Anatomical Characteristics Predict Response to Transcranial Direct Current Stimulation (tDCS): Development of a Computational Pipeline for Optimizing tDCS Protocols
Abstract
1. Introduction
2. Materials and Methods
2.1. Databases–Anatomical Modelling
2.2. Electromagnetic Characterization and Simulations Settings
2.3. Analyzed Quantities and Statistical Analysis
- Anatomical quantities: Cerebrospinal fluid volume, which due to its high conductivity shunts the injected current away from the target region; grey and white matter volumes (cm3); semi-circumference of the head (mm) in correspondence to the EEG 10-10 system points Fp and O, considered as a directly measurable anatomical characteristic of head size [65]; distance of P2–grey matter (mm) considered as a surrogate of the skull thickness (Table 1), one of the major determinants of current’s passage from the skin into the brain.
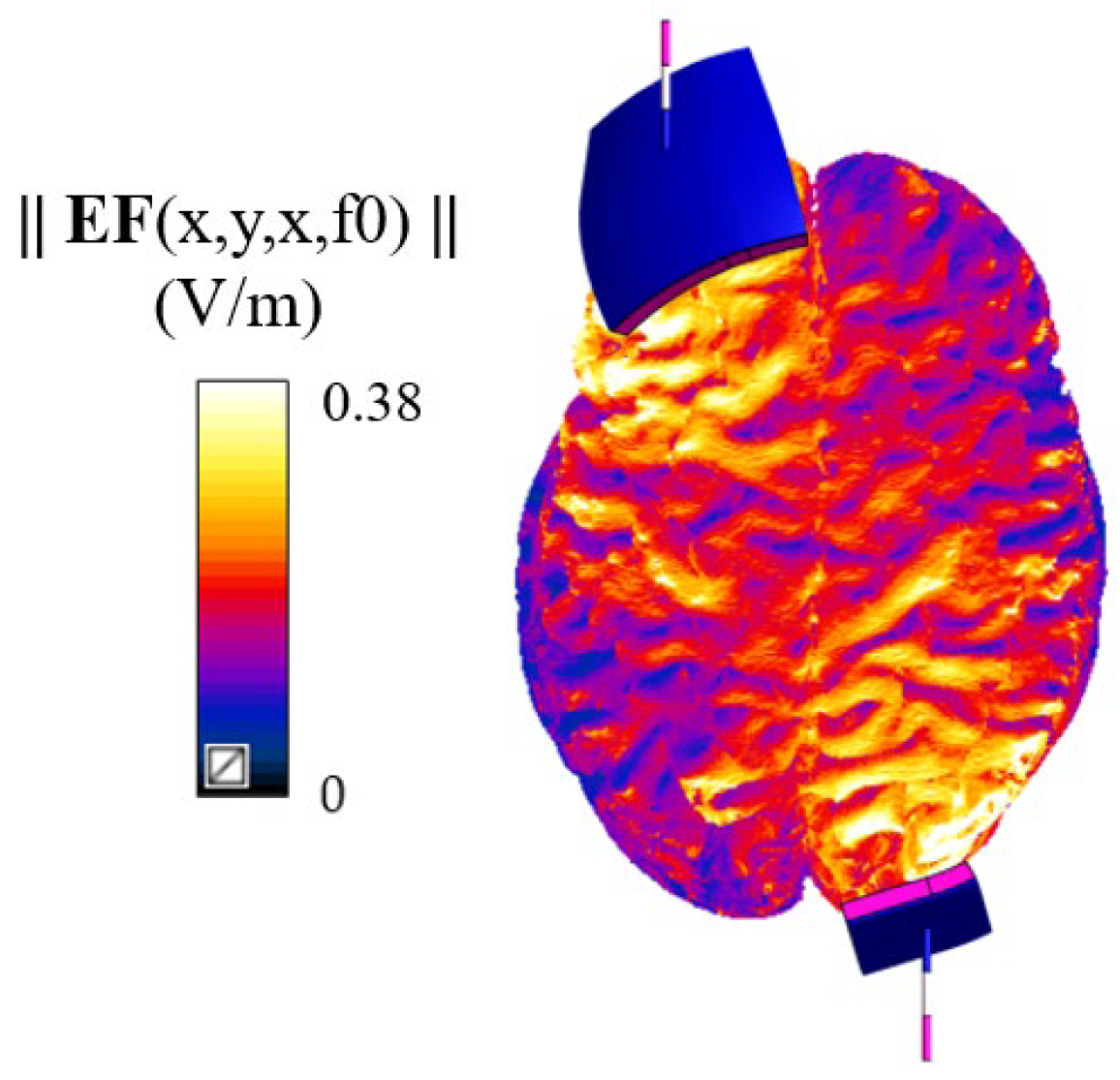
- Electric field distribution: Amplitude of the electric field distribution in grey and white matter.
