Different Expression of Vascularization and Inflammatory Regulators in Cells Derived from Oral Mucosa and Limbus
Abstract
1. Introduction
2. Materials and Methods
2.1. Donor Tissue
2.2. Cell Culture, Growth, and Viability
2.3. Cell Lysis and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.4. Statistical Analysis
3. Results
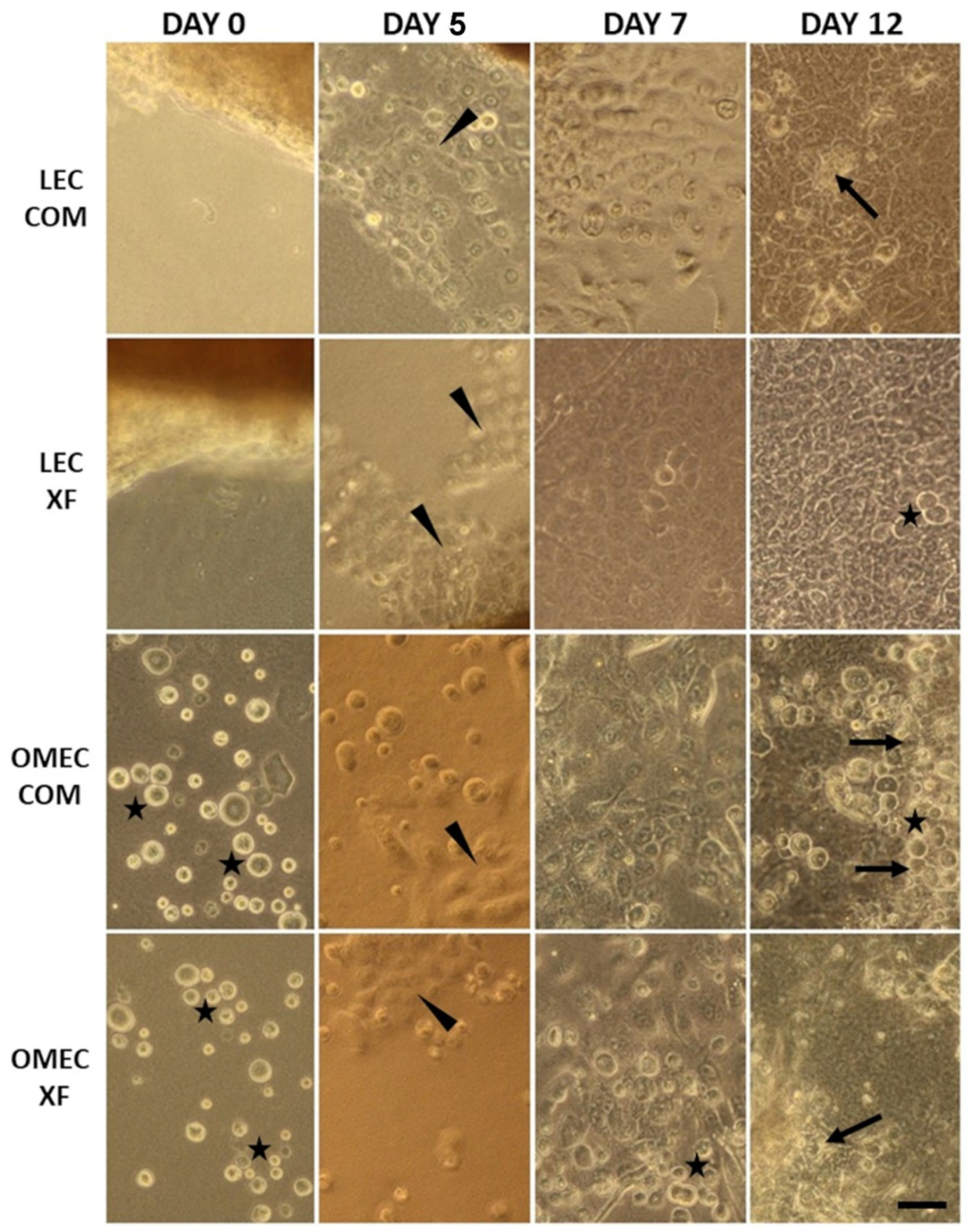
3.1. Cell Growth and Morphology
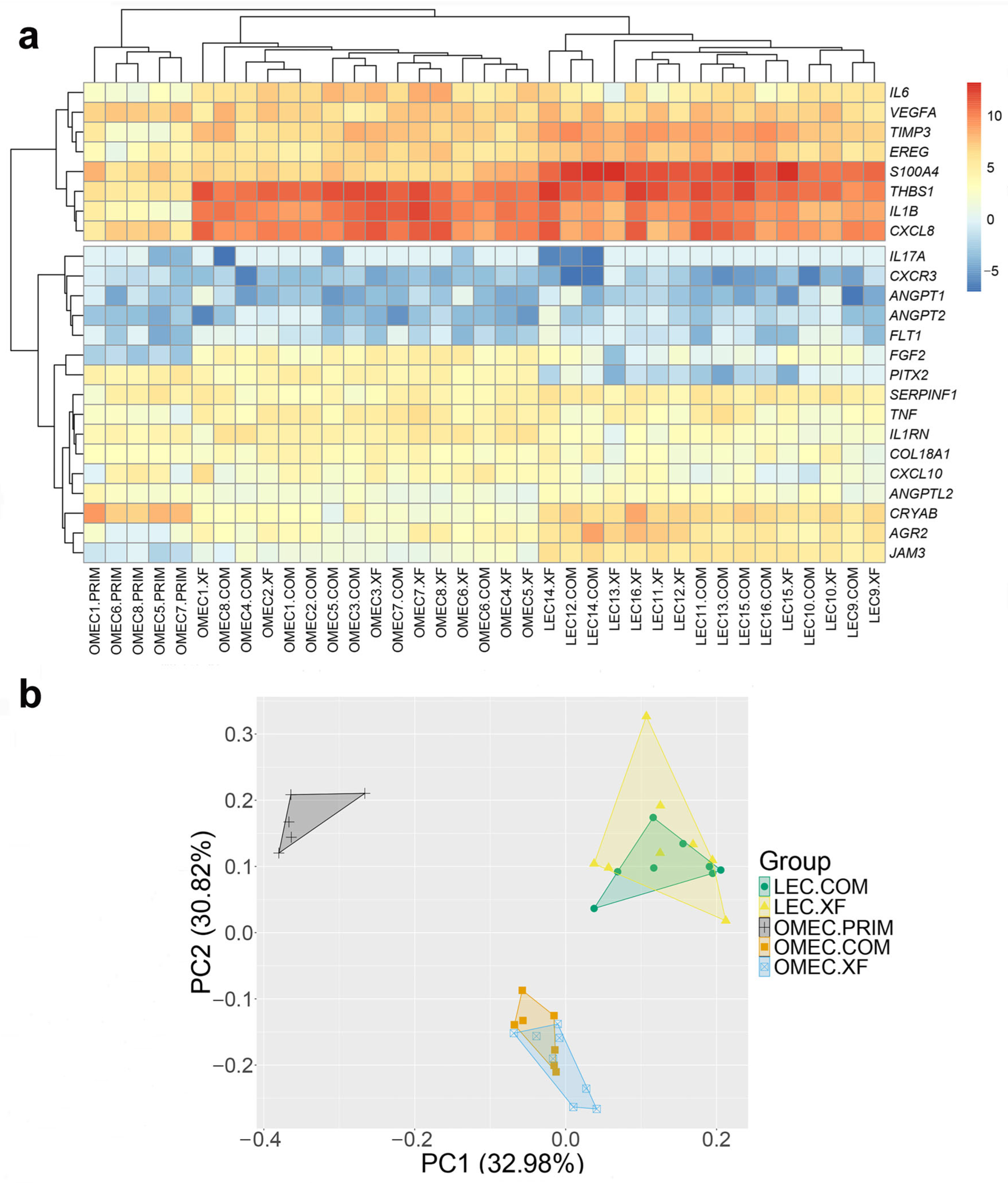
3.2. Angiogenic and Immunomodulatory Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COM | Conventional culture medium |
| COMET | Oral mucosa epithelial cell transplantation |
| FGF2 | Fibroblast growth factor 2 |
| LECs | Limbal epithelial cells |
| LESC | Limbal epithelial stem cell |
| LSCD | Bilateral limbal stem cell deficiency |
| PCA | Principal component analysis |
| PEDF | Pigment epithelium-derived factor |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| THBS1 | Thrombospondin |
| TIMP3 | Tissue inhibitor of metalloproteinase 3 |
| VEGFA | Vascular endothelial growth factor A |
| XF | Xenobiotic-free culture medium |
References
- Bonnet, C.; Roberts, J.S.; Deng, S.X. Limbal Stem Cell Diseases. Exp. Eye Res. 2021, 205, 108437. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-Term Restoration of Damaged Corneal Surfaces with Autologous Cultivated Corneal Epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Amemiya, T.; Kanamura, N.; Kinoshita, S. Transplantation of Cultivated Autologous Oral Mucosal Epithelial Cells in Patients with Severe Ocular Surface Disorders. Br. J. Ophthalmol. 