A 3D SVZonChip Model for In Vitro Mimicry of the Subventricular Zone Neural Stem Cell Niche
Abstract
1. Introduction
2. Materials and Methods
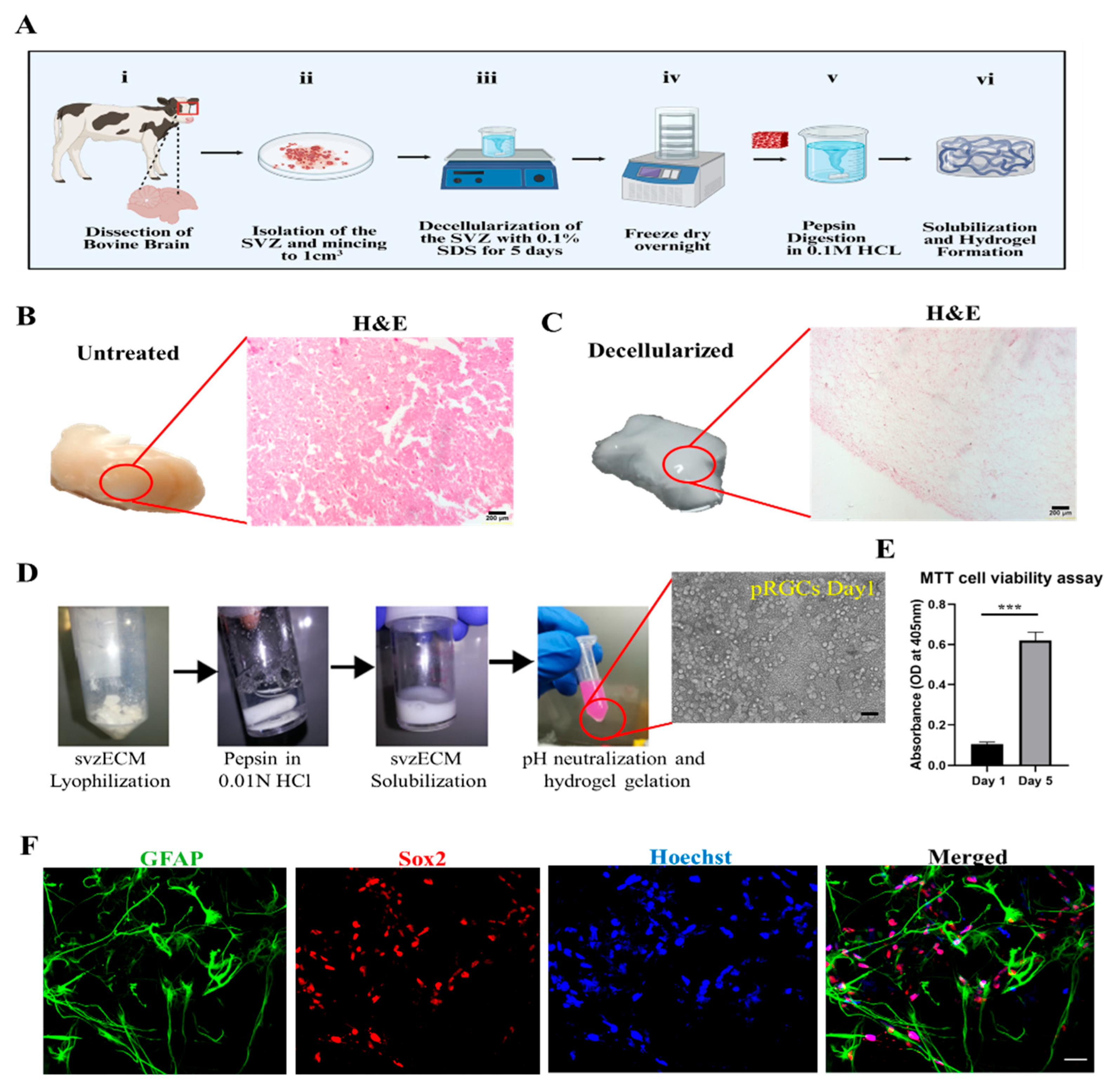
2.1. Decellularization of the Bovine SVZ
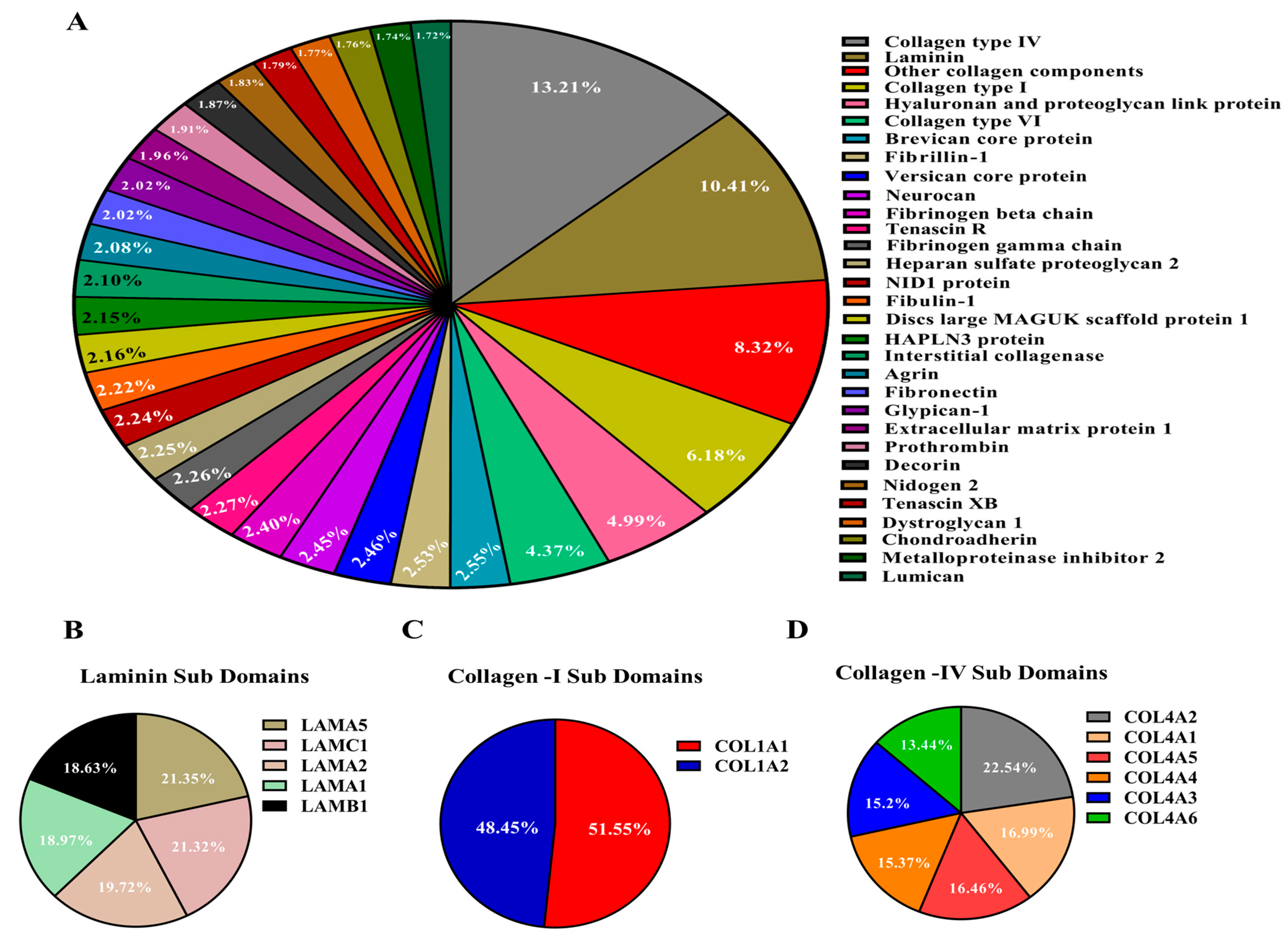
2.2. Characterization and Relative Quantification of the Protein Composition of the Bovine svzECM Decellularized Tissue
2.3. Fabrication of the Bovine svzECM Hydrogel
2.4. Characterization of the Bovine svzECM Hydrogel
2.5. Rheological Characterization of the Bovine svzECM Hydrogel
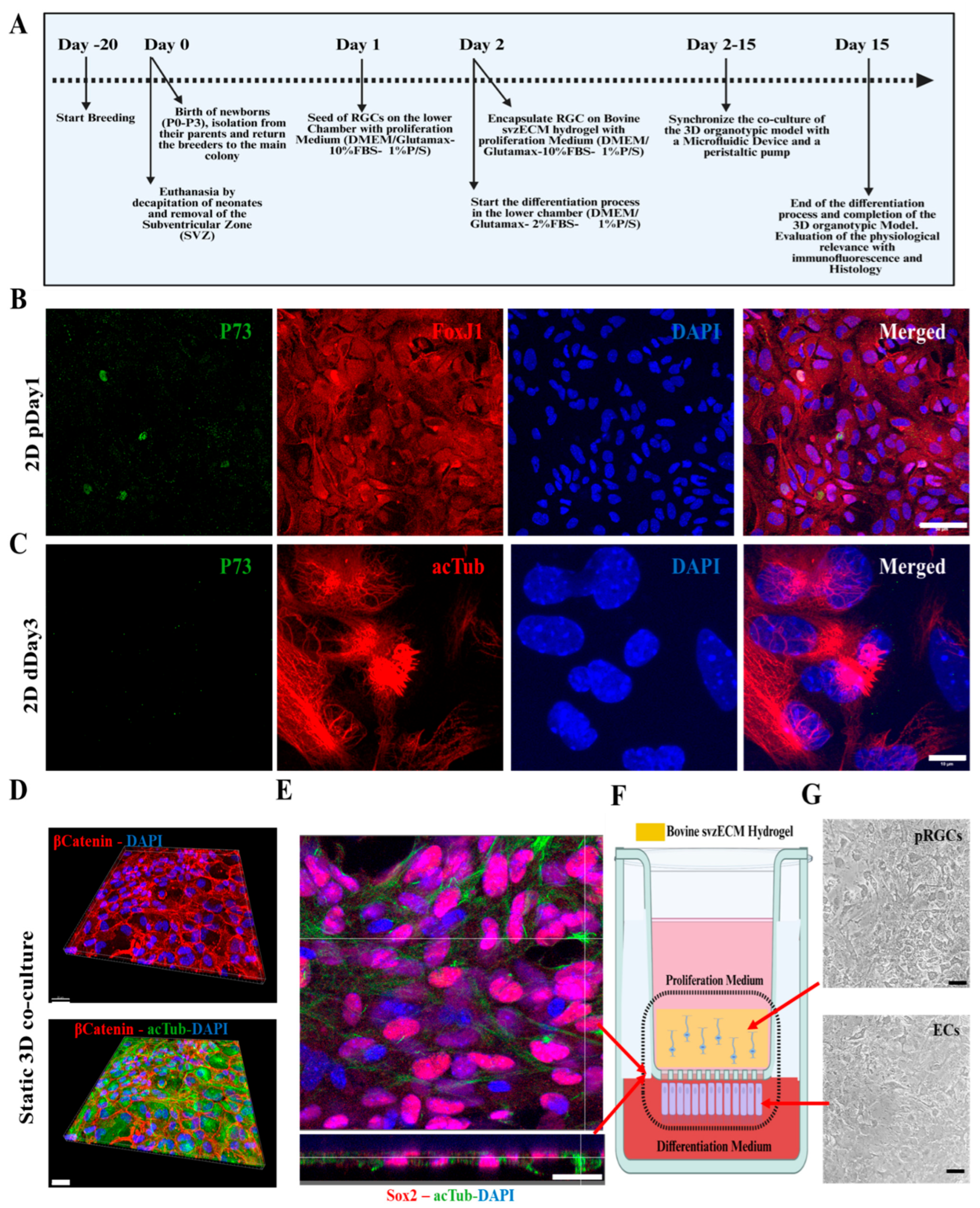
2.6. Mouse SVZ Isolation and Postnatal RGC Cell Culture
2.7. Cell Proliferation Assay
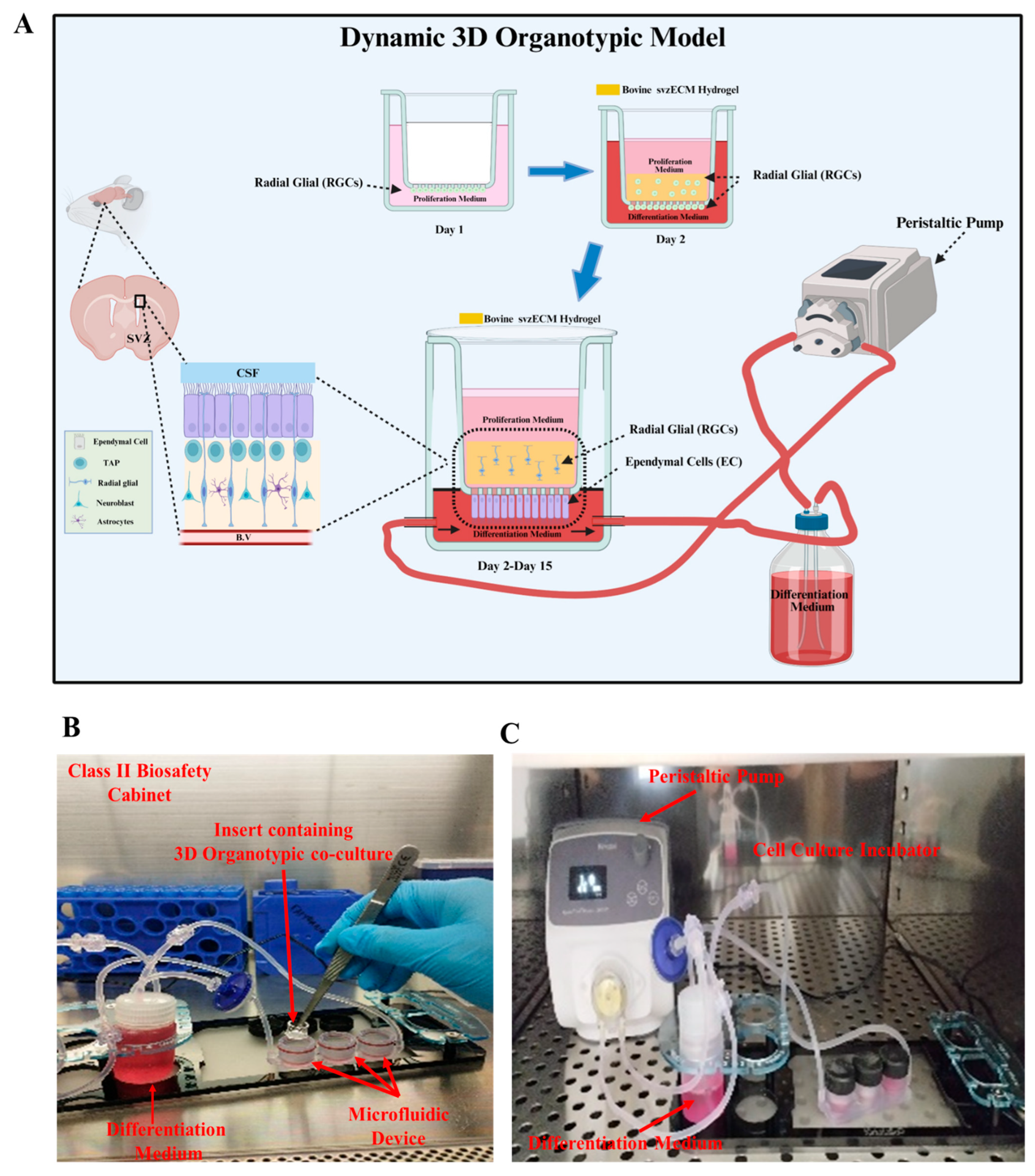
2.8. Static and Dynamic 3D Organotypic Models
2.9. Immunocytochemistry
2.10. Histology and Immunohistochemistry
2.11. Statistical Analysis
3. Results
3.1. Bovine SVZ Decellularization
3.2. Quantitative Characterization of the Decellularized Bovine svzECM
3.3. Characterization of the Decellularized Bovine svzECM Hydrogel
3.4. Developing a Static 3D Organotypic SVZ Model
3.5. Developing a Dynamic 3D Organotypic SVZ Model by Integrating Uniaxial Microfluidic Flow
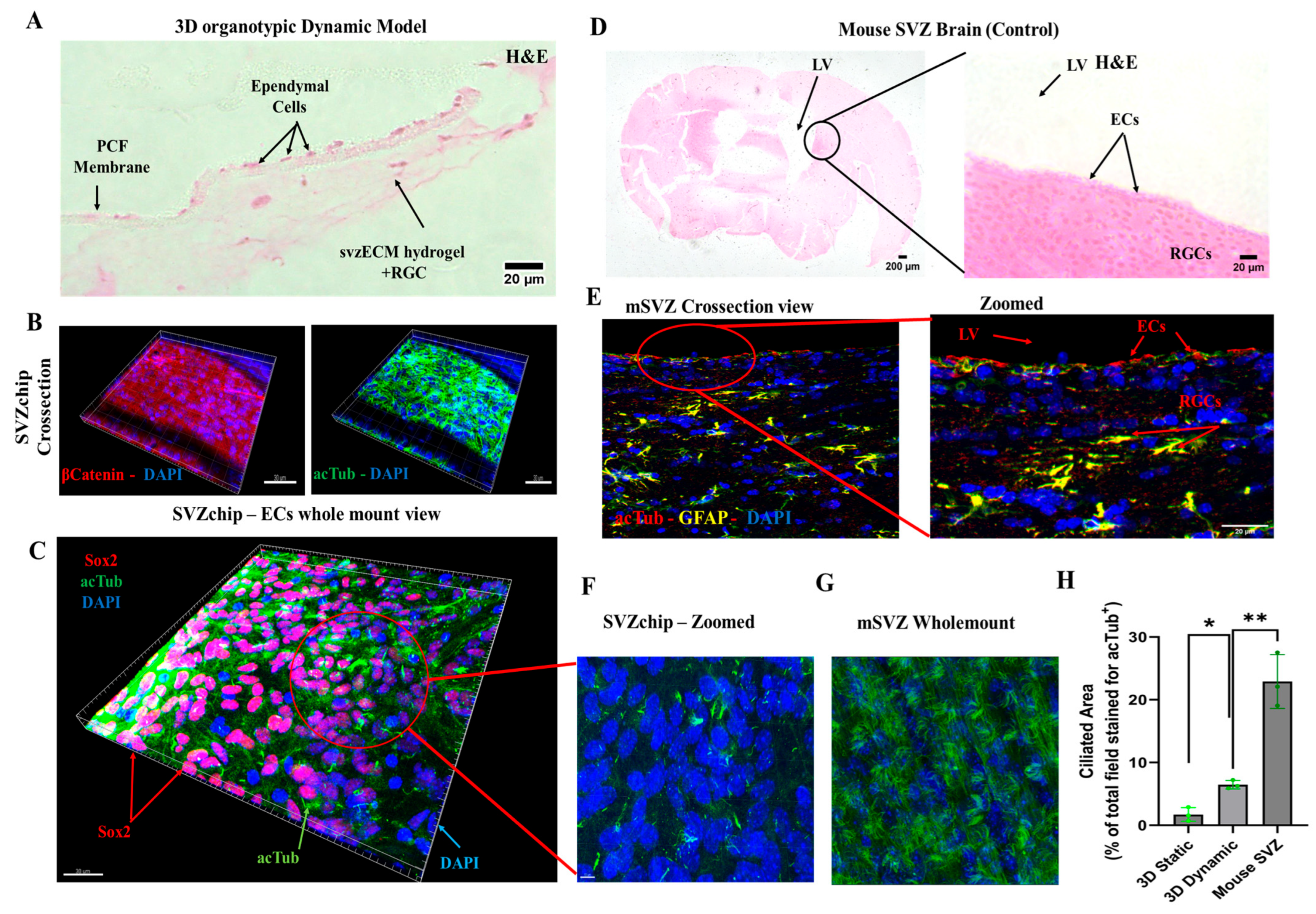
3.6. Characterization of the Dynamic SVZonChip and Comparison with the Mouse SVZ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cadwell, C.R.; Bhaduri, A.; Mostajo-Radji, M.A.; Keefe, M.G.; Nowakowski, T.J. Development and Arealization of the Cerebral Cortex. Neuron 2019, 103, 980–1004. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, J.P.; Silva, W.N.; Costa, A.C.; Picoli, C.C.; Bitencourt, F.C.O.; Coimbra-Campos, L.M.C.; Resende, R.; Magno, L.V.; Romano-Silva, M.A.; Bureš, A.; et al. Neural stem cell niche heterogeneity. Semin. Cell Dev. Biol. 2019, 95, 42–53. [Google Scholar] [CrossRef]
- Angelopoulos, I.; Gakis, G.; Birmpas, K.; Kyrousi, C.; Habeos, E.E.; Kaplani, K.; Lygerou, Z.; Habeos, I.; Taraviras, S. Metabolic regulation of the neural stem cell fate: Unraveling new connections, establishing new concepts. Front. Neurosci. 2022, 16, 1009125. [Google Scholar] [CrossRef]
- Altmann, C.; Keller, S.; Schmidt, M.H.H. The Role of SVZ Stem Cells in Glioblastoma. Cancers 2019, 11, 448. [Google Scholar] [CrossRef]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Med. 2015, 7, a018812. [Google Scholar] [CrossRef]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef]
