Biomechanical Design and Validation of a Novel Elliptical Sleeve Pedicle Screw for Enhanced Spinal Fixation Stability
Abstract
1. Introduction
2. Materials and Methods
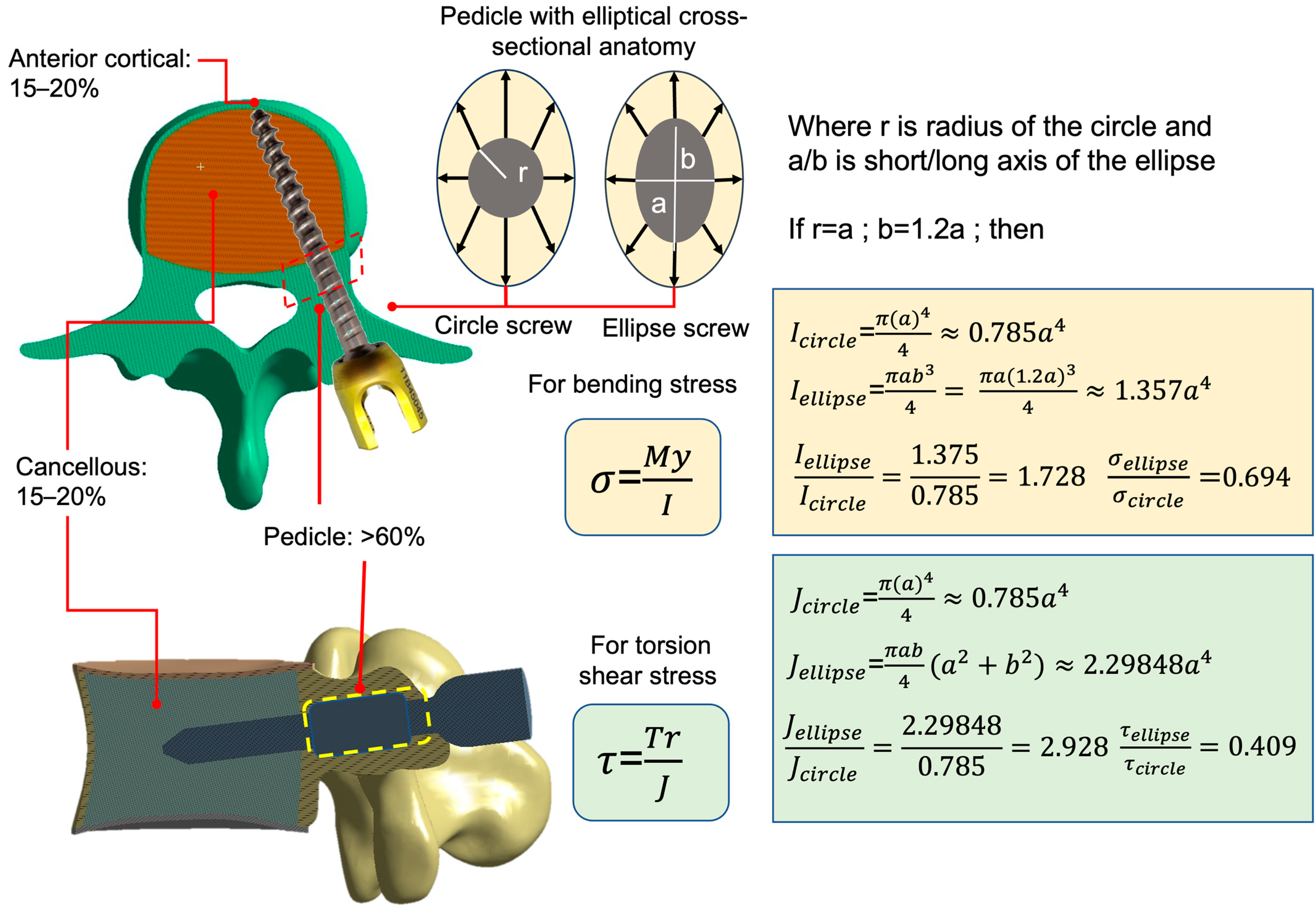
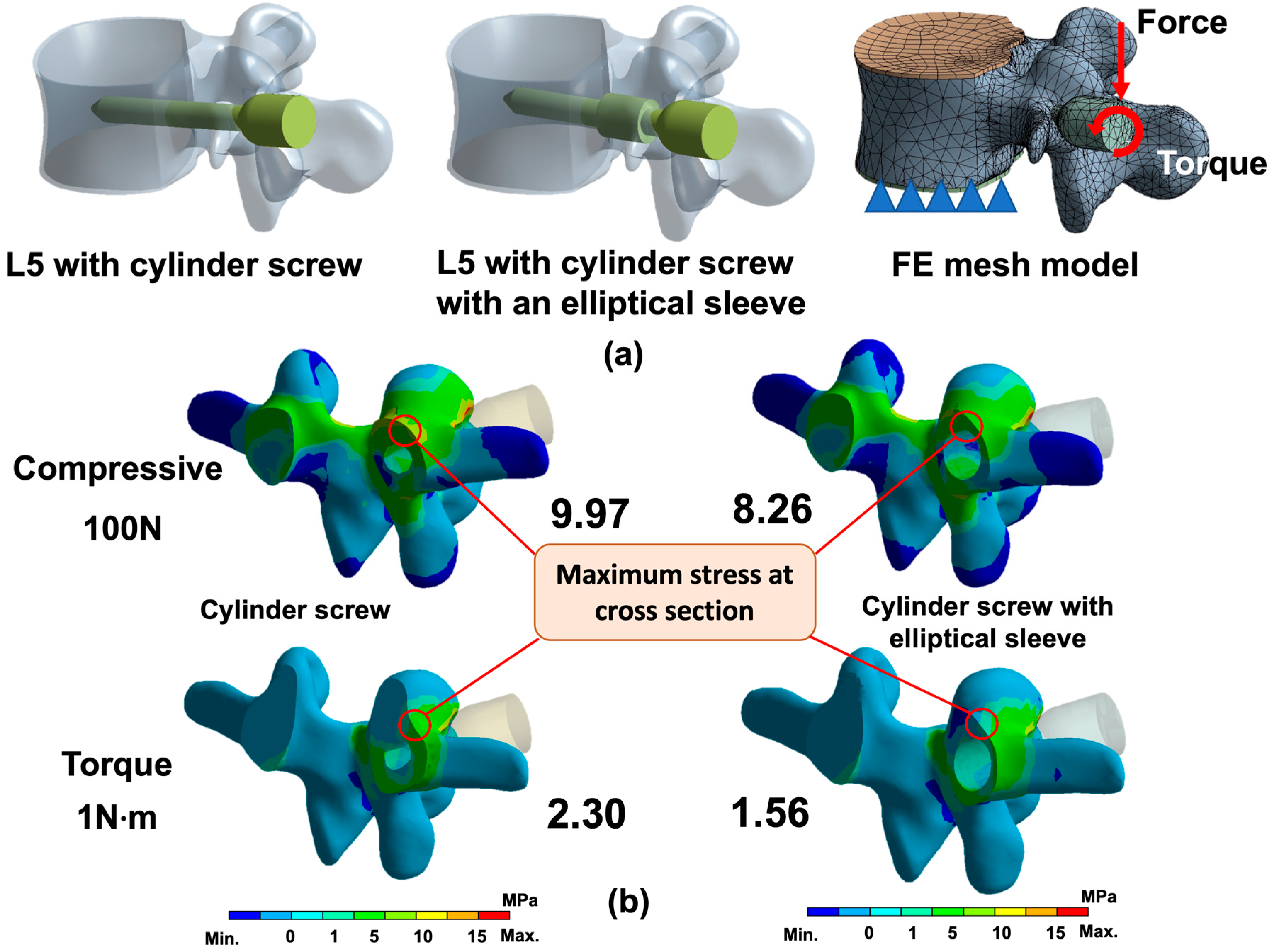
2.1. FE Analysis of Cylinder and Ellipse Pedicle Screws
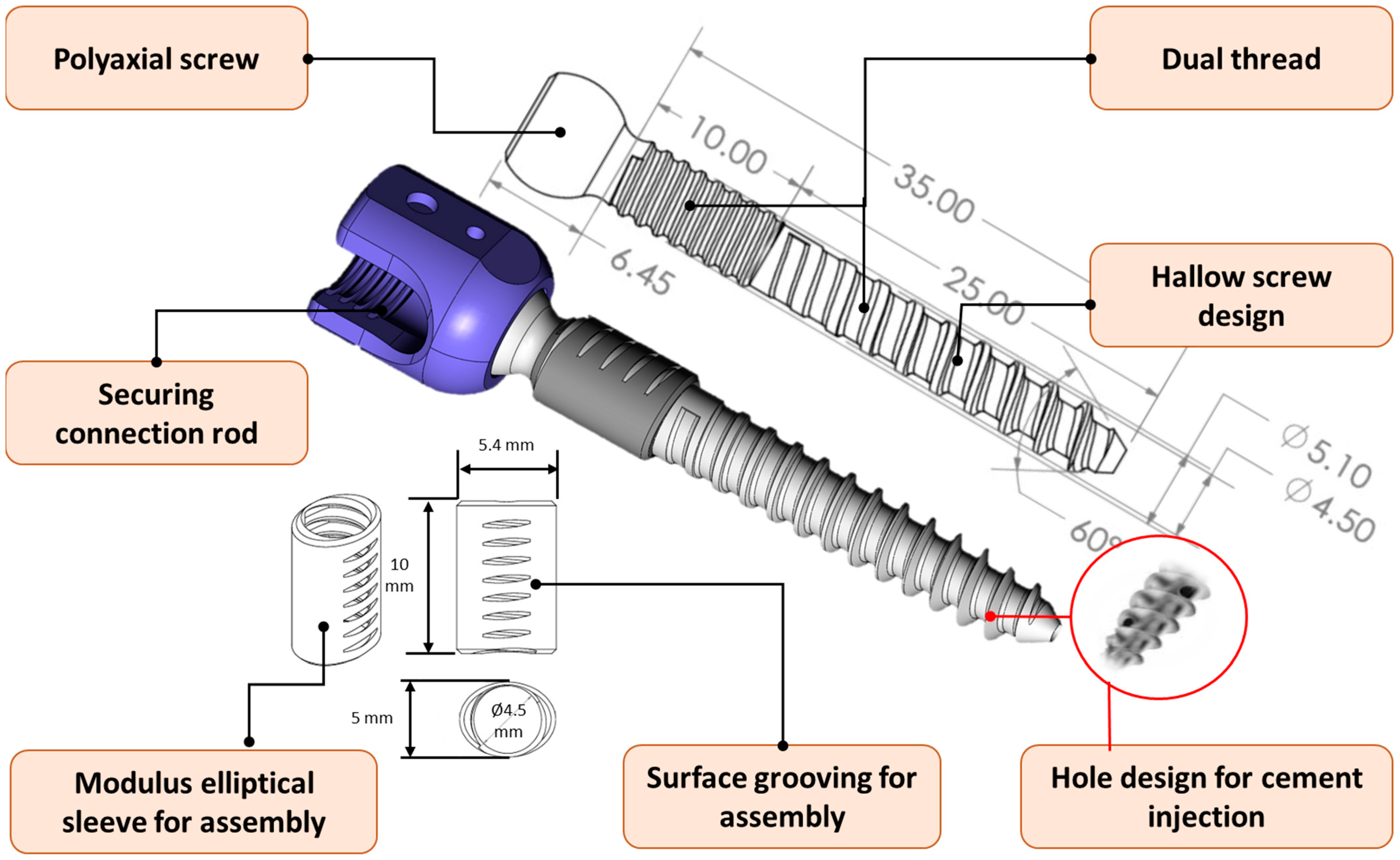
2.2. Design and Manufacture of Novel Pedicle Screw System
2.3. Design of the Specific Instrument & Implantation Procedure
- Positioning the instrument: Intraoperative imaging (e.g., X-ray or C-arm) was used to align the tip of the bone-shaping instrument with the pedicle entry point, and a small hole was created to mark the insertion site (Step 1 in Figure 5).
- Cortex opening and initial insertion: After confirming the entry point, the front end of the bone-shaping instrument was used to open the surface cortex. The instrument was then inserted