Steal Syndrome in Free Flap Microvascular Reconstruction of the Lower Extremity: Systematic Review of Incidence, Risk Factors, and Surgical Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Guidelines and Ethics
2.2. Literature Search
- Terms related to free flap microvascular reconstruction: “Free Tissue Flaps,” “Microvascular Surgery,” “Microanastomosis,” “ Microvascular Reconstruction,” “Free Flap,” and “Free Tissue Graft.”
- Terms related to steal syndrome: “Steal Phenomenon,” “Steal Syndrome,” “Arterial Steal,” “Vascular Steal,” and “Subclavian Steal Syndrome.”
- Search operators were applied to refine results, such as steal NEXT/1 (syndrome* OR phenomenon*)) OR ((arter* OR vascular OR Subclavian) NEXT/1 steal).
2.3. Study Selection
- Clinical studies investigating the presence of steal syndrome in free flap microvascular reconstruction of the lower extremities.
- Studies reporting quantitative or qualitative data on steal syndrome, including predictors, implicated flaps, and recipient vessels.
- Prospective, retrospective, observational studies, case series, and case reports providing relevant data.
- Studies published in English.
- Animal studies or in vitro research.
- Studies focusing on non-free flaps (e.g., pedicled flaps), or reporting the occurrence of steal syndrome unrelated to free flap surgery.
- Studies without clinical relevance or those failing to report outcomes related to steal syndrome.
- Non-English language studies without available translations.
3. Quality Assessment and Risk of Bias
Statistical Analysis
4. Results
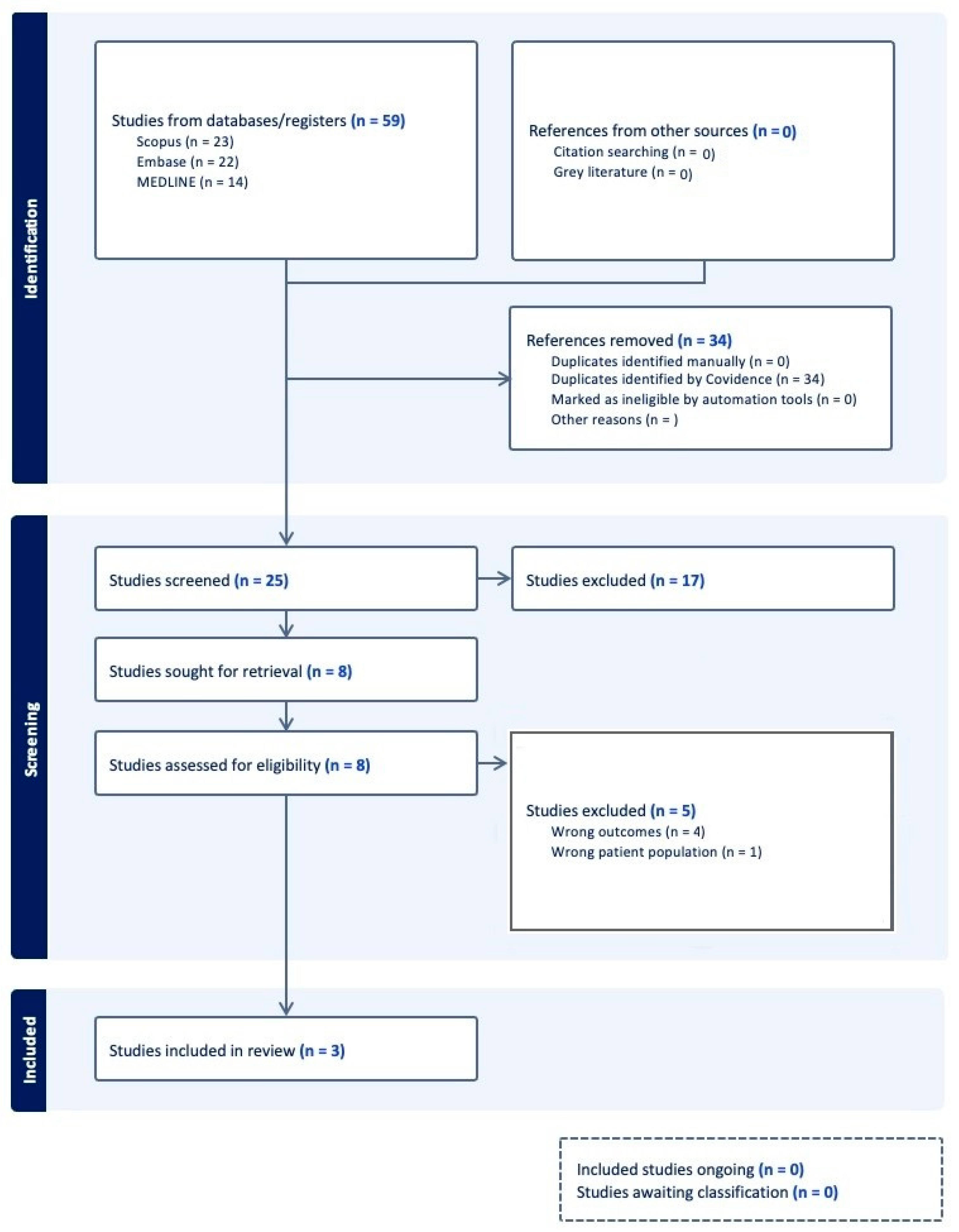
4.1. Study Selection
4.2. Study Characteristics
4.3. Patient Demographics
4.4. Clinical Characteristics
4.5. Microvascular Reconstruction Characteristics
4.6. Steal Syndrome Occurrence and Management
4.7. Risk of Bias and Level of Evidence
5. Discussion
6. Limitations
7. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lachica, R.D. Evidence-based medicine: Management of acute lower extremity trauma. Plast. Reconstr. Surg. 2017, 139, 287e–301e. [Google Scholar] [CrossRef] [PubMed]
- Bosse, M.J.; MacKenzie, E.J.; Kellam, J.F.; Burgess, A.R.; Webb, L.X.; Swiontkowski, M.F.; Sanders, R.W.; Jones, A.L.; McAndrew, M.P.; Patterson, B.M.; et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. N. Engl. J. Med. 2002, 347, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Chung, C.H.; Chang, Y.J.; Kim, K.H. Reconstruction of the lower extremity using free flaps. Arch. Plast. Surg. 2013, 40, 575–583. [Google Scholar] [CrossRef]
- Haddock, N.T.; Weichman, K.E.; Reformat, D.D.; Kligman, B.E.; Levine, J.P.; Saadeh, P.B. Lower extremity arterial injury patterns and reconstructive outcomes in patients with severe lower extremity trauma: A 26-year review. J. Am. Coll. Surg. 2010, 210, 66–72. [Google Scholar] [CrossRef]