- Del Bigio, M.R. The ependyma: A protective barrier between brain and cerebrospinal fluid. Glia 1995, 14, 1–13. [Google Scholar] [CrossRef]
- Murphy, A.R.; Laslett, A.; O’Brien, C.M.; Cameron, N.R. Scaffolds for 3D in vitro culture of neural lineage cells. Acta Biomater. 2017, 54, 1–20. [Google Scholar] [CrossRef]
- Jovanov Milošević, N.; Judaš, M.; Aronica, E.; Kostovic, I. Chapter 7–Neural ECM in Laminar Organization and Connectivity Development in Healthy and Diseased Human Brain. In Progress in Brain Research; Dityatev, A., Wehrle-Haller, B., Pitkänen, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 159–178. [Google Scholar]
- Urbán, N.; Guillemot, F.; Schwamborn, J.C. Cellular Neuroscience Neurogenesis in the embryonic and adult brain: Same regulators, different roles. Front. Cell. Neurosci. 2014, 8, 396. [Google Scholar] [CrossRef]
- Zappaterra, M.W.; Lehtinen, M.K. The cerebrospinal fluid: Regulator of neurogenesis, behavior, and beyond. Cell. Mol. Life Sci. 2012, 69, 2863–2878. [Google Scholar] [CrossRef]
- Nishimura, T.; Honda, H.; Takeichi, M. Planar Cell Polarity Links Axes of Spatial Dynamics in Neural-Tube Closure. Cell 2012, 149, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Morita, H.; Ueno, N. Molecular mechanisms of cell shape changes that contribute to vertebrate neural tube closure. Dev. Growth Differ. 2012, 54, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Desmond, M.E.; Knepper, J.E.; Dibenedetto, A.J.; Malaugh, E.; Callejo, S.; Carretero, R.; Alonso, M.-I.; Gato, A. Focal adhesion kinase as a mechanotransducer during rapid brain growth of the chick embryo. Int. J. Dev. Biol. 2014, 58, 35–43. [Google Scholar] [CrossRef]
- Neuhaus, J.; Risau, W.; Wolburg, H. Induction of Blood-Brain Barrier Characteristics in Bovine Brain Endothelial Cells by Rat Astroglial Cells in Transfilter Coculture. Ann. N. Y. Acad. Sci. 1991, 633, 578–580. [Google Scholar] [CrossRef]
- Gaillard, P.J.; Voorwinden, L.H.; Nielsen, J.L.; Ivanov, A.; Atsumi, R.; Engman, H.; Ringbom, C.; de Boer, A.G.; Breimer, D.D. Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur. J. Pharm. Sci. 2001, 12, 215–222. [Google Scholar] [CrossRef]
- Hatherell, K.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Pilkington, G.J. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods 2011, 199, 223–229. [Google Scholar] [CrossRef]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. A novel transwell blood brain barrier model using primary human cells. Front. Cell. Neurosci. 2019, 13, 230. [Google Scholar] [CrossRef]