- Correlation between the anatomical quantities and the percentiles of the EF distribution across each individual’s brain from both databases (Clinical Outcome and Integrated Databases). By including all twenty-three simulations, the post hoc power statistics for the two main correlations investigated reached a power of 99% for a significance level alpha of 0.05. Additionally, correlations between anatomical quantities and age were also explored for all twenty-three subjects.
- Correlation between TEPs and the EF quantities across subjects only from the Clinical Outcome Database (twelve subjects): to assess which of the EF quantities analyzed—percentiles or spread—affects more the clinical outcome and then the subjects’ responsiveness.
- Correlation between the anatomical quantities and the EF quantity found in step 2 across the models of the Clinical Outcome Database (twelve subjects). Then a multiple regression model, solely on the models of the Clinical Outcome Database, was investigated [43] to assess a priori whether a subject will be respondent or not with the fixed dose of 0.03 mA/cm2.
3. Results
- a.
- Inter-variability of electric field distribution and anthropometric quantities
- b.
- Correlations between anthropomorphic quantities and electric field distribution percentiles
- c.
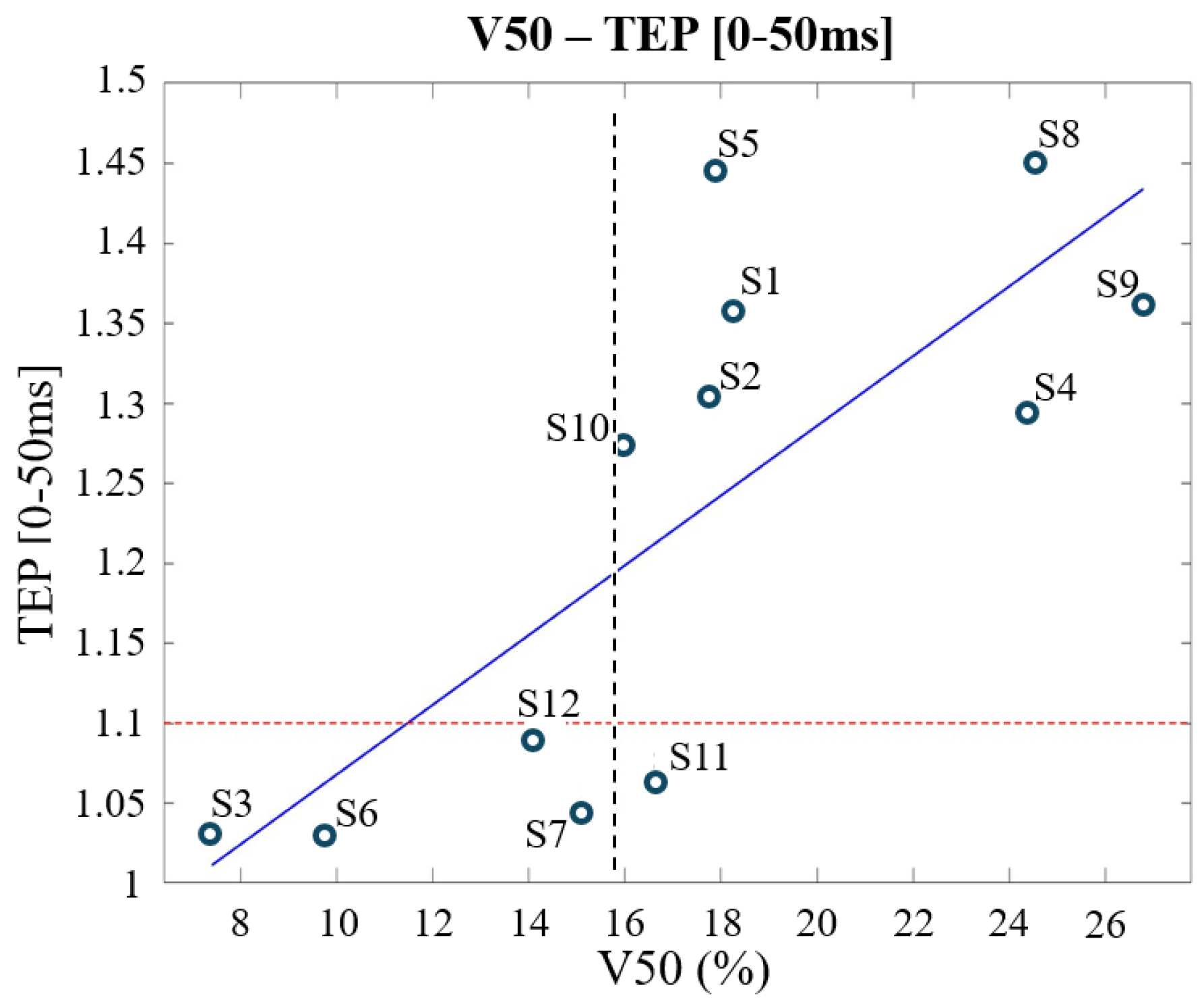
- Correlations between electric field quantities and TMS evoked potentials (TEPs) across Clinical Outcome Database
- d.