- Lerman, O.Z.; Kovach, S.J.; Levin, L.S. The respective roles of plastic and orthopedic surgery in limb salvage. Plast. Reconstr. Surg. 2011, 127, 215S–227S. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, L.J.; Krieger, L.M. From the reconstructive ladder to the reconstructive elevator. Plast. Reconstr. Surg. 1994, 93, 1503–1504. [Google Scholar] [CrossRef]
- Busse, J.W.; Jacobs, C.L.; Swiontkowski, M.F.; Bosse, M.J.; Bhandari, M.; Evidence-Based Orthopaedic Trauma Working Group. Complex limb salvage or early amputation for severe lower-limb injury: A meta-analysis of observational studies. J. Orthop. Trauma 2007, 21, 70–76. [Google Scholar] [CrossRef]
- Xiong, L.; Gazyakan, E.; Kremer, T.; Hernekamp, F.J.; Harhaus, L.; Cyr, M.S.; Kneser, U.; Hirche, C. Free flaps for reconstruction of soft tissue defects in lower extremity: A meta-analysis on microsurgical outcome and safety. Microsurgery 2016, 36, 511–524. [Google Scholar] [CrossRef]
- Engel, H.; Lin, C.-H.; Wei, F.-C. Role of microsurgery in lower extremity reconstruction. Plast. Reconstr. Surg. 2011, 127, 228S–238S. [Google Scholar] [CrossRef]
- Ong, Y.S.; Levin, L.S. Lower limb salvage in trauma. Plast. Reconstr. Surg. 2010, 125, 582–588. [Google Scholar] [CrossRef]
- Saad, N.; McGill, M.; Karamitros, G.; Cromack, D.; Wang, H.; Fisher, S.; Karamanos, E. Surface to perforator index: Assessing the importance of the number of perforators in successful harvesting of the anterolateral thigh flap. J. Reconstr. Microsurg. 2024, 40, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kozusko, S.D.; Liu, X.; Riccio, C.A.; Chang, J.; Boyd, L.C.; Kokkalis, Z.; Konofaos, P. Selecting a free flap for soft tissue coverage in lower extremity reconstruction. Injury 2019, 50, S32–S39. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.H.; Shammas, R.L.; Carney, M.J.; Weissler, J.M.; Bauder, A.R.; Glener, A.D.; Kovach, S.J.; Hollenbeck, S.T.; Levin, L.S. Muscle versus fasciocutaneous free flaps in lower extremity traumatic reconstruction: A multicenter outcomes analysis. Plast. Reconstr. Surg. 2018, 141, 191–199. [Google Scholar] [CrossRef]
- Karamitros, G.; Papas, A.; Lykoudis, E.; Grant, M.P.; Lamaris, G.A. Letter comments on “advanced reconstructive techniques following orbital exenteration: The role of LCFA free flaps.” temporalis muscle flap: A simple yet reliable alternative for orbital exenteration reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2025, 101, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Edlich, R.; Wish, J.R.; Britt, L.D.; Long, W.B., III. An organized approach to trauma care: Legacy of R adams cowley. J. Long-Term. Eff. Med. Implants 2004, 14, 481–511. [Google Scholar] [CrossRef]