- Li, Q.; Ford, M.C.; Lavik, E.B.; Madri, J.A. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: An in vitro study. J. Neurosci. Res. 2006, 84, 1656–1668. [Google Scholar] [CrossRef]
- Kokovay, E.; Goderie, S.; Wang, Y.; Lotz, S.; Lin, G.; Sun, Y.; Roysam, B.; Shen, Q.; Temple, S. Adult svz lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell 2010, 7, 163–173. [Google Scholar] [CrossRef]
- Wang, Y.; Abaci, H.; Shuler, M. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2016, 114, 184–194. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015, 15, 2688–2699. [Google Scholar] [CrossRef]
- Sung, J.H.; Esch, M.B.; Prot, J.-M.; Long, C.J.; Smith, A.; Hickman, J.J.; Shuler, M.L. Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip 2013, 13, 1201–1212. [Google Scholar] [CrossRef]
- Sutherland, M.L.; Fabre, K.M.; Tagle, D.A. The National Institutes of Health Microphysiological Systems Program focuses on a critical challenge in the drug discovery pipeline. Stem Cell Res. Ther. 2013, 4, I1. [Google Scholar] [CrossRef]
- Wikswo, J.P. The relevance and potential roles of microphysiological systems in biology and medicine. Exp. Biol. Med. 2014, 239, 1061–1072. [Google Scholar] [CrossRef]
- Youhanna, S.; Lauschke, V. The Past, Present and Future of Intestinal In Vitro Cell Systems for Drug Absorption Studies. J. Pharm. Sci. 2020, 110, 50–65. [Google Scholar] [CrossRef]
- Hubrecht, C. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef]
- Zemanova, M.A. Towards more compassionate wildlife research through the 3Rs principles: Moving from invasive to non-invasive methods. Wildl. Biol. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Kilkenny, C.; Parsons, N.; Kadyszewski, E.; Festing, M.F.W.; Cuthill, I.C.; Fry, D.; Hutton, J.; Altman, D.G. Survey of the Quality of Experimental Design, Statistical Analysis and Reporting of Research Using Animals. PLoS ONE 2009, 4, e7824. [Google Scholar] [CrossRef]
- Slob, W. Benchmark dose and the three Rs. Part II. Consequences for study design and animal use. Crit. Rev. Toxicol. 2014, 44, 568–580. [Google Scholar] [CrossRef]
- Franco, N.; Olsson, A. Scientists and the 3Rs: Attitudes to animal use in biomedical research and the effect of mandatory training in laboratory animal science. Lab Anim. 2013, 48, 50–60. [Google Scholar] [CrossRef]
