1. Introduction
Rib fractures occur in up to 43% of cases involving blunt chest injuries [
1], with incidence rates increasing by 64.1% since 1990 [
2]. Flail chest, resulting from multiple rib fractures, often leads to complications such as abnormal chest wall movement, pain, and decreased respiratory function. Subsequently, this increases the risk of pulmonary infection and acute respiratory distress syndrome, contributing to a mortality rate of up to 33% [
3]. For most patients with rib fractures, conservative treatment options including multimodal pain management, inhaled bronchodilators, and ventilator support, are the treatments of choice [
4]. However, conservative treatment is associated with an increased risk of complications, including chronic pain, chest wall deformity, and bone nonunion [
5,
6]. The surgical stabilization of rib fractures (SSRF) significantly improves the short- and long-term prognoses of patients compared with conservative treatment. Specifically, the SSRF plays a key role in promoting fracture healing, relieving pain, restoring chest wall stability, and reducing mortality [
7,
8].
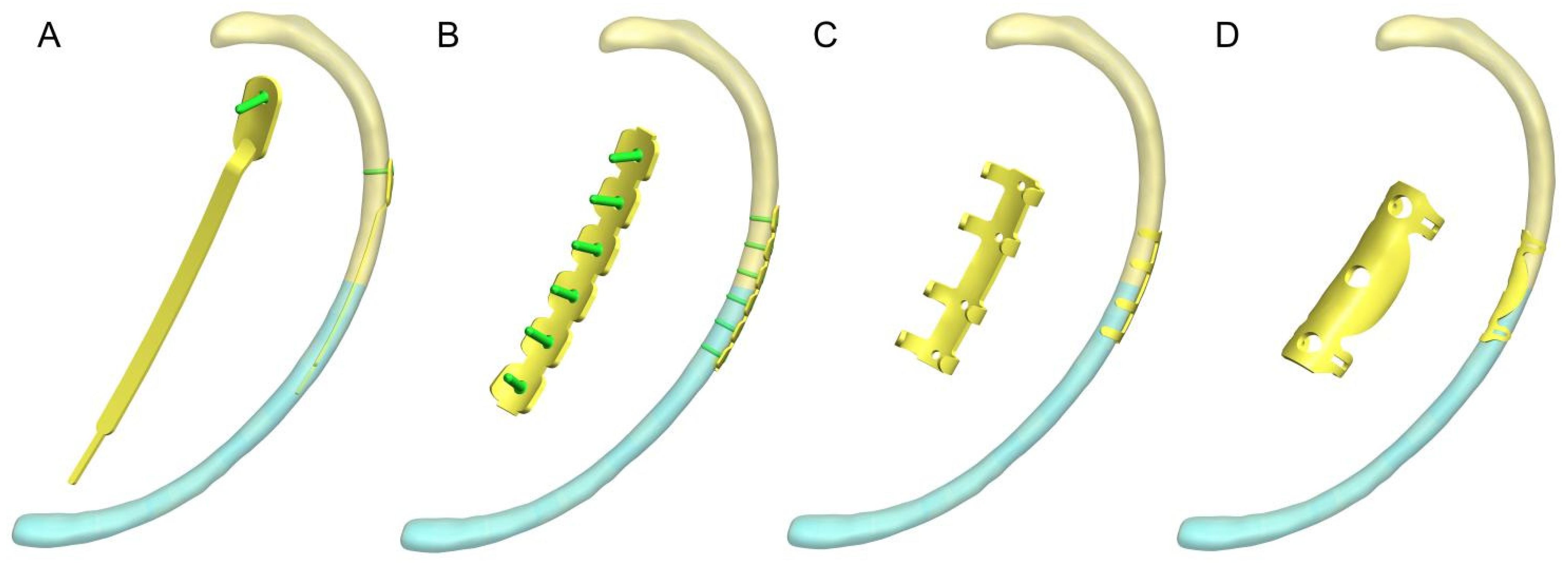
With advances in implant design and surgical techniques, the fixation techniques for rib fractures are becoming increasingly diverse. Intramedullary splints, claw plates, and locking plates are widely used to treat rib fractures [
9]. Recently, minimally invasive rib fracture fixation techniques, assisted by thoracoscopy, have been introduced. These techniques offer the advantages of minimizing soft tissue injury and accelerating the patient’s recovery [
10]. Regardless of the fixation system used, the rib fracture stabilization methods have historically been divided into two categories: cortical and intramedullary fixation [
11]. Although biomechanical experiments and clinical studies have reported the mechanical properties and clinical outcomes of different rib fracture fixation devices, a consensus on the optimal fixation protocol remains lacking [
12].