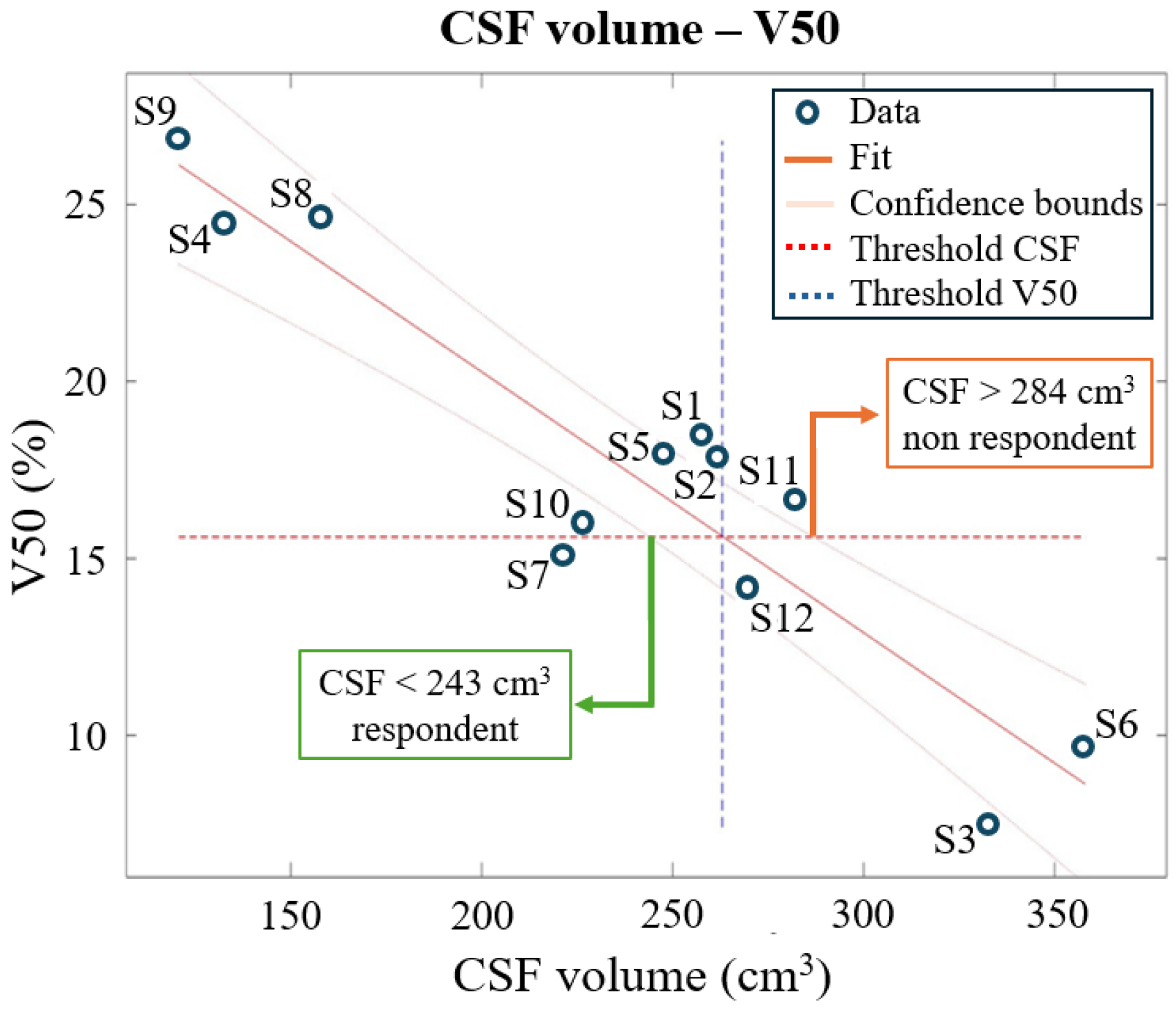
- Correlations between anatomical quantities and V50 (volume of brain tissues over 50% of the threshold 0.227 V/m) and multiple regression model
4. Discussion
- CSF < 243 cm3: Subject is likely to respond.