into and through the isthmus of the pedicle with appropriate torque and pressure. The medio-lateral projection angles should be adjusted according to the trajectory of the pedicle to avoid the tip of the bone-shaping instrument penetrating beyond the medial wall of the pedicle cortex. At this stage, the elliptical portion of the instrument remained outside the pedicle (Step 2 in Figure 5).
- Shaping the pedicle tunnel: A mallet was used to drive the bone-shaping instrument further into the pedicle, sculpting the bone into an elliptical tunnel. Alignment lines on the instrument ensured that the long axis of the elliptical tunnel was parallel to the long axis of the pedicle. (Step 03 in Figure 5).
- Removing the positioning needle: Once the shaping was complete, the internal positioning needle was removed from the pedicle channel. A guide pin was inserted into the tunnel to guide the subsequent screw implantation. (Step 04 in Figure 5).
- Removing the bone-shaping instrument: The bone-shaping instrument was removed, leaving an elliptical cross-section in the pedicle. This tunnel was now ready for the integration of the elliptical sleeve and screw (Step 05 in Figure 5).
- Implanting the screw and elliptical sleeve: The pedicle screw, with its coarse-threaded portion, was screwed in until the elliptical sleeve contacted the pedicle. The sleeve was then press-fitted into the elliptical cavity without rotation, ensuring a parallel fit. Simultaneously, the fine-threaded portion of the screw engaged with the grooves of the elliptical sleeve. The screw was advanced further, fully seating the sleeve into the pedicle until it reached the intended position. Finally, the guide pin was removed, completing the implantation process (Step 06, 06-1, and 06-2 in Figure 5).