Fracture fixation stability is a crucial biomechanical factor that affects fracture healing and clinical outcomes [
13]. Improvement in the respiratory function of the patient is strongly correlated with fracture fixation stability. Given the challenges of directly studying the biomechanical metrics of fracture stability and implant failure risk directly in vitro or in vivo, finite element analysis (FEA) has emerged as a reliable method for evaluating these parameters [
14]. In analyzing the complex mechanical behavior related to the primary stability of fracture fixation structures, FEA offers distinct advantages over other computational and experimental methods [
15]. The present study aimed to numerically investigate the surgical fixation of rib fractures in specific patients using FEA, assess the biomechanical stability of four different fixation modalities, and provide a biomechanical reference for selecting the appropriate implantation protocols for the SSRF.
4. Discussion
A single rib fracture may lead to chronic pain, decreased respiratory function, and reduced long-term quality of life [
29]. Retrospective studies and randomized controlled trials have demonstrated that the SSRF improves patient clinical outcomes [
30]. Currently, the biomechanical properties of implants for treating rib fractures and the advantages of different fixation methods remain unclear. Appropriate implants foster appropriate mechanical conditions for fracture healing and provide sufficient stability to alleviate pain and dysfunction. Therefore, studying the biomechanical properties of different fixation methods from a biomechanical perspective is crucial to provide a reference for selecting appropriate implants for rib fractures.