- Rusli, S.M.; Metz, A.; Steinbacher, J.; Roka-Palkovits, J.; Hu, N.; Tinhofer, I.; Chieh-Han, J.T. Free flap steal syndrome and the loss of free lymphatic tissue flap transplantation in lymphoedema surgery: A case report. J. Lymphoedema 2023, 18, 63. [Google Scholar]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of prospero: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Deeks, J.J.; Bossuyt, P.M.; Leeflang, M.M.; Takwoingi, Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid.-Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- Sullivan, D.; Chung, K.C.; Eaves, F.F., III; Rohrich, R.J. The level of evidence pyra- mid: Indicating levels of evidence in plastic and reconstructive surgery articles. Plast. Reconstr. Surg. 2011, 128, 311–314. [Google Scholar] [CrossRef]
- Sonntag, B.V.; Murphy, R.X., Jr.; Chernofsky, M.A.; Chowdary, R.P. Microvascular steal phenomenon in lower extremity reconstruction. Ann. Plast. Surg. 1995, 34, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Musser, D.J.; Berger, A.; Hallock, G.G. Free flap “steal” hastening amputation of a revascularized lower limb. Eur. J. Plast. Surg. 1995, 18, 311–313. [Google Scholar] [CrossRef]
- Rainer, C.; Schwabegger, A.H.; Meirer, R.; Perkmann, R.; Ninkovic, M.; Ninkovic, M. Microsurgical management of the diabetic foot. J. Reconstr. Microsurg. 2003, 19, 543–553. [Google Scholar]
- Garcia, L.A. Epidemiology and pathophysiology of lower extremity peripheral arterial disease. J. Endovasc. Ther. 2006, 13 (Suppl. 2), II3–II9. [Google Scholar] [CrossRef]
- Weber, B.; Mahapatra, S.; Ryu, H.; Lee, S.; Fuhrer, A.; Reusch, T.C.; Thompson, D.L.; Lee, W.C.; Klimeck, G.; Hollenberg, L.C.L.; et al. Ohm’s law survives to the atomic scale. Science 2012, 335, 64–67. [Google Scholar] [CrossRef]
- Sutera, S.P.; Skalak, R. The history of poiseuille’s law. Annu. Rev. Fluid Mech. 1993, 25, 1–20. [Google Scholar] [CrossRef]
- Skelly, C.L.; Meyerson, S.L.; Curi, M.A.; Loth, F.; Schwartz, L.B. The hemodynamics of vein grafts: Measurement and meaning. Ann. Vasc. Surg. 2001, 15, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Zilla, P.; Bezuidenhout, D.; Human, P. Prosthetic vascular grafts: Wrong models, wrong questions and no healing. Biomaterials 2007, 28, 5009–5027. [Google Scholar] [CrossRef]
- Nasir, S.; Baykal, B.; Altunta, S.; Aydin, M.A. Hemodynamic differences in blood flow between free skin and muscles flaps: Prospective study. J. Reconstr. Microsurg. 2009, 25, 355–360. [Google Scholar] [CrossRef]
- Motomiya, M.; Watanabe, N.; Nakamura, S.; Kameda, Y.; Kawamura, D.; Iwasaki, N. Blood flow distribution after end-to-side anastomosis with wide arteriotomy in extremity free flap surgery. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2495–2503. [Google Scholar] [CrossRef]
- Nasir, S.; Aydin, M.A.; Sonmez, E.; Baykal, B. Flow-through free latissimus dorsi flap for reconstruction of injured limbs: Evaluation of hemodynamic effects on extremity circulation. Ann. Plast. Surg. 2010, 65, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Nissen, T.; Wynn, R. The clinical case report: A review of its merits and limitations. BMC Res. Notes 2014, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Karamitros, G.; Grant, M.P.; Lamaris, G.A. Enhancing the QUINTET study: Exploring quality of life after open lower limb trauma while strengthening research methodology. J. Plast. Reconstr. Aesthet. Surg. 2025, 100, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Karamitros, G.; Grant, M.P.; Lamaris, G.A. Associations in medical research can be misleading: A clinician’s guide to causal inference. J. Surg. Res. 2025, 310, 145–154. [Google Scholar] [CrossRef]