- CSF > 284 cm3: Subject is likely to be non-respondent.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| tDCS | Transcranial direct current stimulation |
| CSF | Cerebrospinal fluid |
| EF | Electric field |
| MaxEF | 99th percentile of the electric field distribution in the white and grey matter |
| CODb | Clinical Outcome Database |
| IDb | Integrated Database |
References
- Liu, Y.; Ouyang, P.; Zheng, Y.; Mi, L.; Zhao, J.; Ning, Y.; Guo, W. A Selective Review of the Excitatory-Inhibitory Imbalance in Schizophrenia: Underlying Biology, Genetics, Microcircuits, and Symptoms. Front. Cell Dev. Biol. 2021, 9, 664535. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef] [PubMed]
- Palm, U.; Ayache, S.S.; Padberg, F.; Lefaucheur, J.-P. Non-invasive brain stimulation therapy in multiple sclerosis: A review of tDCS, rTMS and ECT results. Brain Stimul. 2014, 7, 849–854. [Google Scholar] [CrossRef]
- Grigorescu, C.; Chalah, M.A.; Lefaucheur, J.-P.; Kümpfel, T.; Padberg, F.; Ayache, S.S.; Palm, U. Effects of Transcranial Direct Current Stimulation on Information Processing Speed, Working Memory, Attention, and Social Cognition in Multiple Sclerosis. Front. Neurol. 2020, 11, 545377. [Google Scholar] [CrossRef]
- Ferreri, F.; Guerra, A.; Vollero, L.; Ponzo, D.; Maatta, S.; Mervaala, E.; Iannello, G.; Di Lazzaro, V. Age-related changes of cortical excitability and connectivity in healthy humans: Non-invasive evaluation of sensorimotor network by means of TMS-EEG. Neuroscience 2017, 357, 255–263. [Google Scholar] [CrossRef][Green Version]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef]
- Priori, A. Brain polarization in humans: A reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin. Neurophysiol. 2003, 114, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabré, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef]
- Bikson, M.; Rahman, A.; Datta, A. Computational models of transcranial direct current stimulation. Clin. EEG Neurosci. 2012, 43, 176–183. [Google Scholar] [CrossRef]
- Neuling, T.; Wagner, S.; Wolters, C.H.; Zaehle, T.; Herrmann, C.S. Finite-Element Model Predicts Current Density Distribution for Clinical Applications of tDCS and tACS. Front. Psychiatry 2012, 3, 83. [Google Scholar] [CrossRef] [PubMed]
- Bindman, L.J.; Lippold, O.C.J.; Redfearn, J.W.T. Long-lasting Changes in the Level of the Electrical Activity of the Cerebral Cortex produced by Polarizing Currents. Nature 1962, 196, 584–585. [Google Scholar] [CrossRef] [PubMed]
- Purpura, D.P.; McMurtry, J.G. INTRACELLULAR ACTIVITIES AND EVOKED POTENTIAL CHANGES DURING POLARIZATION OF MOTOR CORTEX. J. Neurophysiol. 1965, 28, 166–185. [Google Scholar] [CrossRef]
- Liu, A.; Vöröslakos, M.; Kronberg, G.; Henin, S.; Krause, M.R.; Huang, Y.; Opitz, A.; Mehta, A.; Pack, C.C.; Krekelberg, B.; et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat. Commun. 2018, 9, 5092. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Lu, M.-K.; Antal, A.; Classen, J.; Nitsche, M.; Ziemann, U.; Ridding, M.; Hamada, M.; Ugawa, Y.; Jaberzadeh, S.; et al. Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clin. Neurophysiol. 2017, 128, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.-F.; Paulus, W.; Nitsche, M.A. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage 2014, 85, 948–960. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Santos, M.C.; Lima, M.; Vieira, A.L.; Rigonatti, S.P.; Silva, M.T.A.; Barbosa, E.R.; Nitsche, M.A.; Pascual-Leone, A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov. Disord. 2006, 21, 1693–1702. [Google Scholar] [CrossRef]
- Ferrucci, R.; Bortolomasi, M.; Vergari, M.; Tadini, L.; Salvoro, B.; Giacopuzzi, M.; Barbieri, S.; Priori, A. Transcranial direct current stimulation in severe, drug-resistant major depression. J. Affect. Disord. 2009, 118, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Narmashiri, A.; Akbari, F. The Effects of Transcranial Direct Current Stimulation (tDCS) on the Cognitive Functions: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2023, 35, 126–152. [Google Scholar] [CrossRef]
- Razza, L.B.; Palumbo, P.; Moffa, A.H.; Carvalho, A.F.; Solmi, M.; Loo, C.K.; Brunoni, A.R. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress. Anxiety 2020, 37, 594–608. [Google Scholar] [CrossRef]