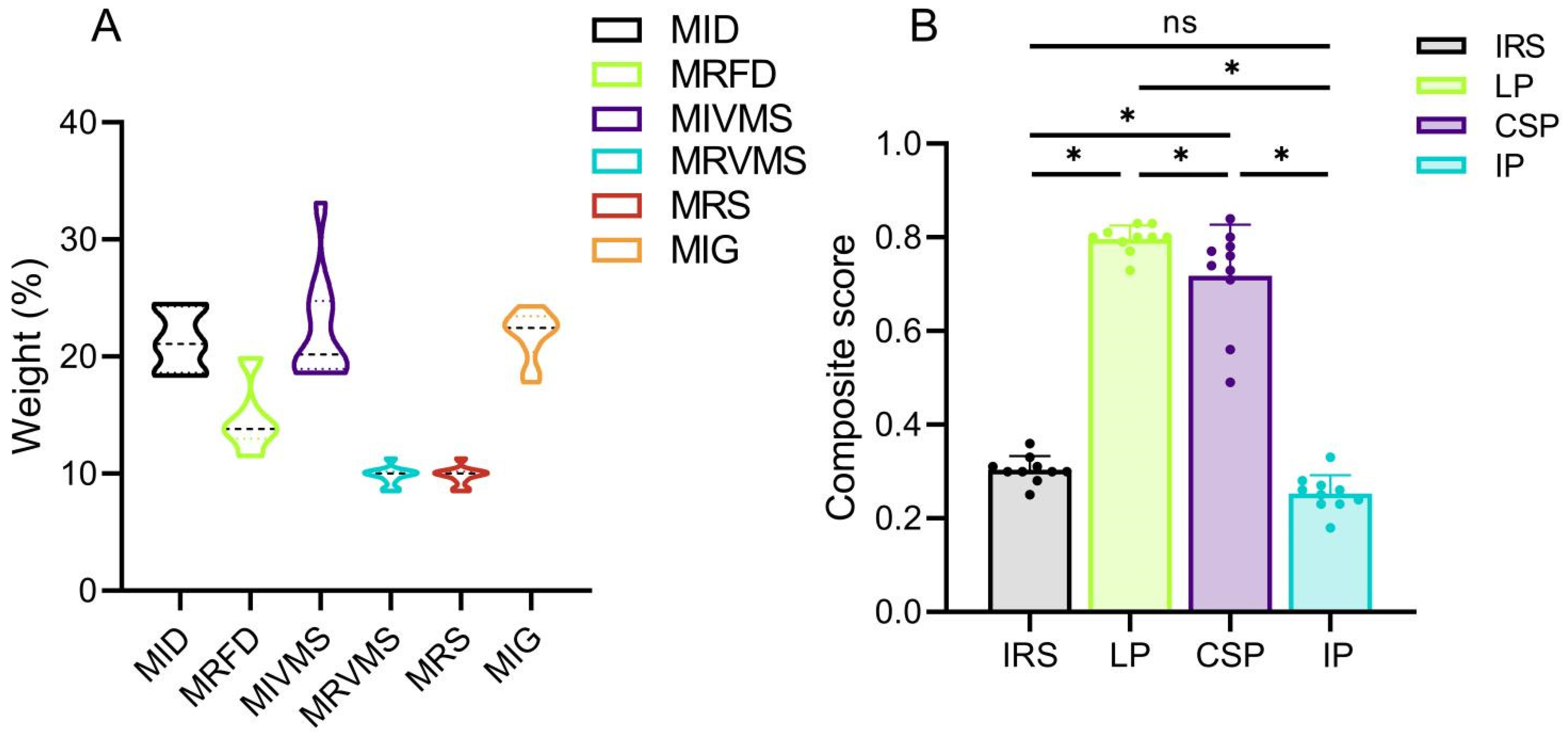
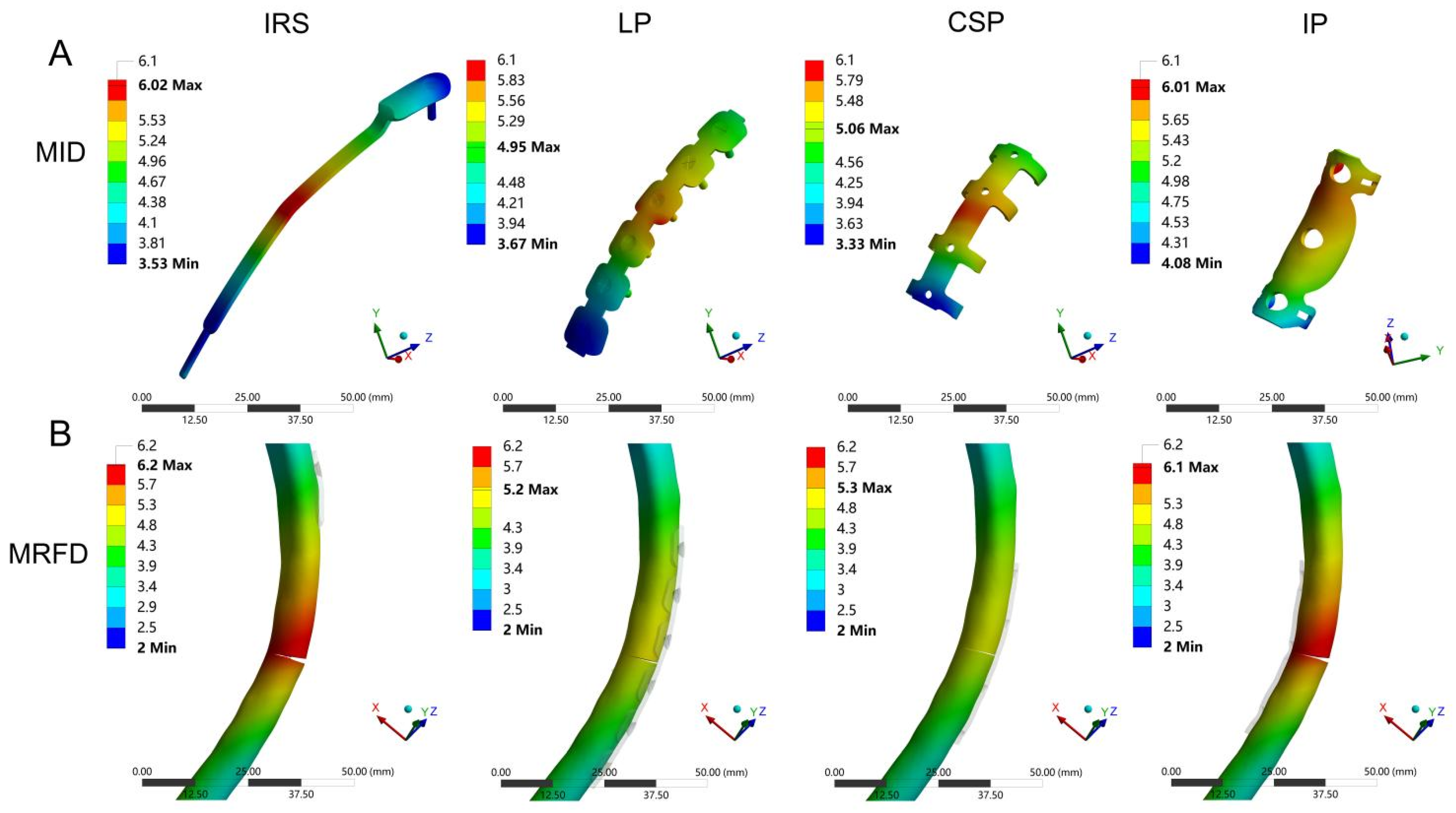
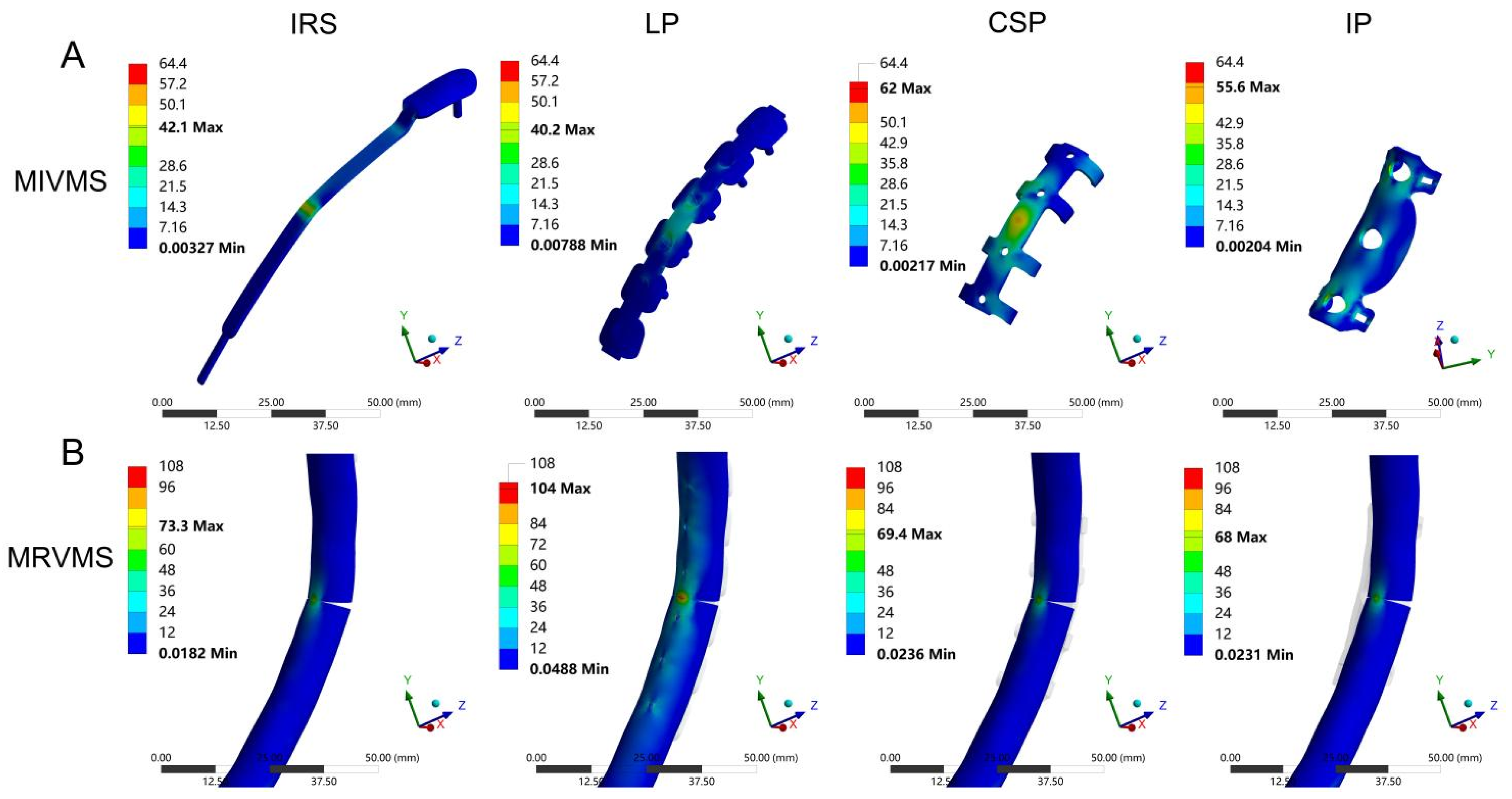
To the best of our knowledge, this is the first comprehensive biomechanical study of the SSRF to compare six biomechanical parameters of 10 patient-specific models stabilized using four different implants. Previous studies have assessed the biomechanical stability of rib fracture fixation using parameters such as implant and bone displacements and stresses [
31]. Notably, the variability of these outcome measures must be recognized, particularly for parameters such as the MIVMS and MRVMS, which exhibit significant standard errors [
32]. Therefore, biomechanical outcomes may vary among patients, even with the identical fixation structures. These variations stem from individual differences in bone mineral density and anatomical morphology. Consequently, constructing patient-specific FE models with a substantial number of samples is crucial to significantly eliminate the impact of individual differences on experimental outcomes. A desirable performance of a model is characterized by low values for each of the six biomechanical parameters (MID, MRFD, MIVMS, MRVMS, MRS, and MIG). The entropy method enables the synthesis of all the biomechanical parameter results and facilitates the comparison of the stability among various fixation modalities, while identifying the significant biomechanical parameters [
32]. Our comprehensive scoring results, based on independent biomechanical parameters, demonstrated better biomechanical stability with the LP compared with the other fixation methods for treating rib fractures.