2004, 88, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Kohji, N.; Masayuki, Y.; Yasutaka, H.; Katsuhiko, W.; Kazuaki, Y.; Eijiro, A.; Shigeru, N.; Akihiko, K.; Naoyuki, M.; Hitoshi, W.; et al. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Autologous Oral Mucosal Epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef]
- Cabral, J.V.; Jackson, C.J.; Utheim, T.P.; Jirsova, K. Ex Vivo Cultivated Oral Mucosal Epithelial Cell Transplantation for Limbal Stem Cell Deficiency: A Review. Stem Cell Res. Ther. 2020, 11, 301. [Google Scholar] [CrossRef]
- Inatomi, T.; Nakamura, T.; Kojyo, M.; Koizumi, N.; Sotozono, C.; Kinoshita, S. Ocular Surface Reconstruction with Combination of Cultivated Autologous Oral Mucosal Epithelial Transplantation and Penetrating Keratoplasty. Am. J. Ophthalmol. 2006, 142, 757–764. [Google Scholar] [CrossRef]
- Inatomi, T.; Nakamura, T.; Koizumi, N.; Sotozono, C.; Yokoi, N.; Kinoshita, S. Midterm Results on Ocular Surface Reconstruction Using Cultivated Autologous Oral Mucosal Epithelial Transplantation. Am. J. Ophthalmol. 2006, 141, 267–275. [Google Scholar] [CrossRef]
- O’Callaghan, A.R.; Daniels, J.T. Concise Review: Limbal Epithelial Stem Cell Therapy: Controversies and Challenges. Stem Cells 2011, 29, 1923–1932. [Google Scholar] [CrossRef]