- Pihl, J.; Karlsson, M.; Chiu, D.T. Microfluidic technologies in drug discovery. Drug Discov. Today 2005, 10, 1377–1383. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Pihl, J.; Sinclair, J.; Karlsson, M.; Orwar, O. Microfluidics for cell-based assays. Mater. Today 2005, 8, 46–51. [Google Scholar] [CrossRef]
- Acevedo, J.P.; Angelopoulos, I.; Van Noort, D.; Khoury, M. Microtechnology applied to stem cells research and development. Regen. Med. 2018, 13, 233–248. [Google Scholar] [CrossRef]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 2012, 12, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Griep, L.M.; Wolbers, F.; de Wagenaar, B.; ter Braak, P.M.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Vermes, I.; van der Meer, A.D.; van den Berg, A.; et al. BBB ON CHIP: Microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed. Microdev. 2013, 15, 145–150. [Google Scholar] [CrossRef]
- Prabhakarpandian, B.; Shen, M.C.; Nichols, J.B.; Mills, I.R.; Sidoryk-Wegrzynowicz, M.; Aschner, M.; Pant, K. SyM-BBB: A microfluidic blood brain barrier model. Lab Chip 2013, 13, 1093–1101. [Google Scholar] [CrossRef]
- Hudson, T.W.; Liu, S.Y.; Schmidt, C.E. Engineering an Improved Acellular Nerve Graft via Optimized Chemical Processing. Tissue Eng. 2004, 10, 1346–1358. [Google Scholar] [CrossRef]
- Hudson, T.W.; Zawko, S.; Deister, C.; Lundy, S.; Hu, C.Y.; Lee, K.; Schmidt, C.E. Optimized Acellular Nerve Graft Is Immunologically Tolerated and Supports Regeneration. Tissue Eng. 2004, 10, 1641–1651. [Google Scholar] [CrossRef]
- Borschel, G.H.; Dennis, R.G.; Kuzon, W.M., Jr. Contractile Skeletal Muscle Tissue-Engineered on an Acellular Scaffold. Plast. Reconstr. Surg. 2004, 113, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.-L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef]
- Ott, H.C.; Clippinger, B.; Conrad, C.; Schuetz, C.; Pomerantseva, I.; Ikonomou, L.; Calle, E.A.; Wobma, H.M.; Gilpin, S.E.; Mathisen, D.J.; et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2010, 16, 927–933. [Google Scholar] [CrossRef]
- Nagao, R.J.; Ouyang, Y.; Keller, R.; Lee, C.; Suggs, L.J.; Schmidt, C.E. Preservation of capillary-beds in rat lung tissue using optimized chemical decellularization. J. Mater. Chem. B 2013, 1, 4801–4808. [Google Scholar] [CrossRef][Green Version]
- Peloso, A.; Petrosyan, A.; Da Sacco, S.; Booth, C.; Zambon, J.P.; O’Brien, T.; Aardema, C.; Robertson, J.; De Filippo, R.E.; Soker, S.; et al. Renal Extracellular Matrix Scaffolds from Discarded Kidneys Maintain Glomerular Morphometry and Vascular Resilience and Retains Critical Growth Factors. Transplantation 2015, 99, 1807–1816. [Google Scholar] [CrossRef]