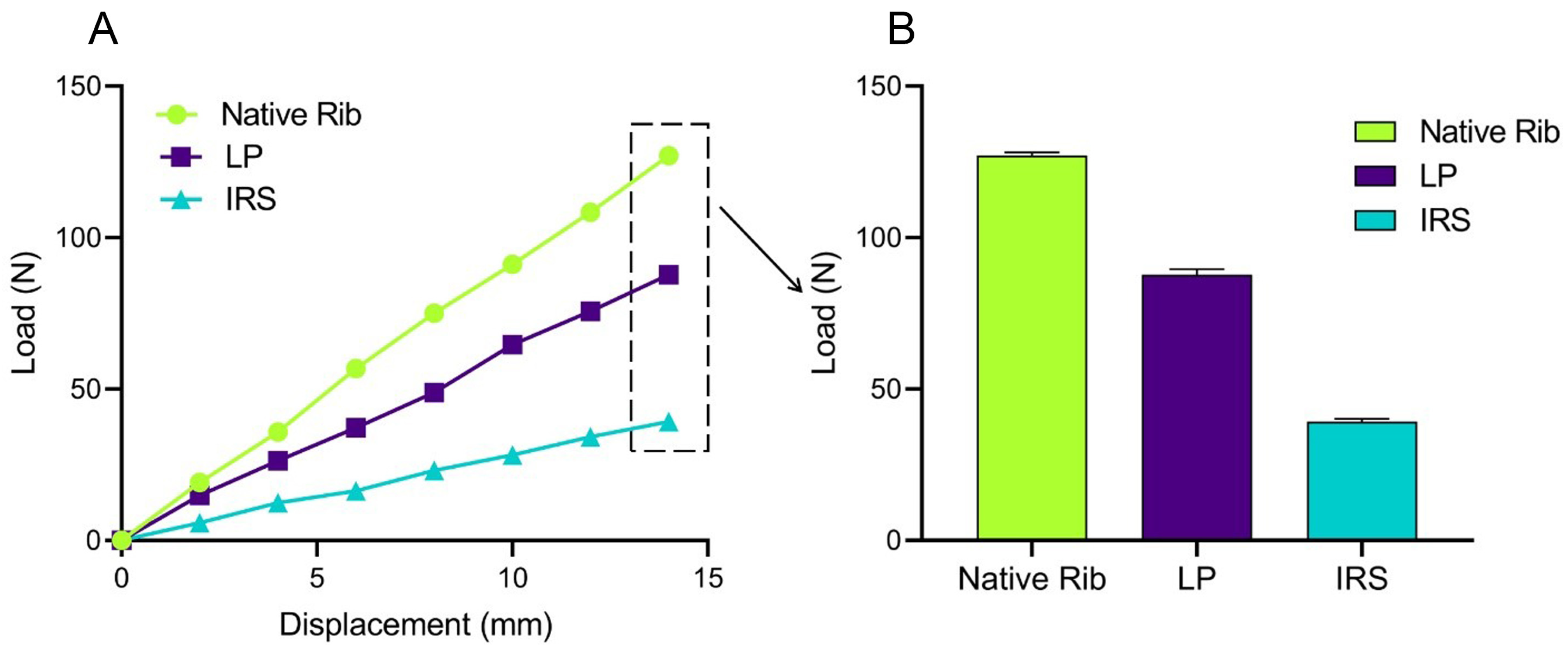
We also verified the effectiveness of the simulation model by comparing the FE simulation results with those of the biomechanical studies reported in the literature [
33,
34]. Thus, under identical experimental conditions, the patient-specific rib stiffness values obtained from the FEA (
Figure A1)—native (9.08 ± 0.08 N/mm), LP fixation (6.27 ± 0.12 N/mm), and IRS fixation (2.81 ± 0.06 N/mm)—were consistent with the biomechanical data reported by Bottlang et al. (native 10.0 ± 6.0 N/mm, LP fixation 7.0 ± 4.0 N/mm, and IRS fixation 2.0 ± 1.0 N/mm), with a standard deviation of ±1, indicating the suitability of our model construction method for further analysis.