- Lim, P.; Fuchsluger, T.A.; Jurkunas, U.V. Limbal Stem Cell Deficiency and Corneal Neovascularization. Semin. Ophthalmol. 2009, 24, 139–148. [Google Scholar] [CrossRef]
- Chen, H.-C.J.; Yeh, L.-K.; Tsai, Y.-J.; Lai, C.-H.; Chen, C.-C.; Lai, J.-Y.; Sun, C.-C.; Chang, G.; Hwang, T.-L.; Chen, J.-K.; et al. Expression of Angiogenesis-Related Factors in Human Corneas after Cultivated Oral Mucosal Epithelial Transplantation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5615–5623. [Google Scholar] [CrossRef]
- Kanayama, S.; Nishida, K.; Yamato, M.; Hayashi, R.; Sugiyama, H.; Soma, T.; Maeda, N.; Okano, T.; Tano, Y. Analysis of Angiogenesis Induced by Cultured Corneal and Oral Mucosal Epithelial Cell Sheets in Vitro. Exp. Eye Res. 2007, 85, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, S.; Nishida, K.; Yamato, M.; Hayashi, R.; Maeda, N.; Okano, T.; Tano, Y. Analysis of Soluble Vascular Endothelial Growth Factor Receptor-1 Secreted from Cultured Corneal and Oral Mucosal Epithelial Cell Sheets in Vitro. Br. J. Ophthalmol. 2009, 93, 263–267. [Google Scholar] [CrossRef]
- Attico, E.; Galaverni, G.; Torello, A.; Bianchi, E.; Bonacorsi, S.; Losi, L.; Manfredini, R.; Lambiase, A.; Rama, P.; Pellegrini, G. Comparison between Cultivated Oral Mucosa and Ocular Surface Epithelia for COMET Patients Follow-Up. Int. J. Mol. Sci. 2023, 24, 11522. [Google Scholar] [CrossRef] [PubMed]
