.W.; Seol, Y.J.; Zhang, Y.S.; Shin, S.R.; Zhao, L.; Aleman, J.; et al. Multi-Tissue Interactions in an Integrated Three-Tissue Organ-on-a-Chip Platform. Sci. Rep. 2017, 7, 8837. [Google Scholar] [CrossRef]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and Its Applications in Tissue Engineering and Regenerative Medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef]
- Kalwar, K.; Sun, W.X.; Li, D.L.; Zhang, X.J.; Shan, D. Coaxial Electrospinning of Polycaprolactone@chitosan: Characterization and Silver Nanoparticles Incorporation for Antibacterial Activity. React. Funct. Polym. 2016, 107, 87–92. [Google Scholar] [CrossRef]
- Jamieson, C.; Keenan, P.; Kirkwood, D.; Oji, S.; Webster, C.; Russell, K.A.; Koch, T.G. A Review of Recent Advances in 3D Bioprinting With an Eye on Future Regenerative Therapies in Veterinary Medicine. Front. Vet. Sci. 2021, 7, 584193. [Google Scholar] [CrossRef]
- Lei, C.; Mei, S.; Zhou, C.; Xia, C. Decellularized Tracheal Scaffolds in Tracheal Reconstruction: An Evaluation of Different Techniques. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211064948. [Google Scholar] [CrossRef]
- Galliger, Z.; Vogt, C.D.; Helms, H.R.; Panoskaltsis-Mortari, A. Extracellular Matrix Microparticles Improve GelMA Bioink Resolution for 3D Bioprinting at Ambient Temperature. Macromol. Mater. Eng. 2022, 307, 2200196. [Google Scholar] [CrossRef]
- Park, J.H.; Ahn, M.; Park, S.H.; Kim, H.; Bae, M.; Park, W.; Hollister, S.J.; Kim, S.W.; Cho, D.W. 3D Bioprinting of a Trachea-Mimetic Cellular Construct of a Clinically Relevant Size. Biomaterials 2021, 279, 121246. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Sing, S.L.; Zhou, M.; Yeong, W.Y. 3D Bioprinting Processes: A Perspective on Classification and Terminology. Int. J. Bioprint. 2018, 4, 151. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, Y.M.; Lai, Y.L.; Lee, S.Y. Mechanical Properties, Accuracy, and Cytotoxicity of UV-Polymerized 3D Printing Resins Composed of Bis-EMA, UDMA, and TEGDMA. J. Prosthet. Dent. 2020, 123, 349–354. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Goering, P.L.; Elespuru, R.K.; Das, S.S.; Narayan, R.J. The Photoinitiator Lithium Phenyl (2,4,6-Trimethylbenzoyl) Phosphinate with Exposure to 405 Nm Light Is Cytotoxic to Mammalian Cells but Not Mutagenic in Bacterial Reverse Mutation Assays. Polymers 2020, 12, 1489. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Ghadiri, E.; Ramesh, H.; Kengla, C.; Kassis, J.; Calvert, P.; Williams, D.; Khademhosseini, A.; Narayan, R.; Forgacs, G.; et al. Physics of Bioprinting. Appl. Phys. Rev. 2019, 6, 021315. [Google Scholar] [CrossRef]
- Phillippi, J.A.; Miller, E.; Weiss, L.; Huard, J.; Waggoner, A.; Campbell, P. Microenvironments Engineered by Inkjet Bioprinting Spatially Direct Adult Stem Cells Toward Muscle-and Bone-Like Subpopulations. Stem Cells 2008, 26, 127–134. [Google Scholar] [CrossRef]
- Walczak, R.; Adamski, K. Inkjet 3D Printing of Microfluidic Structures—On the Selection of the Printer towards Printing Your Own Microfluidic Chips. J. Micromech. Microeng. 2015, 25, 085013. [Google Scholar] [CrossRef]
- Williams, D.; Thayer, P.; Martinez, H.; Gatenholm, E.; Khademhosseini, A. A Perspective on the Physical, Mechanical and Biological Specifications of Bioinks and the Development of Functional Tissues in 3D Bioprinting. Bioprinting 2018, 9, 19–36. [Google Scholar] [CrossRef]
- Sun, Y.; Huo, Y.; Ran, X.; Chen, H.; Pan, Q.; Chen, Y.; Zhang, Y.; Ren, W.; Wang, X.; Zhou, G.; et al. Instant Trachea Reconstruction Using 3D-Bioprinted C-Shape Biomimetic Trachea Based on Tissue-Specific Matrix Hydrogels. Bioact. Mater. 2024, 32, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Yi, H.; Est-Witte, S.; George, S.; Kengla, C.; Lee, S.J.; Atala, A.; Murphy, S.V. Bioprinted Trachea Constructs with Patient Matched Design, Mechanical and Biological Properties. Mater. Today Proc. 2020, 12, 16–20. [Google Scholar] [CrossRef]