- Karamitros, G.; Lamaris, G.A.; Goulas, S. US air pollution and increased incidence of non-syndromic cleft lip/palate: Association does not imply causality. J. Plast. Reconstr. Aesthet. Surg. 2024, 90, 23–24. [Google Scholar] [CrossRef]
- Rizzi, D.A.; Pedersen, S.A. Causality in medicine: Towards a theory and terminology. Theor. Med. 1992, 13, 233–254. [Google Scholar] [CrossRef]
- Karamitros, G.; Papas, A.; Grant, M.P.; Lamaris, G.A. Letter comments on: Prevalence of body dysmorphic disorder in plastic surgery: Addressing biases and improving screening approaches. J. Plast. Reconstr. Aesthet. Surg. 2025, 101, 190–191. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Rutjes, A.W.; Whiting, P.; Vlassov, V.V.; Leeflang, M.M.; Deeks, J.J. Assessing methodological quality. In Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version; Cochrane: London, UK, 2009; Volume 1, pp. 1–28. [Google Scholar]
- Karamitros, G.; Grant, M.P.; Lamaris, G.A. Reevaluating the impact of scars on satisfaction and HR-QoLafter DIEP-flap breast reconstruction: Methodological concerns and future directions. Aesthet. Plast. Surg. 2024, 49, 733–740. [Google Scholar] [CrossRef]
| Study | Journal | Study Design | Country | Patients | Number of Flaps | Level of Evidence |
|---|---|---|---|---|---|---|
| Sonntag et al., 1995 [22] | Annals of Plastic Surgery | USA | Retrospective case series | 3 | 3 | IV |
| Musser et al., 1995 [23] | European Journal of Plastic Surgery | USA | Case report | 1 | 1 | V |
| Rainer et al., 2003 [24] | Journal of Reconstructive Microsurgery | Austria | Retrospective case series | 9 | 10 | IV |
| Study | Mean Age (±SD) | Indication | Flap Type | Number of Patients Developing Steal Syndrome | Patient Comorbidities | Vascular Pedicle | Recipient Vessel |
|---|---|---|---|---|---|---|---|
| Sonntag et al., 1995 [22] | 69.67 ± 4.04 | Non-healing heel ulcer and necrosis of the 5th toe, non-healing heel ulcer, non-healing heel ulcer, and exposed Achilles tendon | LD (2) and rectus abdominis (1) | 3 | HTN, DM, PVD, and RF | Thoracodorsal and inferior epigastric | Popliteal (3) |
| Musser et al., 1995 [23] | 73 | Non-healing forefoot ulcer | LD | 1 | HTN, DM, and PVD | Thoracodorsal | Anterior tibial |
| Rainer et al., 2003 [24] | 54.3 ± 9.37 | Diabetic foot ulcer | LD (2) and gracilis (1) | 3 | DM, HTN, PVD, and PN | Thoracodorsal, medial circumflex femoral | Anterior tibial (1) and dorsal pedis (2) |
| Study | Anastomotic Configuration | Artery Inflow (Recipient Artery) | Venous Outflow (Single/Double Vein Anastomosis) | Vein Graft Used (Yes/No) |