- Sekiyama, E.; Nakamura, T.; Kawasaki, S.; Sogabe, H.; Kinoshita, S. Different Expression of Angiogenesis-Related Factors between Human Cultivated Corneal and Oral Epithelial Sheets. Exp. Eye Res. 2006, 83, 741–746. [Google Scholar] [CrossRef]
- Brejchova, K.; Trosan, P.; Studeny, P.; Skalicka, P.; Utheim, T.P.; Bednar, J.; Jirsova, K. Characterization and Comparison of Human Limbal Explant Cultures Grown under Defined and Xeno-Free Conditions. Exp. Eye Res. 2018, 176, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, L. Systematic Review and Meta-Analysis on Transplantation of Ex Vivo Cultivated Limbal Epithelial Stem Cell on Amniotic Membrane in Limbal Stem Cell Deficiency. Cornea 2015, 34, 592–600. [Google Scholar] [CrossRef]
- Cabral, J.V.; Voukali, E.; Smorodinova, N.; Balogh, L.; Kolin, V.; Studeny, P.; Netukova, M.; Jirsova, K. Cultivation and Characterization of Oral Mucosal Epithelial Cells on Fibrin Gel in a Xenobiotic-Free Medium for the Treatment of Limbal Stem Cell Deficiency. Exp. Eye Res. 2025, 253, 110300. [Google Scholar] [CrossRef]
- Trousil, J.; Cabral, J.V.; Voukali, E.; Nováčková, J.; Pop-Georgievski, O.; Vacík, T.; Studený, P.; Studenovska, H.; Jirsova, K. Electrospun Poly(l-Lactide-Co-Dl-Lactide) Nanofibrous Scaffold as Substrate for Ex Vivo Limbal Epithelial Cell Cultivation. Heliyon 2024, 10, e30970. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 22 June 2025).