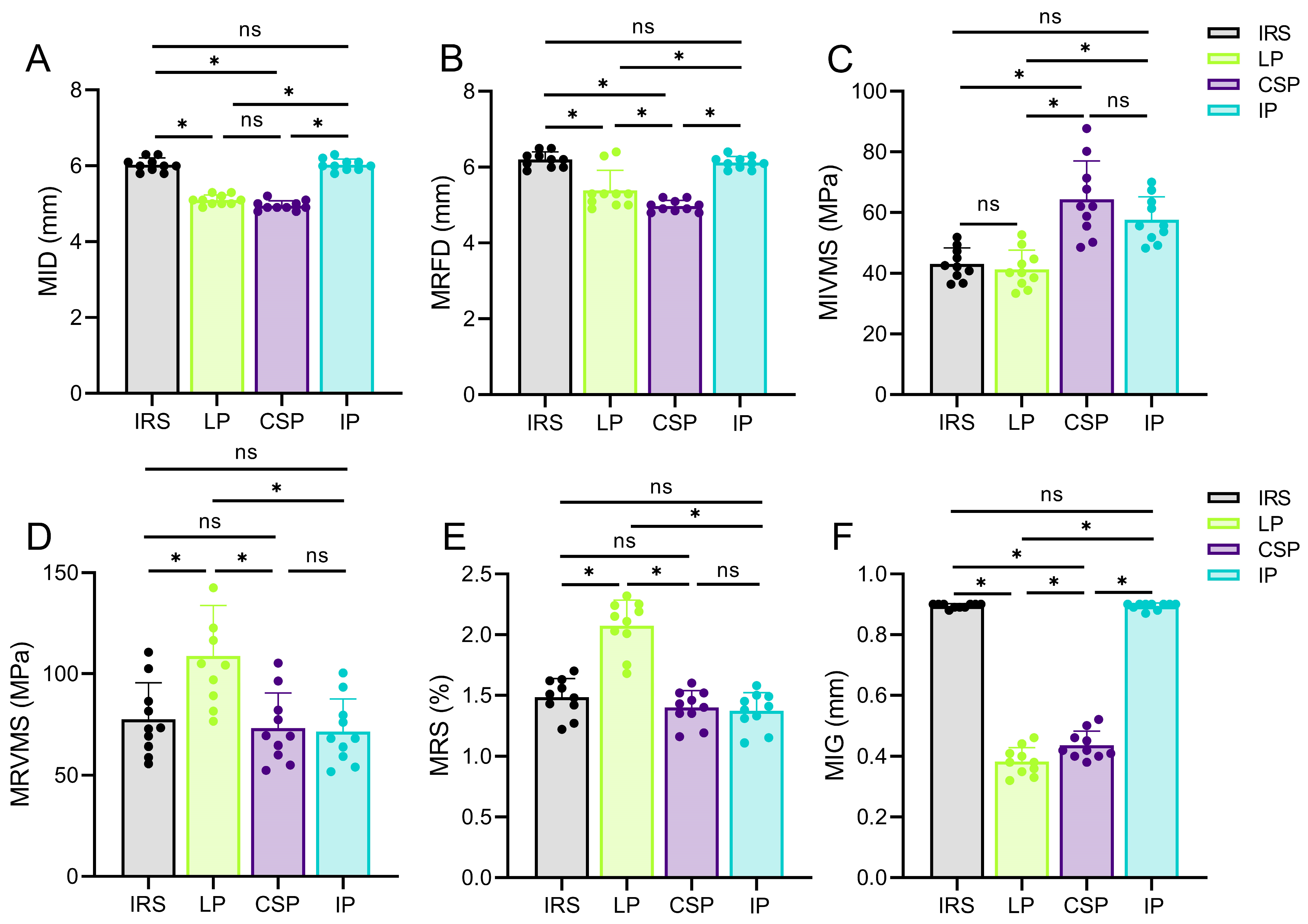
Implant and bone displacements are typical outcome parameters for the FEA and have been widely evaluated in in-silico studies [
31,
35]. Our results revealed higher fixation stabilities with the CSP and LP, owing to their lower MID and MRFD values. In contrast, the IRS and IP fixation exhibited a lower stability compared with previous findings [
20,
36]. This may be related to the anatomical characteristics of the ribs, as the maximum bending moment occurs in the lateral region of the rib under an anteroposterior load. The lateral cortical fixation techniques of the CSP and LP enhanced the lateral cortical stability of the ribs, resulting in smaller bone and implant displacements during rib collisions. Therefore, the CSP and LP were more reliable in terms of fixation strength. Furthermore, in addition to reduction and firm fixation, the blood supply to the fracture end is critical for fracture healing [
37]. Compared with the CSP, smaller bone and implant displacements were observed with the LP. The CSP may compress the intercostal nerves and blood vessels during the fracture end fixation, causing chest pain and affecting the blood supply. Typically, the LP does not require freeing the vascular and nerve bundles at the lower edge of the ribs during implantation, offering certain advantages in preserving the blood supply [
38]. Therefore, a LP is an appropriate implant for the treatment of rib fractures, whether for fixation stability or protection of blood supply.