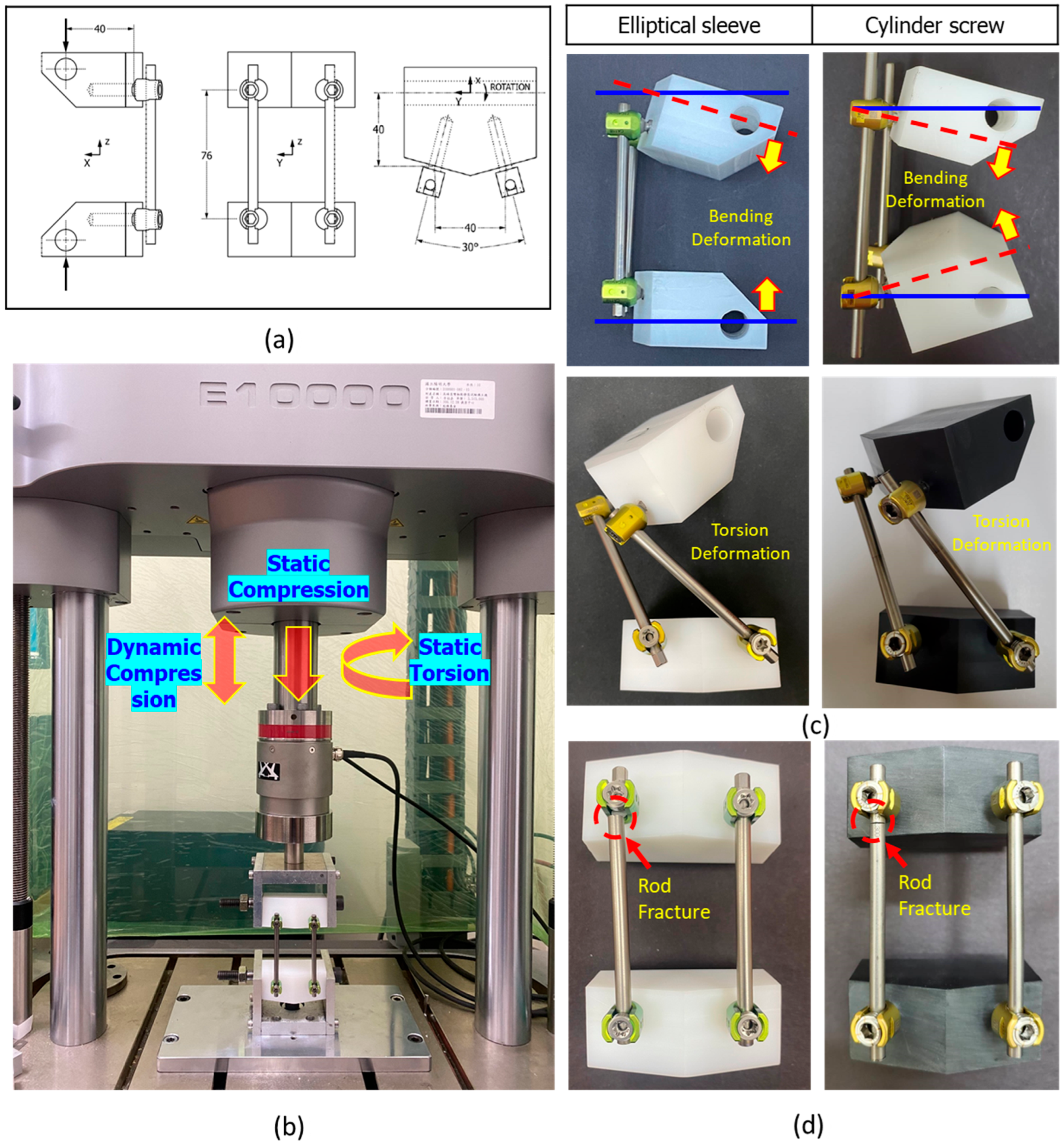
2.4. ASTM F1717 Testing for Static/Fatigue Compression Bending and Static Torsion
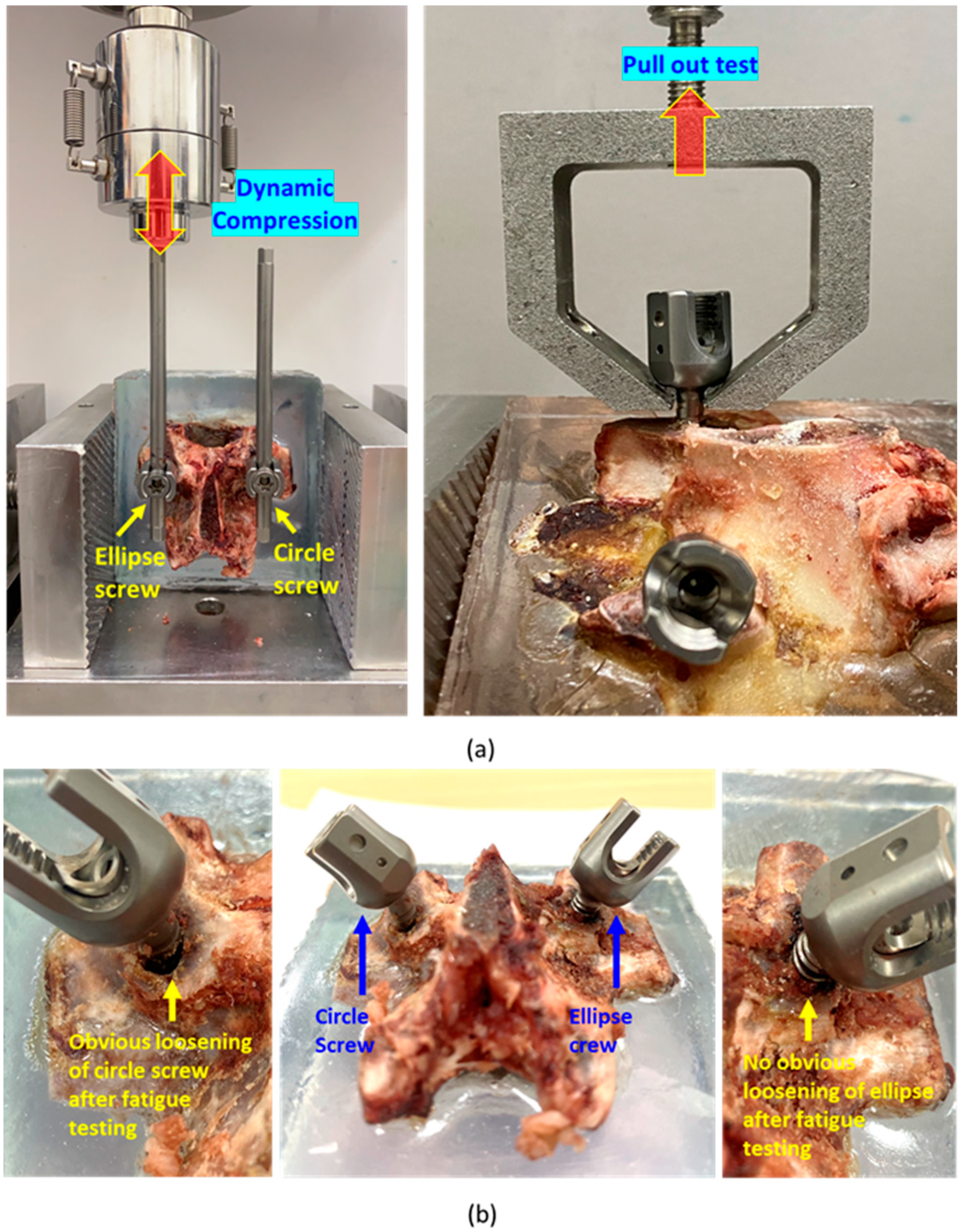
2.5. Biomechanical Compression Fatigue and Pull-Out Testing
3. Results
3.1. FE Analysis
3.2. ASTM F1717 Testing
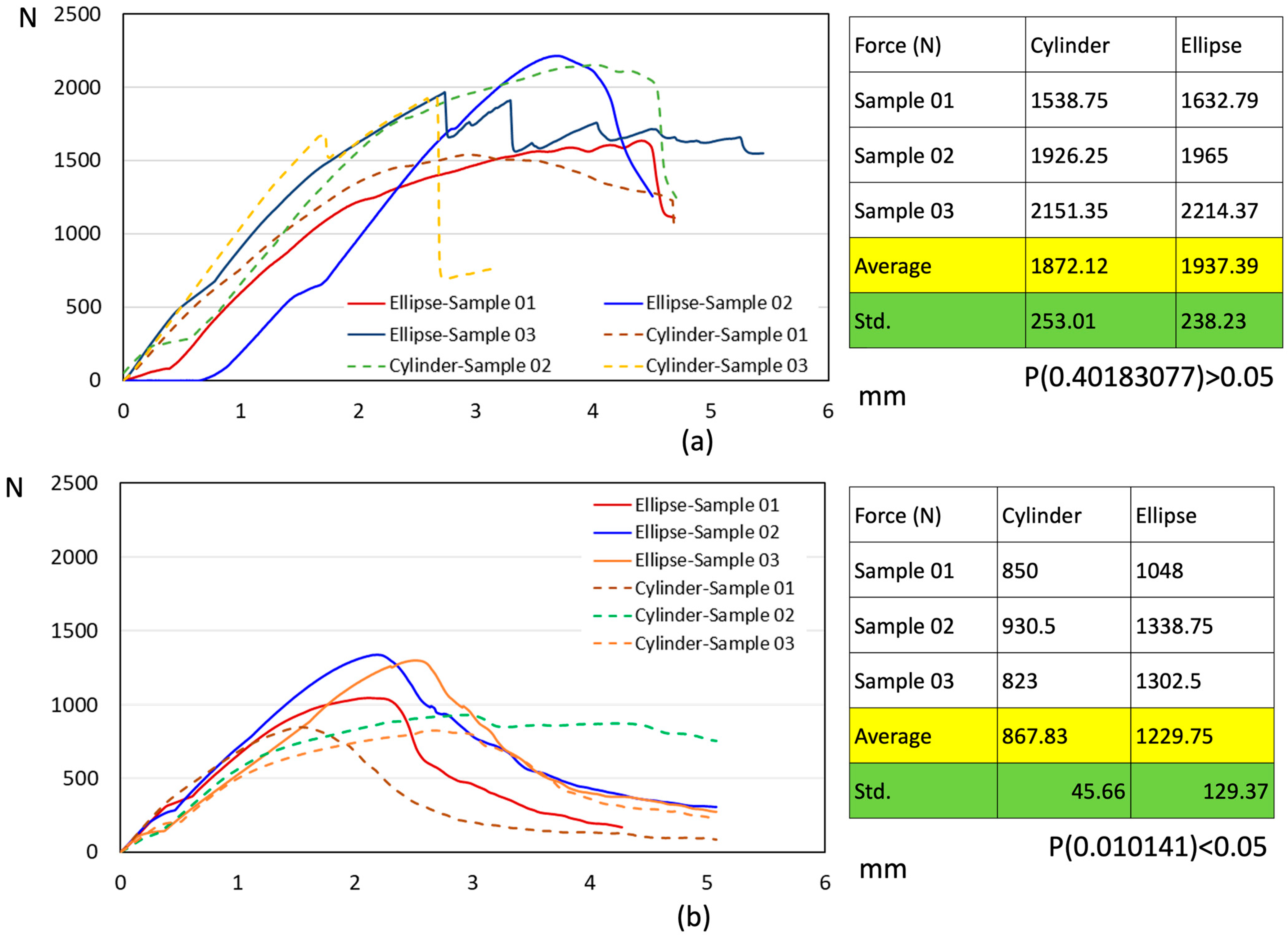
3.3. Biomechanical Pull-Out Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, L.; Xu, H.J.; Yu, Y.K.; Tang, H.H.; Fang, B.H.; Chen, K. Systematic review and meta-analysis comparative analysis of the safety and efficacy of fenestrated pedicle screw with cement and conventional pedicle screw with cement in the treatment of osteoporotic vertebral fractures: A meta-analysis. Chin. J. Traumatol. 2024, 24, 00176–00177. [Google Scholar]
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18. [Google Scholar] [PubMed]
- Greiner-Perth, A.K.; Wilke, H.J.; Liebsch, C. Which spinal fixation technique achieves which degree of stability after thoracolumbar trauma? A systematic quantitative review. Spine J. 2024, 24, 01095–01097. [Google Scholar] [CrossRef]
- Zhong, R.; Xue, X.; Wang, R.; Dan, J.; Wang, C.; Liu, D. Safety and efficacy of unilateral and bilateral pedicle screw fixation for lumbar degenerative diseases by transforaminal lumbar interbody fusion: An updated systematic review and meta-analysis. Front. Neurol. 2022, 13, 998173. [Google Scholar] [CrossRef]