- Ma, D.H.-K.; Chen, J.-K.; Zhang, F.; Lin, K.-Y.; Yao, J.-Y.; Yu, J.-S. Regulation of Corneal Angiogenesis in Limbal Stem Cell Deficiency. Prog. Retin. Eye Res. 2006, 25, 563–590. [Google Scholar] [CrossRef]
- Okada-Ban, M.; Thiery, J.P.; Jouanneau, J. Fibroblast Growth Factor-2. Int. J. Biochem. Cell Biol. 2000, 32, 263–267. [Google Scholar] [CrossRef]
- Toyono, T.; Usui, T.; Yokoo, S.; Kimakura, M.; Nakagawa, S.; Yamagami, S.; Miyata, K.; Oike, Y.; Amano, S. Angiopoietin-like Protein 2 Is a Potent Hemangiogenic and Lymphangiogenic Factor in Corneal Inflammation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4278–4285. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G. Angiopoietin-Like Proteins: A Comprehensive Look. Front. Endocrinol. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Giacomini, C.; Bignami, F.; Moi, D.; Ranghetti, A.; Doglioni, C.; Naldini, L.; Rama, P.; Mazzieri, R. Angiopoietin 2 Expression in the Cornea and Its Control of Corneal Neovascularisation. Br. J. Ophthalmol. 2016, 100, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Suri, C.; Jones, P.F.; Patan, S.; Bartunkova, S.; Maisonpierre, P.C.; Davis, S.; Sato, T.N.; Yancopoulos, G.D. Requisite Role of Angiopoietin-1, a Ligand for the TIE2 Receptor, during Embryonic Angiogenesis. Cell 1996, 87, 1171–1180. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, Q.; Yu, X.; Merugu, S.B.; Mangukiya, H.B.; Smith, N.; Li, Z.; Zhang, B.; Negi, H.; Rong, R.; et al. Tumor-Secreted Anterior Gradient-2 Binds to VEGF and FGF2 and Enhances Their Activities by Promoting Their Homodimerization. Oncogene 2017, 36, 5098–5109. [Google Scholar] [CrossRef]
- Delom, F.; Mohtar, M.A.; Hupp, T.; Fessart, D. The Anterior Gradient-2 Interactome. Am. J. Physiol. Cell Physiol. 2020, 318, C40–C47. [Google Scholar] [CrossRef]
- Kase, S.; He, S.; Sonoda, S.; Kitamura, M.; Spee, C.; Wawrousek, E.; Ryan, S.J.; Kannan, R.; Hinton, D.R. αB-Crystallin Regulation of Angiogenesis by Modulation of VEGF. Blood 2010, 115, 3398–3406. [Google Scholar] [CrossRef]
- Boelens, W.C. Cell Biological Roles of αB-Crystallin. Prog. Biophys. Mol. Biol. 2014, 115, 3–10. [Google Scholar] [CrossRef]
- Morita, S.; Shirakata, Y.; Shiraishi, A.; Kadota, Y.; Hashimoto, K.; Higashiyama, S.; Ohashi, Y. Human Corneal Epithelial Cell Proliferation by Epiregulin and Its Cross-Induction by Other EGF Family Members. Mol. Vis. 2007, 13, 2119–2128. [Google Scholar]
- Zhang, Y.; Kobayashi, T.; Hayashi, Y.; Yoshioka, R.; Shiraishi, A.; Shirasawa, S.; Higashiyama, S.; Ohashi, Y. Important Role of Epiregulin in Inflammatory Responses during Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2414–2423. [Google Scholar] [CrossRef]
- Li, C.; Zhang, F.; Wang, Y. S100A Proteins in the Pathogenesis of Experimental Corneal Neovascularization. Mol. Vis. 2010, 16, 2225–2235. [Google Scholar]
- Ambartsumian, N.; Klingelhöfer, J.; Grigorian, M.; Christensen, C.; Kriajevska, M.; Tulchinsky, E.; Georgiev, G.; Berezin, V.; Bock, E.; Rygaard, J.; et al. The Metastasis-Associated Mts1(S100A4) Protein Could Act as an Angiogenic Factor. Oncogene 2001, 20, 4685–4695. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, N.; Zenkel, M.; Menzel-Severing, J.; Kruse, F.E.; Schlötzer-Schrehardt, U. Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem Cells 2016, 34, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Rabquer, B.J.; Amin, M.A.; Teegala, N.; Shaheen, M.K.; Tsou, P.-S.; Ruth, J.H.; Lesch, C.A.; Imhof, B.A.; Koch, A.E. Junctional Adhesion Molecule-C Is a Soluble Mediator of Angiogenesis. J. Immunol. 2010, 185, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.W.; Volpert, O.V.; Gillis, P.; Crawford, S.E.; Xu, H.; Benedict, W.; Bouck, N.P. Pigment Epithelium-Derived Factor: A Potent Inhibitor of Angiogenesis. Science 1999, 285, 245–248. [Google Scholar] [CrossRef]