- Moffa, A.H.; Martin, D.; Alonzo, A.; Bennabi, D.; Blumberger, D.M.; Benseñor, I.M.; Daskalakis, Z.; Fregni, F.; Haffen, E.; Lisanby, S.H.; et al. Efficacy and acceptability of transcranial direct current stimulation (tDCS) for major depressive disorder: An individual patient data meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 99, 109836. [Google Scholar] [CrossRef] [PubMed]
- Mosayebi-Samani, M.; Jamil, A.; Salvador, R.; Ruffini, G.; Haueisen, J.; Nitsche, M.A. The impact of individual electrical fields and anatomical factors on the neurophysiological outcomes of tDCS: A TMS-MEP and MRI study. Brain Stimul. 2021, 14, 316–326. [Google Scholar] [CrossRef]
- Lauro, L.J.R.; Pisoni, A.; Rosanova, M.; Casarotto, S.; Mattavelli, G.; Bolognini, N.; Vallar, G. Localizing the effects of anodal tDCS at the level of cortical sources: A Reply to Bailey et al., 2015. Cortex 2016, 74, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Lauro, L.J.R.; Rosanova, M.; Mattavelli, G.; Convento, S.; Pisoni, A.; Opitz, A.; Bolognini, N.; Vallar, G. TDCS increases cortical excitability: Direct evidence from TMS–EEG. Cortex 2014, 58, 99–111. [Google Scholar] [CrossRef]
- Saari, J.; Kallioniemi, E.; Tarvainen, M.; Julkunen, P. Oscillatory TMS-EEG-Responses as a Measure of the Cortical Excitability Threshold. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Esser, S.; Huber, R.; Massimini, M.; Peterson, M.; Ferrarelli, F.; Tononi, G. A direct demonstration of cortical LTP in humans: A combined TMS/EEG study. Brain Res. Bull. 2006, 69, 86–94. [Google Scholar] [CrossRef]
- Miniussi, C.; Thut, G. Combining TMS and EEG offers new prospects in cognitive neuroscience. Brain Topogr. 2010, 22, 249–256. [Google Scholar] [CrossRef]
- Ikkai, A.; Curtis, C.E. Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia 2011, 49, 1428–1434. [Google Scholar] [CrossRef]
- Fogassi, L.; Luppino, G. Motor functions of the parietal lobe. Curr. Opin. Neurobiol. 2005, 15, 626–631. [Google Scholar] [CrossRef]
- Shomstein, S. Cognitive functions of the posterior parietal cortex: Top-down and bottom-up attentional control. Front. Integr. Neurosci. 2012, 6, 38. [Google Scholar] [CrossRef]
- Wright, J.M.; Krekelberg, B. Transcranial direct current stimulation over posterior parietal cortex modulates visuospatial localization. J. Vis. 2014, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.B.; Sparing, R.; Fink, G.R.; Hesse, M.D. Modulation of attention functions by anodal tDCS on right PPC. Neuropsychologia 2015, 74, 96–107. [Google Scholar] [CrossRef]
- Ghanavati, E.; Nejati, V.; Salehinejad, M.A. Transcranial Direct Current Stimulation over the Posterior Parietal Cortex (PPC) Enhances Figural Fluency: Implications for Creative Cognition. J. Cogn. Enhanc. 2018, 2, 88–96. [Google Scholar] [CrossRef]
- Chalah, M.A.; Riachi, N.; Ahdab, R.; Mhalla, A.; Abdellaoui, M.; Créange, A.; Lefaucheur, J.-P.; Ayache, S.S. Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J. Neurol. Sci. 2017, 372, 131–137. [Google Scholar] [CrossRef]
- Salehinejad, M.A.; Ghayerin, E.; Nejati, V.; Yavari, F.; Nitsche, M.A. Domain-specific Involvement of the Right Posterior Parietal Cortex in Attention Network and Attentional Control of ADHD: A Randomized, Cross-over, Sham-controlled tDCS Study. Neuroscience 2020, 444, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Sparing, R.; Thimm, M.; Hesse, M.D.; Küst, J.; Karbe, H.; Fink, G.R. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain 2009, 132, 3011–3020. [Google Scholar] [CrossRef] [PubMed]
- Filmer, H.L.; Mattingley, J.B.; Dux, P.E. Modulating brain activity and behaviour with tDCS: Rumours of its death have been greatly exaggerated. Cortex 2020, 123, 141–151. [Google Scholar] [CrossRef]
- Evans, C.; Bachmann, C.; Lee, J.S.; Gregoriou, E.; Ward, N.; Bestmann, S. Dose-controlled tDCS reduces electric field intensity variability at a cortical target site. Brain Stimul. 2020, 13, 125–136. [Google Scholar] [CrossRef]