- Wu, J.C.; Huang, W.C.; Tsai, H.W.; Ko, C.C.; Wu, C.L.; Tu, T.H.; Cheng, H. Pedicle screw loosening in dynamic stabilization: Incidence, risk, and outcome in 126 patients. Neurosurg. Focus. 2011, 4, E9. [Google Scholar] [CrossRef]
- Kil, J.S.; Park, J.T. Simple New Screw Insertion Technique without Extraction for Broken Pedicle Screws. World Neurosurg. 2018, 113, 125–128. [Google Scholar] [CrossRef]
- Hicks, J.M.; Singla, A.; Shen, F.H.; Arlet, V. Complications of pedicle screw fixation in scoliosis surgery: A systematic review. Spine 2010, 35, E465–E470. [Google Scholar] [CrossRef]
- Chong, X.L.; Kumar, A.; Yang, E.W.R.; Kaliya-Perumal, A.K.; Oh, J.Y. Incidence of pedicle breach following open and minimally invasive spinal instrumentation: A postoperative CT analysis of 513 pedicle screws applied under fluoroscopic guidance. Biomedicine. 2020, 10, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Choy, W.J.; Walsh, W.R.; Phan, K.; Mobbs, R.J. Technical Note: Pedicle Cement Augmentation with Proximal Screw Toggle and Loosening. Orthop. Surg. 2019, 11, 510–515. [Google Scholar] [CrossRef]
- Sensale, M.; Vendeuvre, T.; Schilling, C.; Grupp, T.; Rochette, M.; Dall’Ara, E. Patient-Specific Finite Element Models of Posterior Pedicle Screw Fixation: Effect of Screw’s Size and Geometry. Front. Bioeng. Biotechnol. 2021, 9, 643154. [Google Scholar] [CrossRef]
- Williams, A.L.; Gornet, M.F.; Burkus, J.K. CT Evaluation of Lumbar Interbody Fusion: Current Concepts. AJNR Am. J. Neuroradiol. 2005, 26, 2057–2066. [Google Scholar] [PubMed]