- Pellicciotta, N.; Hamilton, E.; Kotar, J.; Faucourt, M.; Delgehyr, N.; Spassky, N.; Cicuta, P. Entrainment of mammalian motile cilia in the brain with hydrodynamic forces. Proc. Natl. Acad. Sci. USA 2020, 117, 8315–8325. [Google Scholar] [CrossRef]
- Cuartero, M.I.; García-Culebras, A.; Torres-López, C.; Medina, V.; Fraga, E.; Vázquez-Reyes, S.; Jareño-Flores, T.; García-Segura, J.M.; Lizasoain, I.; Moro, M.Á.; et al. Post-stroke Neurogenesis: Friend or Foe? Front. Cell Dev. Biol. 2021, 9, 657846. [Google Scholar] [CrossRef]
- Bardella, C.; Al-Shammari, A.R.; Soares, L.; Tomlinson, I.; O’Neill, E.; Szele, F.G. The role of inflammation in subventricular zone cancer. Prog. Neurobiol. 2018, 170, 37–52. [Google Scholar] [CrossRef]
- Zheng, Y.; Hiraki, H.L.; Nagao, R.J.; Himmelfarb, J. Fabricating a Kidney Cortex Extracellular Matrix-Derived Hydrogel. JoVE 2018, 140, e58314. [Google Scholar] [CrossRef]
- Bekas, N.; Samiotaki, M.; Papathanasiou, M.; Mokos, P.; Pseftogas, A.; Xanthopoulos, K.; Thanos, D.; Mosialos, G.; Dafou, D. Inactivation of Tumor Suppressor CYLD Inhibits Fibroblast Reprogramming to Pluripotency. Cancers 2023, 15, 4997. [Google Scholar] [CrossRef]
- Demichev, V.; Messner, C.; Vernardis, S.; Lilley, K.; Ralser, M. DIA-NN: Neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 2020, 17, 41–44. [Google Scholar] [CrossRef]
- Delgehyr, N.; Meunier, A.; Faucourt, M.; Bosch Grau, M.; Strehl, L.; Janke, C.; Spassky, N. Ependymal Cell Differentiation, from Monociliated to Multiciliated Cells. In Methods in Cell Biology; Basto, R., Marshall, W.F., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 19–35. [Google Scholar]
- Mirzadeh, Z.; Merkle, F.T.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Neural Stem Cells Confer Unique Pinwheel Architecture to the Ventricular Surface in Neurogenic Regions of the Adult Brain. Cell Stem Cell 2008, 3, 265–278. [Google Scholar] [CrossRef]
- Paez-Gonzalez, P.; Abdi, K.; Luciano, D.; Liu, Y.; Soriano-Navarro, M.; Rawlins, E.; Bennett, V.; Garcia-Verdugo, J.M.; Kuo, C.T. Ank3-Dependent SVZ Niche Assembly Is Required for the Continued Production of New Neurons. Neuron 2011, 71, 61–75. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.N.; Rassmann, S.; Stüven, B.; Jurisch-Yaksi, N.; Wachten, D. CiliaQ: A simple, open-source software for automated quantification of ciliary morphology and fluorescence in 2D, 3D, and 4D images. Eur. Phys. J. E 2021, 44, 18. [Google Scholar] [CrossRef]