Peak stress is associated with static yield or cyclic fatigue failure, and fixation failure often begins at the stress concentration site [
39]. In the present study, the MIVMS of the LP was lower than that of the other groups, indicating more uniform stress distribution in the LP with a lower risk of fixation failure. This may be associated with the stress-transfer modes of the different fixation methods. The LP acts as a stress-transfer bridge between the fractured ends of the anterior and posterior ribs. The stress is evenly shared by the LP and the screws, thereby reducing bone stress and displacement [
34]. The CSP stabilizes the fracture ends via the clasp mechanism of the circlip, counteracting the bending moment generated by the anteroposterior load of the ribs and reducing fixation stress [
38]. The IRS restores the continuity of rib fractures via an intramedullary implant for elastic fixation [
33]. The IP restores the load transfer by enhancing the medial cortical stability, effectively reducing the MRVMS value in the medial cortex [
40]. Existing implants are sufficient for maintaining the stability of the chest wall under normal physiological breathing conditions. Meanwhile, accident-induced fractures around implants during postoperative rehabilitation are a significant concern. Therefore, suitable fixation devices should prevent new fractures in vulnerable areas of the chest wall when subjected to external forces. The stress distribution results show that the LP fixation structure not only firmly stabilizes the fracture ends but also effectively reduces the stress on the fixation structure and provides better stability and safety in some cases, such as minor impacts.
Bone strain is a biomechanical parameter commonly used to predict bone failure. By measuring the axial and shear components at the end of the fracture fragment, the interfragmentary strain and stability of the fracture fixation can be evaluated in a normalized manner, which is of great predictive significance for fracture healing [
41,
42]. Our findings revealed that the CSP and LP exhibited lower MIG values than the IRS and IP when subjected to a frontal collision. This implies that the intramedullary and medial rib cortical fixations are less stable than lateral rib cortical fixation under extreme conditions. Elkins et al. [
43], through the FEA of patient-specific fracture healing based on radiographic data, demonstrated that appropriate axial micromotion at the fracture site promotes fracture healing, whereas excessive separation motion inhibits fracture healing. Under the mechanical effect of the implant, the MIG at the fracture site exhibited a state of “relative stability”. As the fracture healing progresses, the callus gradually hardens, and the variable gap decreases, ultimately restoring the load transmission through the fracture [
44]. In case of an external impact, lateral rib cortical fixation methods, such as the LP and CSP, exhibit lower MIG values, indicating a greater potential for promoting fracture healing. However, further validation of this finding is warranted using clinical studies with large sample sizes. In addition, the MIG value of the CSP was higher than that of the LP, which is consistent with the results of biomechanical experiments conducted by Huang et al. [
38]. As long as the fracture fixation provides sufficient stability, more flexible structures can be beneficial for fracture healing [
45]. Therefore, in addition to the LP, lateral cortical fixation strategies, such as the CSP, also appear to exhibit unique advantages in treating rib fractures.
The present study is the first to use FEA to comprehensively evaluate the biomechanical effects of four different fixation methods on the stability after rib fracture fixation. Nevertheless, this study has some limitations. First, despite our efforts to mitigate the impact of variations in rib morphology and material properties on experimental outcomes by increasing the sample size, the number of patient samples remains limited. Consequently, the trends observed in this study may not be applicable to all patients. Second, not including the cartilage, thoracic organs, and vertebrae in our FEA model poses challenges to our ability to fully evaluate the effect of fixation structures on the overall stability of the chest wall. Third, the scope of this study was confined to the assessment of a single loading condition and, as such, neglects other clinically relevant conditions, such as thoracic forces on the ribs, sternum, and costal cartilage during respiration.