- Frejo, L.; Grande, D.A. 3D-Bioprinted Tracheal Reconstruction: An Overview. Bioelectron. Med. 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Whitaker, I.S. To Print or Not to Print, That Is the Question: How Close Are We to Clinical Translation of Contemporary Bioinks? J. 3D Print. Med. 2018, 2, 1–3. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D Bioprinting System to Produce Human-Scale Tissue Constructs with Structural Integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Choe, G.; Lee, M.; Oh, S.; Seok, J.M.; Kim, J.; Im, S.; Park, S.A.; Lee, J.Y. Three-Dimensional Bioprinting of Mesenchymal Stem Cells Using an Osteoinductive Bioink Containing Alginate and BMP-2-Loaded PLGA Nanoparticles for Bone Tissue Engineering. Biomater. Adv. 2022, 136, 212789. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing Three-Dimensional Tissue Analogues with Decellularized Extracellular Matrix Bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Zolfagharian, A.; Bodaghi, M. 4D Bioprinting of Smart Polymers for Biomedical Applications: Recent Progress, Challenges, and Future Perspectives. React. Funct. Polym. 2022, 179, 105374. [Google Scholar] [CrossRef]
- Chiesa, I.; Esposito, A.; Vozzi, G.; Gottardi, R.; De Maria, C. 4D Bioprinted Self-Folding Scaffolds Enhance Cartilage Formation in the Engineering of Trachea. Adv. Mater. Res. 2025, 10, 2401210. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Xia, T.; Wan, H.; Lu, Y.; Ding, C.; Zhang, F.; Shen, Z.; Pan, S. The Effect of Bioactivity of Airway Epithelial Cells Using Methacrylated Gelatin Scaffold Loaded with Exosomes Derived from Bone Marrow Mesenchymal Stem Cells. J. Biomed. Mater. Res. A 2024, 112, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.C.; Lynch, T.J.; Ahlers, B.A.; Ievlev, V.; Engelhardt, J.F.; Parekh, K.R. A Novel Bioreactor for Reconstitution of the Epithelium and Submucosal Glands in Decellularized Ferret Tracheas. Cells 2022, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Matias, G.d.S.S. Recellularization of Biological Scaffolds from Canine Trachea for Tissue Bioengineering; Biblioteca Digitais de Teses e Dissertação da USP: São Paulo, Brazil, 2021. [Google Scholar]
- Dang, L.H.; Hung, S.-H.; Tseng, Y.; Quang, L.X.; Le, N.T.N.; Fang, C.-L.; Tseng, H. Partial Decellularized Scaffold Combined with Autologous Nasal Epithelial Cell Sheet for Tracheal Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 10322. [Google Scholar] [CrossRef]
- Cheng, S.F.; Wu, S.; Li, Q.P.; Sang, H.Y.; Fan, Z.Y. Airway Reconstruction Using Decellularized Aortic Xenografts in a Dog Model. Organogenesis 2020, 16, 73–82. [Google Scholar] [CrossRef]
- Viet-Nhi, N.K.; Chen, Y.C.; Dang, L.H.; Tseng, H.; Hung, S.H. Degassing a Decellularized Scaffold Enhances Wound Healing and Reduces Fibrosis during Tracheal Defect Reconstruction: A Preliminary Animal Study. J. Funct. Biomater. 2023, 14, 147. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
| Stage | Description |
|---|---|
| Review Type | Scoping review following the PRISMA-ScR protocol. |
| Databases | PubMed, Google Scholar, VHL, LILACS, SciELO, VETLib, Semantic Scholar. |
| Timeframe | 2015–2025 |
| Inclusion Criteria | Experimental studies with animal models or in vitro, focused on tracheal substitutes and bioengineering. |
| Exclusion Criteria | Reviews, studies without empirical data, or unrelated to the trachea. |