- Parazzini, M.; Fiocchi, S.; Ravazzani, P. Electric field and current density distribution in an anatomical head model during transcranial direct current stimulation for tinnitus treatment. Bioelectromagnetics 2012, 33, 476–487. [Google Scholar] [CrossRef]
- Fiocchi, S.; Ravazzani, P.; Priori, A.; Parazzini, M. Cerebellar and spinal direct current stimulation in children: Computational modeling of the induced electric field. Front. Hum. Neurosci. 2016, 10, 522. [Google Scholar] [CrossRef]
- Parazzini, M.; Fiocchi, S.; Liorni, I.; Priori, A.; Ravazzani, P. Computational modeling of transcranial direct current stimulation in the child brain: Implications for the treatment of refractory childhood focal epilepsy. Int. J. Neural Syst. 2014, 24, 1430006. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, M.; Fiocchi, S.; Rossi, E.; Paglialonga, A.; Ravazzani, P. Transcranial direct current stimulation: Estimation of the electric field and of the current density in an anatomical human head model. IEEE Trans. Biomed. Eng. 2011, 58, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Opitz, A.; Paulus, W.; Will, S.; Antunes, A.; Thielscher, A. Determinants of the electric field during transcranial direct current stimulation. NeuroImage 2015, 109, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Beumer, S.; Boon, P.; Klooster, D.C.W.; van Ee, R.; Carrette, E.; Paulides, M.M.; Mestrom, R.M.C. Personalized tDCS for Focal Epilepsy—A Narrative Review: A Data-Driven Workflow Based on Imaging and EEG Data. Brain Sci. 2022, 12, 610. [Google Scholar] [CrossRef]
- Peterchev, A.V.; Wagner, T.A.; Miranda, P.C.; Nitsche, M.A.; Paulus, W.; Lisanby, S.H.; Pascual-Leone, A.; Bikson, M. Fundamentals of transcranial electric and magnetic stimulation dose: Definition, selection, and reporting practices. Brain Stimul. 2012, 5, 435–453. [Google Scholar] [CrossRef]
- Ridding, M.C.; Ziemann, U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010, 588, 2291–2304. [Google Scholar] [CrossRef]
- Vergallito, A.; Feroldi, S.; Pisoni, A.; Lauro, L.J.R. Inter-Individual Variability in tDCS Effects: A Narrative Review on the Contribution of Stable, Variable, and Contextual Factors. Brain Sci. 2022, 12, 522. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Kashyap, R.; Goodwill, A.M.; O’BRien, B.A.; Rapp, B.; Oishi, K.; Desmond, J.E.; Chen, S.A. Sex difference in tDCS current mediated by changes in cortical anatomy: A study across young, middle and older adults. Brain Stimul. 2022, 15, 125–140. [Google Scholar] [CrossRef]
- Zanto, T.P.; Jones, K.T.; Ostrand, A.E.; Hsu, W.-Y.; Campusano, R.; Gazzaley, A. Individual differences in neuroanatomy and neurophysiology predict effects of transcranial alternating current stimulation. Brain Stimul. 2021, 14, 1317–1329. [Google Scholar] [CrossRef]
- Sun, W.; Wang, H.; Zhang, J.; Yan, T.; Pei, G. Multi-layer skull modeling and importance for tDCS simulation. In Proceedings of the 2021 International Conference on Bioinformatics and Intelligent Computing, Harbin, China, 22–24 January 2021; ACM: New York, NY, USA, 2021; pp. 250–256. [Google Scholar]
- Ono, S.M.; Kubik, C.D. Abernathey. In Atlas of the Cerebral Sulci; Thieme: New York, NY, USA, 1990. [Google Scholar]
- Rademacher, J.; Caviness, V.S.; Steinmetz, H.; Galaburda, A.M. Topographical variation of the human primary cortices: Implications for neuroimaging, brain mapping, and neurobiology. Cereb. Cortex 1993, 3, 313–329. [Google Scholar] [CrossRef]
- Dahnke, R.; Yotter, R.A.; Gaser, C. Cortical thickness and central surface estimation. NeuroImage 2013, 65, 336–348. [Google Scholar] [CrossRef]
- Datta, A.; Bansal, V.; Diaz, J.; Patel, J.; Reato, D.; Bikson, M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009, 2, 201–207.e1. [Google Scholar] [CrossRef]
- Huang, Y.; Datta, A.; Bikson, M.; Parra, L.C. Realistic volumetric-approach to simulate transcranial electric stimulation—ROAST—A fully automated open-source pipeline. J. Neural Eng. 2019, 16, 056006. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, E. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin. Neurophysiol. 2002, 113, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Sim4life by ZMT Zurich Med Tech AG, Zurich, Switzerland. Available online: www.zurichmedtech.com (accessed on 12 June 2025).