- Lauring, M.C.; Zhu, T.; Luo, W.; Wu, W.; Yu, F.; Toomre, D. New software for automated cilia detection in cells (ACDC). Cilia 2019, 8, 1. [Google Scholar] [CrossRef]
- Walsh, R.M.; Luongo, R.; Giacomelli, E.; Ciceri, G.; Rittenhouse, C.; Verrillo, A.; Galimberti, M.; Dickinson Bocchi, V.; Wu, Y.; Xu, N.; et al. Generation of human cerebral organoids with a structured outer subventricular zone. Cell Rep. 2024, 43, 114031. [Google Scholar] [CrossRef]
- Li, R.; Sun, L.; Fang, A.; Li, P.; Wu, Q.; Wang, X. Recapitulating cortical development with organoid culture in vitro and modeling abnormal spindle-like (ASPM related primary) microcephaly disease. Protein Cell 2017, 8, 823–833. [Google Scholar] [CrossRef]
- Gonzalez-Perez, O.; Alvarez-Buylla, A. Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res. Rev. 2011, 67, 147–156. [Google Scholar] [CrossRef]
- Ming, G.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Lee, D.-C.; Hsu, Y.-C.; Chung, Y.-F.; Hsiao, C.-Y.; Chen, S.-L.; Chen, M.-S.; Lin, H.-K.; Chiu, I.-M. Isolation of neural stem/progenitor cells by using EGF/FGF1 and FGF1B promoter-driven green fluorescence from embryonic and adult mouse brains. Mol. Cell. Neurosci. 2009, 41, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Park, M.G.; Jang, H.; Lee, S.-H.; Lee, C.J. Flow shear stress enhances the proliferative potential of cultured radial glial cells possibly via an activation of mechanosensitive calcium channel. Exp. Neurobiol. 2017, 26, 71–81. [Google Scholar] [CrossRef]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef]
- McMurtrey, R.J. Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control. J. Neural. Eng. 2014, 11, 066009. [Google Scholar] [CrossRef]
- Song, J.J.; Guyette, J.P.; Gilpin, S.E.; Gonzalez, G.; Vacanti, J.P.; Ott, H.C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med. 2013, 19, 646–651. [Google Scholar] [CrossRef]
- Quinteira, R.; Gimondi, S.; Monteiro, N.O.; Sobreiro-Almeida, R.; Lasagni, L.; Romagnani, P.; Neves, N.M. Decellularized kidney extracellular matrix-based hydrogels for renal tissue engineering. Acta Biomater. 2024, 180, 295–307. [Google Scholar] [CrossRef]
- Bandtlow, C.E.; Zimmermann, D.R. Proteoglycans in the developing brain: New conceptual insights for old proteins. Physiol. Rev. 2000, 80, 1267–1290. [Google Scholar] [CrossRef]