- Matsui, T.; Nishino, Y.; Maeda, S.; Yamagishi, S. PEDF-Derived Peptide Inhibits Corneal Angiogenesis by Suppressing VEGF Expression. Microvasc. Res. 2012, 84, 105–108. [Google Scholar] [CrossRef]
- Ambati, B.K.; Nozaki, M.; Singh, N.; Takeda, A.; Jani, P.D.; Suthar, T.; Albuquerque, R.J.C.; Richter, E.; Sakurai, E.; Newcomb, M.T.; et al. Corneal Avascularity Is Due to Soluble VEGF Receptor-1. Nature 2006, 443, 993–997. [Google Scholar] [CrossRef]
- Kenney, M.C.; Chwa, M.; Alba, A.; Saghizadeh, M.; Huang, Z.-S.; Brown, D.J. Localization of TIMP-1, TIMP-2, TIMP-3, Gelatinase A and Gelatinase B in Pathological Human Corneas. Curr. Eye Res. 1998, 17, 238–246. [Google Scholar] [CrossRef]
- Ebrahem, Q.; Qi, J.H.; Sugimoto, M.; Ali, M.; Sears, J.E.; Cutler, A.; Khokha, R.; Vasanji, A.; Anand-Apte, B. Increased Neovascularization in Mice Lacking Tissue Inhibitor of Metalloproteinases-3. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6117–6123. [Google Scholar] [CrossRef]
- Cursiefen, C.; Masli, S.; Ng, T.F.; Dana, M.R.; Bornstein, P.; Lawler, J.; Streilein, J.W. Roles of Thrombospondin-1 and -2 in Regulating Corneal and Iris Angiogenesis. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1117–1124. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An Endogenous Inhibitor of Angiogenesis and Tumor Growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; McMullen, T.C.B.; Campbell, I.L.; Rohan, R.; Kaji, Y.; Afshari, N.A.; Usui, T.; Archer, D.B.; Adamis, A.P. The Inflammatory Milieu Associated with Conjunctivalized Cornea and Its Alteration with IL-1 RA Gene Therapy. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2905–2915. [Google Scholar]
- Gao, N.; Liu, X.; Wu, J.; Li, J.; Dong, C.; Wu, X.; Xiao, X.; Yu, F.-S.X. CXCL10 Suppression of Hem- and Lymph-Angiogenesis in Inflamed Corneas through MMP13. Angiogenesis 2017, 20, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Annunziato, F.; Lasagni, L.; Lazzeri, E.; Beltrame, C.; Francalanci, M.; Uguccioni, M.; Galli, G.; Cosmi, L.; Maurenzig, L.; et al. Cell Cycle–Dependent Expression of CXC Chemokine Receptor 3 by Endothelial Cells Mediates Angiostatic Activity. J. Clin. Investig. 2001, 107, 53–63. [Google Scholar] [CrossRef]
- Pauklin, M.; Steuhl, K.-P.; Meller, D. Characterization of the Corneal Surface in Limbal Stem Cell Deficiency and after Transplantation of Cultivated Limbal Epithelium. Ophthalmology 2009, 116, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.S.; Banerjee, K.; Kinchington, P.R.; Rouse, B.T. Involvement of IL-6 in the Paracrine Production of VEGF in Ocular HSV-1 Infection. Exp. Eye Res. 2006, 82, 46–54. [Google Scholar] [CrossRef]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a Macrophage-Derived Mediator of Angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef]
- Strieter, R.M.; Kunkel, S.L.; Elner, V.M.; Martonyi, C.L.; Koch, A.E.; Polverini, P.J.; Elner, S.G. Interleukin-8. A Corneal Factor That Induces Neovascularization. Am. J. Pathol. 1992, 141, 1279–1284. [Google Scholar]