- Klem, G.H.; Lüders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar]
- Christ, A.; Kainz, W.; Hahn, E.G.; Honegger, K.; Zefferer, M.; Neufeld, E.; Rascher, W.; Janka, R.; Bautz, W.; Chen, J.; et al. The Virtual Family—Development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys. Med. Biol. 2010, 55, N23–N38. [Google Scholar] [CrossRef]
- Iacono, M.I.; Neufeld, E.; Akinnagbe, E.; Bower, K.; Wolf, J.; Oikonomidis, I.V.; Sharma, D.; Lloyd, B.; Wilm, B.J.; Wyss, M.; et al. MIDA: A Multimodal Imaging-Based Detailed Anatomical Model of the Human Head and Neck. PLoS ONE 2015, 10, e0124126. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, C.; Hasgall, P.A.; Di Gennaro, F.; Neufeld, E.; Lloyd, B.; Gosselin, M.C.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues. Version 4.1. Available online: http://itis.swiss/database (accessed on 5 June 2024).
- Saturnino, G.B.; Thielscher, A.; Madsen, K.H.; Knösche, T.R.; Weise, K. A principled approach to conductivity uncertainty analysis in electric field calculations. NeuroImage 2019, 188, 821–834. [Google Scholar] [CrossRef]
- Datta, A.; Baker, J.M.; Bikson, M.; Fridriksson, J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul. 2011, 4, 169–174. [Google Scholar] [CrossRef]
- Koessler, L.; Maillard, L.; Benhadid, A.; Vignal, J.P.; Felblinger, J.; Vespignani, H.; Braun, M. Automated cortical projection of EEG sensors: Anatomical correlation via the international 10–10 system. NeuroImage 2009, 46, 64–72. [Google Scholar] [CrossRef]
- Antonenko, D.; Grittner, U.; Puonti, O.; Flöel, A.; Thielscher, A. Estimation of individually induced e-field strength during transcranial electric stimulation using the head circumference. Brain Stimul. 2021, 14, 1055–1058. [Google Scholar] [CrossRef]
- Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson Correlation Coefficient. In Noise Reduction in Speech Processing; Springer Topics in Signal Processing; Springer: Berlin/Heidelberg, Germany, 2009; Volume 2. [Google Scholar] [CrossRef]
- Massey, F.J., Jr. The Kolmogorov-Smirnov Test for Goodness of Fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- Datta, A.; Bikson, M.; Fregni, F. Transcranial direct current stimulation in patients with skull defects and skull plates: High-resolution computational FEM study of factors altering cortical current flow. NeuroImage 2010, 52, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.K.; Minhas, P.; Woods, A.J.; Rosen, A.; Gorman, C.; Bikson, M.; Chambers, C. Dosage Considerations for Transcranial Direct Current Stimulation in Children: A Computational Modeling Study. PLoS ONE 2013, 8, e76112. [Google Scholar] [CrossRef] [PubMed]
- Laakso, I.; Mikkonen, M.; Koyama, S.; Hirata, A.; Tanaka, S. Can electric fields explain inter-individual variability in transcranial direct current stimulation of the motor cortex? Sci. Rep. 2019, 9, 626. [Google Scholar] [CrossRef]
- Chatzi, A.; Doody, O. The one-way ANOVA test explained. Nurse Res. 2023, 31, 8–14. [Google Scholar] [CrossRef]
- Feng, W.; Kautz, S.A.; Schlaug, G.; Meinzer, C.; George, M.S.; Chhatbar, P.Y. Transcranial Direct Current Stimulation for Poststroke Motor Recovery: Challenges and Opportunities. PM&R 2018, 10, S157–S164. [Google Scholar] [CrossRef]