| Selection Process | PRISMA screening (duplicate removal, evaluation by two reviewers, full-text reading). |
| Data Extraction | Organized into a matrix (author, year, animal model, scaffold, method, results). |
| Bias Analysis | Consideration of publication bias, language bias, and methodological heterogeneity. |
| Registration | Protocol registered in PROSPERO. |
| Scaffold Type | Example Biomaterials | Fabrication Methods | Biomechanical Properties (Advantages/Limitations) | Observed Biocompatibility | Vascularization or Cellular Integration | Animal Model Used |
|---|---|---|---|---|---|---|
| Synthetic | Poly(caprolactone) (PCL)([-O-(CH2)5-CO-]n), polyurethane ([-OC-NH-R-NH-CO-O-R’-O-]n), thermoplastic elastomers (PCU), POSS-PCU, PET/PU ([-O-CH2-CH2-O-CO-C6H4-CO-]n) | 3D printing, electrospinning, injection molding | Advantages: High dimensional customization; strong mechanical resistance. Limitations: Excessive stiffness, risk of granulation tissue formation, variable foreign body reaction. | Moderate to low; depends on surface treatment and chemical composition. May trigger chronic inflammation. | Requires angiogenic factor modification or stem cell addition to improve integration. Poor intrinsic vascularization. | Rabbits, rats, sheep |
| Decellularized | ECM from porcine, canine, rabbit tracheas; bladder/mucosa matrix | Chemical (SDS, Triton X-100, SLES), enzymatic (DNase, RNase), physical (ultrasound, freeze–thaw cycles, agitation) | Advantages: Preserves natural structure; retains native ECM cues. Limitations: Structural weakening risk; possible immunogenicity if incomplete decellularization. | High with complete DNA/antigen removal. Lower immune risk when combined with DNase/RNase. | Good integration when pre-seeded with mesenchymal or epithelial cells. Vascularization dependent on implantation strategy. | Rabbits, mice, dogs, ferrets, pig |
| Hybrid | Hydrogels (gelMA, collagen, alginate), PCL-hydrogel combinations with embedded cells | Extrusion bioprinting, inkjet, SLA, DLP, FDM | Advantages: Highly customizable; supports native-like cell distribution. Limitations: Lower initial mechanical strength; long-term viability challenges. | Excellent; live cells improve biocompatibility. Low immune rejection with autologous/immunoprivileged cells. | High cellular integration potential; vascularization possible with suitable bioinks or angiogenic strategies. | Rabbits, ferrets |
| Source | Tracheal Substitute | Biomaterial Characterization | Relevant Results | Biocompatibility Findings |
|---|---|---|---|---|
| [20] | Hybrid scaffolds of thermoresponsive elastomer (PUU-POSS) and type I collagen, fabricated via TIPS and 3D printing | SEM; Confocal microscopy: collagen distribution; Mechanical analysis; Contact angle testing: surface hydrophilicity; XPS; quantification of structural characteristics. | 3D-TIPS scaffolds with collagen improved hydrophilicity, supported epithelial differentiation, and formed a functional barrier with high TEER in coculture. | Scaffolds supported cell adhesion, proliferation, and differentiation, while coculture promoted a mature epithelium with tight junctions, barrier function, and mucus production, indicating potential for mucociliary function. |
| [19] | Electrospun nanofibers of poly(ε-caprolactone) (PCL) and depolymerized chitosan. | SEM, XRD, FTIR, tensile testing, contact angle analysis. | Successful nanofiber fabrication; chitosan influenced mechanical properties and hydrophilicity. | Good cell adhesion, no cytotoxicity, and maintained cell morphology. |