- Kwon, J.; Ha, M.H.; Lee, M.G. Alternative Pedicle Screw Design via Biomechanical Evaluation. Appl. Sci. 2020, 10, 4746. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, D.K.; Shin, W.S.; Kim, M.J.; Lee, H.Y.; Go, Y.R. Clinical Effects and Complications of Pedicle Screw Augmentation with Bone Cement: Comparison of Fenestrated Screw Augmentation and Vertebroplasty Augmentation. Clin. Orthop. Surg. 2020, 12, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Rydevik, B.L.; Rauschning, W. Anatomic and technical considerations of pedicle screw fixation. Clin. Orthop. Relat. Res. 1992, 284, 34–46. [Google Scholar] [CrossRef]
- Hirano, T.; Hasegawa, K.; Takahashi, H.E.; Uchiyama, S.; Hara, T.; Washio, T.; Sugiura, T.; Yokaichiya, M.; Ikeda, M. Structural characteristics of the pedicle and its role in screw stability. Spine 1997, 22, 2504–2509. [Google Scholar] [CrossRef]
- Uvaraj, N.R.; Jill, J. Pedicle screw stacking: A technical note on instrumentation of lumbar spine with a broken pedicle screw. Interdiscip. Neurosurg. 2021, 23, 100893. [Google Scholar] [CrossRef]
- Goodno, B.; Gere, J. Mechanics of Materials SI 9/e. Cengage Learning, Boston, Massachusetts; Cengage Learning: Boston, MA, USA, 2017; ISBN 9781337093354. [Google Scholar]
- Shea, T.M.; Laun, J.; Gonzalez-Blohm, S.A.; Doulgeris, J.J.; Lee, W.E., 3rd; Aghayev, K.; Vrionis, F.D. Designs and Techniques That Improve the Pullout Strength of Pedicle Screws in Osteoporotic Vertebrae: Current Status. BioMed. Res. Int. 2014, 2014, 748393. [Google Scholar] [CrossRef]