- Wagner, S.; Rampersad, S.M.; Aydin, Ü.; Vorwerk, J.; Oostendorp, T.F.; Neuling, T.; Herrmann, C.S.; Stegeman, D.F.; Wolters, C.H. Investigation of tDCS volume conduction effects in a highly realistic head model. J. Neural Eng. 2014, 11, 016002. [Google Scholar] [CrossRef]
- Klassen, T.P.; Hartling, L.; Craig, J.C.; Offringa, M. Children are not just small adults: The urgent need for high-quality trial evidence in children. PLoS Med. 2008, 5, e172. [Google Scholar] [CrossRef]
- Kambeitz, J.; Goerigk, S.; Gattaz, W.; Falkai, P.; Benseñor, I.M.; Lotufo, P.A.; Bühner, M.; Koutsouleris, N.; Padberg, F.; Brunoni, A.R. Clinical patterns differentially predict response to transcranial direct current stimulation (tDCS) and escitalopram in major depression: A machine learning analysis of the ELECT-TDCS study. J. Affect. Disord. 2020, 265, 460–467. [Google Scholar] [CrossRef]


| Age (y) | Semi-Circumf Head (mm) | Skull Thickness (mm) | CSF Volume (cm3) | Grey Matter Volume (cm3) | White Matter Volume (cm3) | |
|---|---|---|---|---|---|---|
| CODb 1 | 27 ± 6 | 277.22 ± 12.03 | 16.83 ± 2.17 | 238.97 ± 73.06 | 674.78 ± 116.61 | 562.9 ± 73.68 |
| IDb over20 2 | 34 ± 6 | 305.71 ± 41.96 | 18.83 ± 3.11 | 233.04 ± 35.78 | 598.82 ± 94.55 | 512.8 ± 81.08 |
| IDb under20 2 | 9 ± 3 | 258.29 ± 9.21 | 11.19 ± 1.73 | 198.48 ± 83.28 | 693.45 ± 49.49 | 370.67 ± 65.38 |
| EF50 | EF75 | Max EF | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| CSF volume | −0.82 | 0.0000 * | −0.791 | 0.0000 * | −0.4024 | 0.057 |
| White matter volume | −0.6512 | 0.0008 * | −0.6605 | 0.0006 * | −0.7536 | 0.0000 * |
| Skull thickness | −0.5974 | 0.0026 * | −0.655 | 0.0007 * | −0.7918 | 0.0000 * |
| Semi-circumference | −0.4935 | 0.0196 * | −0.5447 | 0.0088 * | −0.6738 | 0.0006 * |
| Age | −0.5906 | 0.003 * | −0.6476 | 0.0008 * | −0.823 | 0.0000 * |
| TEP (ms) | Max EF | V50 | ||
|---|---|---|---|---|
| r | p | r | p | |
| 0–50 | 0.7248 | 0.0077 * | 0.7636 | 0.0039 * |
| 50–100 | 0.5953 | 0.0412 * | 0.5911 | 0.0429 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caiani, G.; Chiaramello, E.; Parazzini, M.; Arrigoni, E.; Lauro, L.J.R.; Pisoni, A.; Fiocchi, S. Anatomical Characteristics Predict Response to Transcranial Direct Current Stimulation (tDCS): Development of a Computational Pipeline for Optimizing tDCS Protocols. Bioengineering 2025, 12, 656. https://doi.org/10.3390/bioengineering12060656
Caiani G, Chiaramello E, Parazzini M, Arrigoni E, Lauro LJR, Pisoni A, Fiocchi S. Anatomical Characteristics Predict Response to Transcranial Direct Current Stimulation (tDCS): Development of a Computational Pipeline for Optimizing tDCS Protocols. Bioengineering. 2025; 12(6):656. https://doi.org/10.3390/bioengineering12060656
Chicago/Turabian StyleCaiani, Giulia, Emma Chiaramello, Marta Parazzini, Eleonora Arrigoni, Leonor J. Romero Lauro, Alberto Pisoni, and Serena Fiocchi. 2025. "Anatomical Characteristics Predict Response to Transcranial Direct Current Stimulation (tDCS): Development of a Computational Pipeline for Optimizing tDCS Protocols" Bioengineering 12, no. 6: 656. https://doi.org/10.3390/bioengineering12060656
APA StyleCaiani, G., Chiaramello, E., Parazzini, M., Arrigoni, E., Lauro, L. J. R., Pisoni, A., & Fiocchi, S. (2025). Anatomical Characteristics Predict Response to Transcranial Direct Current Stimulation (tDCS): Development of a Computational Pipeline for Optimizing tDCS Protocols. Bioengineering, 12(6), 656. https://doi.org/10.3390/bioengineering12060656