- Shabani, Z.; Ghadiri, T.; Karimipour, M.; Sadigh-Eteghad, S.; Mahmoudi, J.; Mehrad, H.; Farhoudi, M. Modulatory properties of extracellular matrix glycosaminoglycans and proteoglycans on neural stem cells behavior: Highlights on regenerative potential and bioactivity. Int. J. Biol. Macromol. 2021, 171, 366–381. [Google Scholar] [CrossRef]
- Nagao, R.J.; Xu, J.; Luo, P.; Xue, J.; Wang, Y.; Kotha, S.; Zeng, W.; Fu, X.; Himmelfarb, J.; Zheng, Y.; et al. Decellularized Human Kidney Cortex Hydrogels Enhance Kidney Microvascular Endothelial Cell Maturation and Quiescence. Tissue Eng. Part A 2016, 22, 1140–1150. [Google Scholar] [CrossRef]
- Caralt, M.; Uzarski, J.S.; Iacob, S.; Obergfell, K.P.; Berg, N.; Bijonowski, B.M.; Jeong, J.; Emani, S.; Farkas, A.E.; Somorjai, I.; et al. Optimization and Critical Evaluation of Decellularization Strategies to Develop Renal Extracellular Matrix Scaffolds as Biological Templates for Organ Engineering and Transplantation. Am. J. Transplant. 2015, 15, 64–75. [Google Scholar] [CrossRef]
- Pu, W.; Han, Y.; Yang, M. Human decellularized adipose tissue hydrogels as a culture platform for human adipose-derived stem cell delivery. J. Appl. Biomater. Funct. Mater. 2021, 19, 2280800020988141. [Google Scholar] [CrossRef]
- Jacquet, B.V.; Salinas-Mondragon, R.; Liang, H.; Therit, B.; Buie, J.D.; Dykstra, M.; Campbell, K.; Ostrowski, L.E.; Brody, S.L.; Ghashghaei, H.T.; et al. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development 2009, 136, 4021–4031. [Google Scholar] [CrossRef] [PubMed]
- Maeso-Alonso, L.; López-Ferreras, L.; Marques, M.M.; Marin, M.C. p73 as a Tissue Architect. Front. Cell Dev. Biol. 2021, 9, 716957. [Google Scholar] [CrossRef] [PubMed]
- Siyahhan, B.; Knobloch, V.; de Zélicourt, D.; Asgari, M.; Schmid Daners, M.; Poulikakos, D.; Kurtcuoglu, V. Flow induced by ependymal cilia dominates near-wall cerebrospinal fluid dynamics in the lateral ventricles. J. R. Soc. Interface 2014, 11, 20131189. [Google Scholar] [CrossRef]
- Ringers, C.; Olstad, E.W.; Jurisch-Yaksi, N. The role of motile cilia in the development and physiology of the nervous system. Philos. Trans. R. Soc. B 2020, 375, 20190156. [Google Scholar] [CrossRef]
- Mazzei, D.; Guzzardi, M.A.; Giusti, S.; Ahluwalia, A. A low shear stress modular bioreactor for connected cell culture under high flow rates. Biotechnol. Bioeng. 2010, 106, 127–137. [Google Scholar] [CrossRef]