|---|---|---|---|---|
| Sonntag et al., 1995 [22] | End-to-side | Reverse vein graft between the LD and popliteal artery, vein graft used on PTFE graft between the deep femoral artery and popliteal artery, and vein graft between the popliteal artery and dorsal pedis | Popliteal vein (single vein) | Saphenous vein |
| Musser et al., 1995 [23] | End-to-side | Saphenous vein graft between the superficial femoral to the proximal anterior tibial | Posterior tibial vein (single vein) | Cephalic vein |
| Rainer et al., 2003 [24] | Flow-through and end-to-side | Anterior tibial | Anterior tibial (single vein) | No |
| Study | Patients with Steal Syndrome | Salvage Intervention | Follow-Up (Months) |
|---|---|---|---|
| Sonntag et al., 1995 [22] | 3 | Not attempted, BKA | Not mentioned |
| Musser et al., 1995 [23] | 1 | Not attempted, BKA | Not mentioned |
| Rainer et al., 2003 [24] | 3 | Anastomotic revision of venous thrombosis (post-op day 4), debridement (post-op day 22), | 37.7 ± 4.6 |
| Chopart’s amputation due to progressive ischemic necrosis of the remaining toes (after post-op day 22), | |||
| and debridement of peripheral necrosis (post-op day 58 and 64) |
| Domain for Evaluating the Methodological Quality of Case Reports and Case Series | ||||||||
|---|---|---|---|---|---|---|---|---|
| Selection | Ascertainment | Causality | Reporting | |||||
| Reference | Q.1 | Q.2 | Q.3 | Q.4 | Q.5 | Q.6 | Q.7 | Q.8 |
| Sonntag et al., 1995 [22] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Musser et al., 1995 [23] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Rainer et al., 2003 [24] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamitros, G.; Iliadis, I.; Pensy, R.A.; Lamaris, G.A. Steal Syndrome in Free Flap Microvascular Reconstruction of the Lower Extremity: Systematic Review of Incidence, Risk Factors, and Surgical Management. Bioengineering 2025, 12, 647. https://doi.org/10.3390/bioengineering12060647
Karamitros G, Iliadis I, Pensy RA, Lamaris GA. Steal Syndrome in Free Flap Microvascular Reconstruction of the Lower Extremity: Systematic Review of Incidence, Risk Factors, and Surgical Management. Bioengineering. 2025; 12(6):647. https://doi.org/10.3390/bioengineering12060647
Chicago/Turabian StyleKaramitros, Georgios, Ilias Iliadis, Raymond A. Pensy, and Gregory A. Lamaris. 2025. "Steal Syndrome in Free Flap Microvascular Reconstruction of the Lower Extremity: Systematic Review of Incidence, Risk Factors, and Surgical Management" Bioengineering 12, no. 6: 647. https://doi.org/10.3390/bioengineering12060647
APA StyleKaramitros, G., Iliadis, I., Pensy, R. A., & Lamaris, G. A. (2025). Steal Syndrome in Free Flap Microvascular Reconstruction of the Lower Extremity: Systematic Review of Incidence, Risk Factors, and Surgical Management. Bioengineering, 12(6), 647. https://doi.org/10.3390/bioengineering12060647