- Hsu, C.C.; Chao, C.K.; Wang, J.L.; Hou, S.M.; Tsai, Y.T.; Lin, J. Increase of pullout strength of spinal pedicle screws with conical core: Biomechanical tests and finite element analyses. J. Orthop. Res. 2005, 23, 788–794. [Google Scholar] [CrossRef]
- Tsuang, F.Y.; Chen, C.H.; Wu, L.C.; Kuo, Y.J.; Lin, S.C.; Chiang, C.J. Biomechanical arrangement of threaded and unthreaded portions providing holding power of transpedicular screw fixation. Clin. Biomech. 2016, 39, 71–76. [Google Scholar] [CrossRef]
- Kwan, M.K.; Chan, C.Y.; Saw, L.B.; Rukmanikanthan, S.; Lenke, L.G. The Safety and Strength of a Novel Medial, Partial Nonthreaded Pedicle Screw. Clin. Spine Surg. 2017, 30, E297–E304. [Google Scholar] [CrossRef]
- Gates, T.A.; Moldavsky, M.; Salloum, K.; Dunbar, G.L.; Park, J.; Bucklen, B. Biomechanical Analysis of a Novel Pedicle Screw Anchor Designed for the Osteoporotic Population. World Neurosurg. 2015, 83, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.F.; Chang, C.M.; Liao, C.Y.; Chan, Y.T.; Li, Z.Y.; Lin, C.L. Biomechanical evaluation of an osteo-porotic anatomical 3D printed posterior lumbar interbody fusion cage with internal lattice design based on weighted topology optimization. Int. J. Bioprint. 2023, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- ASTM F1717-21; Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model. ASTM International: West Conshohocken, PA, USA, 2021.