- Miranda-Azpiazu, P.; Panagiotou, S.; Jose, G.; Saha, S. A novel dynamic multicellular co-culture system for studying individual blood-brain barrier cell types in brain diseases and cytotoxicity testing. Sci. Rep. 2018, 8, 8784. [Google Scholar] [CrossRef]
- Chandorkar, P.; Posch, W.; Zaderer, V.; Blatzer, M.; Steger, M.; Ammann, C.G.; Binder, U.; Hermann, M.; Hörtnagl, P.; Lass-Flörl, C.; et al. Fast-track development of an in vitro 3D lung/immune cell model to study Aspergillus infections. Sci. Rep. 2017, 7, 11644. [Google Scholar] [CrossRef]
- Mestre, H.; Tithof, J.; Du, T.; Song, W.; Peng, W.; Sweeney, A.M.; Olveda, G.; Thomas, J.H.; Nedergaard, M.; Kelley, D.H.; et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 2018, 9, 4878. [Google Scholar] [CrossRef]
- Guirao, B.; Meunier, A.; Mortaud, S.; Aguilar, A.; Corsi, J.-M.; Strehl, L.; Hirota, Y.; Desoeuvre, A.; Boutin, C.; Han, Y.-G.; et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 2010, 12, 341–350. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulos, I.; Ioannidis, K.; Lyroni, K.G.; Vlassopoulos, D.; Samiotaki, M.; Pavlidou, E.; Chatzistavrou, X.; Papantoniou, I.; Papageorgiou, K.; Kritas, S.K.; et al. A 3D SVZonChip Model for In Vitro Mimicry of the Subventricular Zone Neural Stem Cell Niche. Bioengineering 2025, 12, 562. https://doi.org/10.3390/bioengineering12060562
Angelopoulos I, Ioannidis K, Lyroni KG, Vlassopoulos D, Samiotaki M, Pavlidou E, Chatzistavrou X, Papantoniou I, Papageorgiou K, Kritas SK, et al. A 3D SVZonChip Model for In Vitro Mimicry of the Subventricular Zone Neural Stem Cell Niche. Bioengineering. 2025; 12(6):562. https://doi.org/10.3390/bioengineering12060562
Chicago/Turabian StyleAngelopoulos, Ioannis, Konstantinos Ioannidis, Konstantina Gr. Lyroni, Dimitris Vlassopoulos, Martina Samiotaki, Eleni Pavlidou, Xanthippi Chatzistavrou, Ioannis Papantoniou, Konstantinos Papageorgiou, Spyridon K. Kritas, and et al. 2025. "A 3D SVZonChip Model for In Vitro Mimicry of the Subventricular Zone Neural Stem Cell Niche" Bioengineering 12, no. 6: 562. https://doi.org/10.3390/bioengineering12060562
APA StyleAngelopoulos, I., Ioannidis, K., Lyroni, K. G., Vlassopoulos, D., Samiotaki, M., Pavlidou, E., Chatzistavrou, X., Papantoniou, I., Papageorgiou, K., Kritas, S. K., & Grivas, I. (2025). A 3D SVZonChip Model for In Vitro Mimicry of the Subventricular Zone Neural Stem Cell Niche. Bioengineering, 12(6), 562. https://doi.org/10.3390/bioengineering12060562