- Yoshida, S.; Ono, M.; Shono, T.; Izumi, H.; Ishibashi, T.; Suzuki, H.; Kuwano, M. Involvement of Interleukin-8, Vascular Endothelial Growth Factor, and Basic Fibroblast Growth Factor in Tumor Necrosis Factor Alpha-Dependent Angiogenesis. Mol. Cell. Biol. 1997, 17, 4015–4023. [Google Scholar] [CrossRef]
- Chi, H.; Wei, C.; Ma, L.; Yu, Y.; Zhang, T.; Shi, W. The Ocular Immunological Alterations in the Process of High-Risk Corneal Transplantation Rejection. Exp. Eye Res. 2024, 245, 109971. [Google Scholar] [CrossRef]
- Volpert, O.V.; Fong, T.; Koch, A.E.; Peterson, J.D.; Waltenbaugh, C.; Tepper, R.I.; Bouck, N.P. Inhibition of Angiogenesis by Interleukin 4. J. Exp. Med. 1998, 188, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Samolov, B.; Kvanta, A.; van der Ploeg, I. Delayed Neovascularization in Inflammation-Induced Corneal Neovascularization in Interleukin-10-Deficient Mice. Acta Ophthalmol. 2010, 88, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.-Y.; Xie, H.-T.; Zhao, X.-Y.; Xu, W.-H.; Zhang, M.-C. Limbal Niche Cells Can Reduce the Angiogenic Potential of Cultivated Oral Mucosal Epithelial Cells. Cell. Mol. Biol. Lett. 2019, 24, 3. [Google Scholar] [CrossRef]
- Hermankova, B.; Javorkova, E.; Palacka, K.; Holan, V. Perspectives and Limitations of Mesenchymal Stem Cell-Based Therapy for Corneal Injuries and Retinal Diseases. Cell Transplant. 2025, 34, 9636897241312798. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR Signaling in Health and Disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Formanek, M.; Knerer, B.; Temmel, A.; Thurnher, D.; Millesi, W.; Kornfehl, J. Oral Keratinocytes Derived from the Peritonsillar Mucosa Express the Proinflammatory Cytokine IL-6 without Prior Stimulation. J. Oral Pathol. Med. 1998, 27, 202–206. [Google Scholar] [CrossRef]
- Fung, G.; Wong, J.; Berhe, F.; Mohamud, Y.; Xue, Y.C.; Luo, H. Phosphorylation and Degradation of αB-Crystallin during Enterovirus Infection Facilitates Viral Replication and Induces Viral Pathogenesis. Oncotarget 2017, 8, 74767–74780. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Harvey, V.S.; Estrella, P.; Brown, L.F.; McDonagh, J.; Dvorak, A.M. Fibrin Containing Gels Induce Angiogenesis. Implications for Tumor Stroma Generation and Wound Healing. Lab. Investig. 1987, 57, 673–686. [Google Scholar]


| Gene | Full Name | Function |
|---|---|---|
| Pro-angiogenic | ||
| FGF2 | Fibroblast growth factor 2 | Promotes angiogenesis, wound healing, and cell proliferation [9,20,21] |
| VEGFA | Vascular endothelial growth factor A | Key driver of blood vessel formation under hypoxic or inflammatory conditions [9,20] |
| ANGPTL2 | Angiopoietin like 2 | Induces inflammation and neovascularization in tissue remodeling and cancer [22,23] |
| ANGPT2 | Angiopoietin 2 | Destabilizes blood vessels, facilitating VEGF-driven angiogenesis [24] |
| ANGPT1 | Angiopoietin 1 | Stabilizes blood vessels and supports endothelial cell survival [25] |
| AGR2 | Anterior gradient 2, protein disulphide isomerase family member | Promotes epithelial cell growth and has roles in wound healing and tumor angiogenesis [26,27] |
| CRYAB | Crystallin alpha B | Acts as a molecular chaperone; involved in protection against stress and may support angiogenesis [28,29] |