- Busscher, I.; Ploegmakers, J.J.; Verkerke, G.J.; Veldhuizen, A.G. Comparative anatomical dimensions of the complete human and porcine spine. Eur. Spine J. 2010, 19, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Kostuik, J.P.; Smith, T.J. Pitfalls of biomechanical testing. Spine 1991, 16, 1233–1235. [Google Scholar] [CrossRef]
- Aycan, M.F.; Yaman, M.E.; Usta, Y.; Demir, T.; Tolunay, T. Investigation of toggling effect on pullout performance of pedicle screws. Proc. Inst. Mech. Eng. H. 2018, 232, 395–402. [Google Scholar] [CrossRef]
- Baluch, D.A.; Patel, A.A.; Lullo, B.; Havey, R.M.; Voronov, L.I.; Nguyen, N.L.; Carandang, G.; Ghanayem, A.J.; Patwardhan, A.G. Effect of physiological loads on cortical and traditional pedicle screw fixa-tion. Spine 2014, 39, E1297–E1302. [Google Scholar] [CrossRef]




| Material | Young’s Modulus (MPa) | Poission’s Ratio |
|---|---|---|
| Cortical bone | 12,000 | 0.3 |
| Cancellous | 100 | 0.2 |
| Endplate | 24 | 0.25 |
| Ti6Al4V | 110,000 | 0.3 |
| Testing Type | Static Compression | Static Torsion | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recorded | Ultimate Strength (N) | Yield Strength (N) | Bending Stiffness (N/mm) | Ultimate Torque (N-m) | Yield Torque (N-m) | Torsional Stiffness (N-m/Degree) | ||||||
| Sample type | Ellipse | Cylinder | Ellipse | Cylinder | Ellipse | Cylinder | Ellipse | Cylinder | Ellipse | Cylinder | Ellipse | Cylinder |
| Sample 01 | 455.82 | 357.20 | 385.42 | 267.90 | 34.97 | 22.14 | 13.82 | 17.05 | 8.82 | 13.8 | 2.81 | 2.14 |
| Sample 02 | 556.40 | 365.70 | 406.89 | 333.30 | 31.20 | 26.36 | 15.49 | 18.99 | 9.30 | 14.04 | 2.56 | 2.00 |
| Sample 03 | 528.68 | 460.90 | 404.51 | 299.90 | 30.72 | 27.82 | 13.81 | 16.38 | 9.79 | 12.75 | 2.32 | 2.07 |
| Sample 04 | 558.68 | 407.90 | 422.16 | 252.10 | 32.31 | 24.85 | 13.80 | 17.14 | 9.25 | 12.96 | 2.41 | 2.13 |
| Sample 05 | 517.60 | 389.90 | 360.42 | 217.90 | 30.24 | 27.19 | 16.32 | 17.77 | 10.45 | 13.4 | 2.36 | 2.07 |
| Average (Std.) | 523.43 (41.72) | 357.20 (41.27) | 395.88 (23.73) | 267.90 (44.30) | 31.89 (1.89) | 22.14 (2.27) | 14.23 (0.84) | 17.47 (0.98) | 9.29 (0.40) | 13.39 (0.54) | 2.41 (0.12) | 2.08 (0.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, T.-S.; Chiang, C.-J.; Wang, H.-W.; Chen, Y.-S.; Lin, C.-L. Biomechanical Design and Validation of a Novel Elliptical Sleeve Pedicle Screw for Enhanced Spinal Fixation Stability. Bioengineering 2025, 12, 668. https://doi.org/10.3390/bioengineering12060668
Hsu T-S, Chiang C-J, Wang H-W, Chen Y-S, Lin C-L. Biomechanical Design and Validation of a Novel Elliptical Sleeve Pedicle Screw for Enhanced Spinal Fixation Stability. Bioengineering. 2025; 12(6):668. https://doi.org/10.3390/bioengineering12060668
Chicago/Turabian StyleHsu, Ting-Shuo, Chang-Jung Chiang, Hsuan-Wen Wang, Yu-San Chen, and Chun-Li Lin. 2025. "Biomechanical Design and Validation of a Novel Elliptical Sleeve Pedicle Screw for Enhanced Spinal Fixation Stability" Bioengineering 12, no. 6: 668. https://doi.org/10.3390/bioengineering12060668
APA StyleHsu, T.-S., Chiang, C.-J., Wang, H.-W., Chen, Y.-S., & Lin, C.-L. (2025). Biomechanical Design and Validation of a Novel Elliptical Sleeve Pedicle Screw for Enhanced Spinal Fixation Stability. Bioengineering, 12(6), 668. https://doi.org/10.3390/bioengineering12060668