| [21] | Bioinspired laryngotracheal patch with PCL-PEG and dexamethasone release. | SEM, laser profilometry, mechanical testing, mucoadhesion, cytotoxicity. | Higher PCL particle density enhanced strength and adhesion. | Biocompatible and promising for tracheal applications. |
| [22] | Decellularized ECMs from porcine trachea and bladder. | DNA quantification, histology, immunohistochemistry, protein quantification. | HBECs exhibited better growth and differentiation on porcine tracheal ECM. | Both ECMs were biocompatible, but the tracheal ECM promoted superior differentiation. |
| [16] | Decellularized extracellular matrix (dECM) from porcine trachea | DNA quantification, histology, structural analysis | Efficient decellularization (>95% DNA removal) with preserved extracellular matrix | Good matrix preservation, indicating potential for biocompatibility. |
| [18] | Decellularized hybrid grafts biofabricated in 3D (PCL + gamma radiation) | Compressive tesing, histology | Decellularized scaffolds showed lower compressive strength; gamma radiation affected mechanical properties | Histology confirmed cell removal; the combination of 3D bioprinting and decellularized ECM is promising |
| [15] | Decellularized rabbit tracheal scaffold | DNA analysis, biochemical assays, histology, polarized microscopy | Effective protocol for cell removal while preserving ECM; protocol variations affected ECM integrity | Decellularization removes immunogenic components while preserving ECM; potential for clinical use |
| [23] | Decellularized rabbit tracheal biomatrix | Histology, DNA and GAG quantification, SEM, and biomechanical tests | Decellularization removed cellular components while preserving structure and ECM; slight reduction in tensile strength | Potential as a biocompatible scaffold; in vivo studies needed for confirmation |
| [24] | Porcine tracheas subjected to different decellularization methods, including supercritical carbon dioxide (scCO2) | Biomaterial characterization after decellularization | Decellularization and sterilization with scCO2 preserved the extracellular matrix, glycosaminoglycans (GAGs), and collagen structure, maintaining suitable mechanical | Scaffolds supported cell adhesion, proliferation, and viability, with efficient DNA removal and GAG retention, indicating a promising method for biomaterials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, A.C.; Machado Holzlsauer, G.; Ruiz Lucio de Lima Parra, J.P.; Querino Candelária, R.A.; Santos da Silva, T.; da Silva Nunes Barreto, R.; Miglino, M.A. Organic and Synthetic Substitutes in Tracheal Reconstruction: A Scoping Review (2015–2025). Bioengineering 2025, 12, 704. https://doi.org/10.3390/bioengineering12070704
dos Santos AC, Machado Holzlsauer G, Ruiz Lucio de Lima Parra JP, Querino Candelária RA, Santos da Silva T, da Silva Nunes Barreto R, Miglino MA. Organic and Synthetic Substitutes in Tracheal Reconstruction: A Scoping Review (2015–2025). Bioengineering. 2025; 12(7):704. https://doi.org/10.3390/bioengineering12070704
Chicago/Turabian Styledos Santos, Ana Caroline, Guilherme Machado Holzlsauer, João Paulo Ruiz Lucio de Lima Parra, Raí André Querino Candelária, Thamires Santos da Silva, Rodrigo da Silva Nunes Barreto, and Maria Angelica Miglino. 2025. "Organic and Synthetic Substitutes in Tracheal Reconstruction: A Scoping Review (2015–2025)" Bioengineering 12, no. 7: 704. https://doi.org/10.3390/bioengineering12070704
APA Styledos Santos, A. C., Machado Holzlsauer, G., Ruiz Lucio de Lima Parra, J. P., Querino Candelária, R. A., Santos da Silva, T., da Silva Nunes Barreto, R., & Miglino, M. A. (2025). Organic and Synthetic Substitutes in Tracheal Reconstruction: A Scoping Review (2015–2025). Bioengineering, 12(7), 704. https://doi.org/10.3390/bioengineering12070704