| EREG | Epiregulin | EGFR ligand that promotes epithelial repair, proliferation, and angiogenesis [30,31] |
| S100A4 | S100 calcium binding protein A4 | Associated with motility, invasion, and angiogenesis in cancer and inflammation [32,33] |
| JAM3 | Junctional adhesion molecule 3 | Mediates cell–cell adhesion and contributes to angiogenesis [34,35] |
| Anti-angiogenic | ||
| SERPINF1 | Serpin family F member 1 (PEDF) | Potent anti-angiogenic factor that inhibits VEGF signaling [36,37] |
| FLT1 | Fms related receptor tyrosine kinase 1 (VEGFR1) | Acts as a decoy receptor for VEGF, limiting angiogenesis [38] |
| TIMP3 | TIMP metallopeptidase inhibitor 3 | Inhibits matrix metalloproteinases and angiogenesis [39,40] |
| THBS1 | Thrombospondin 1 | Suppresses angiogenesis through interaction with CD36 and other receptors [14,41] |
| COL18A1 | Collagen type XVIII alpha 1 chain | Encodes endostatin, a known inhibitor of angiogenesis [42] |
| Immuno-regulatory | ||
| IL1RN | Interleukin 1 receptor antagonist | Anti-inflammatory cytokine that blocks IL-1 signaling [43] |
| CXCL10 | C-X-C motif chemokine ligand 10 | Chemokine that recruits immune cells and can inhibit angiogenesis [44] |
| CXCR3 | C-X-C motif chemokine receptor 3 | Receptor for CXCL9/10/11 involved in T-cell trafficking and inflammation [45] |
| IL1B | Interleukin 1 beta | Key pro-inflammatory cytokine promoting leukocyte recruitment [9,46] |
| IL6 | Interleukin 6 | Pro-inflammatory cytokine with roles in acute phase response and angiogenesis [9,47] |
| CXCL8 | C-X-C motif chemokine ligand 8 (IL-8) | Promotes neutrophil chemotaxis and angiogenesis [9,48,49,50] |
| IL17A | Interleukin 17A | Stimulates pro-inflammatory responses and angiogenesis [51] |
| TNF | Tumor necrosis factor | Master regulator of inflammation, promotes angiogenesis under pathological conditions [9,50] |
| IL4 | Interleukin 4 | Anti-inflammatory cytokine, promotes Th2 immune response [52] |
| IL10 | Interleukin 10 | Anti-inflammatory cytokine that limits immune responses [53] |
| Oral mucosa identity | ||
| PITX2 | Paired like homeodomain 2 | Transcription factor involved in oral epithelial identity and development [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voukali, E.; Cabral, J.V.; Smorodinova, N.; Kolin, V.; Netukova, M.; Vacík, T.; Jirsova, K. Different Expression of Vascularization and Inflammatory Regulators in Cells Derived from Oral Mucosa and Limbus. Bioengineering 2025, 12, 688. https://doi.org/10.3390/bioengineering12070688
Voukali E, Cabral JV, Smorodinova N, Kolin V, Netukova M, Vacík T, Jirsova K. Different Expression of Vascularization and Inflammatory Regulators in Cells Derived from Oral Mucosa and Limbus. Bioengineering. 2025; 12(7):688. https://doi.org/10.3390/bioengineering12070688
Chicago/Turabian StyleVoukali, Eleni, Joao Victor Cabral, Natalia Smorodinova, Vojtech Kolin, Magdalena Netukova, Tomáš Vacík, and Katerina Jirsova. 2025. "Different Expression of Vascularization and Inflammatory Regulators in Cells Derived from Oral Mucosa and Limbus" Bioengineering 12, no. 7: 688. https://doi.org/10.3390/bioengineering12070688
APA StyleVoukali, E., Cabral, J. V., Smorodinova, N., Kolin, V., Netukova, M., Vacík, T., & Jirsova, K. (2025). Different Expression of Vascularization and Inflammatory Regulators in Cells Derived from Oral Mucosa and Limbus. Bioengineering, 12(7), 688. https://doi.org/10.3390/bioengineering12070688






