Integrating Artificial Intelligence and Precision Therapeutics for Advancing the Diagnosis and Treatment of Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methodology
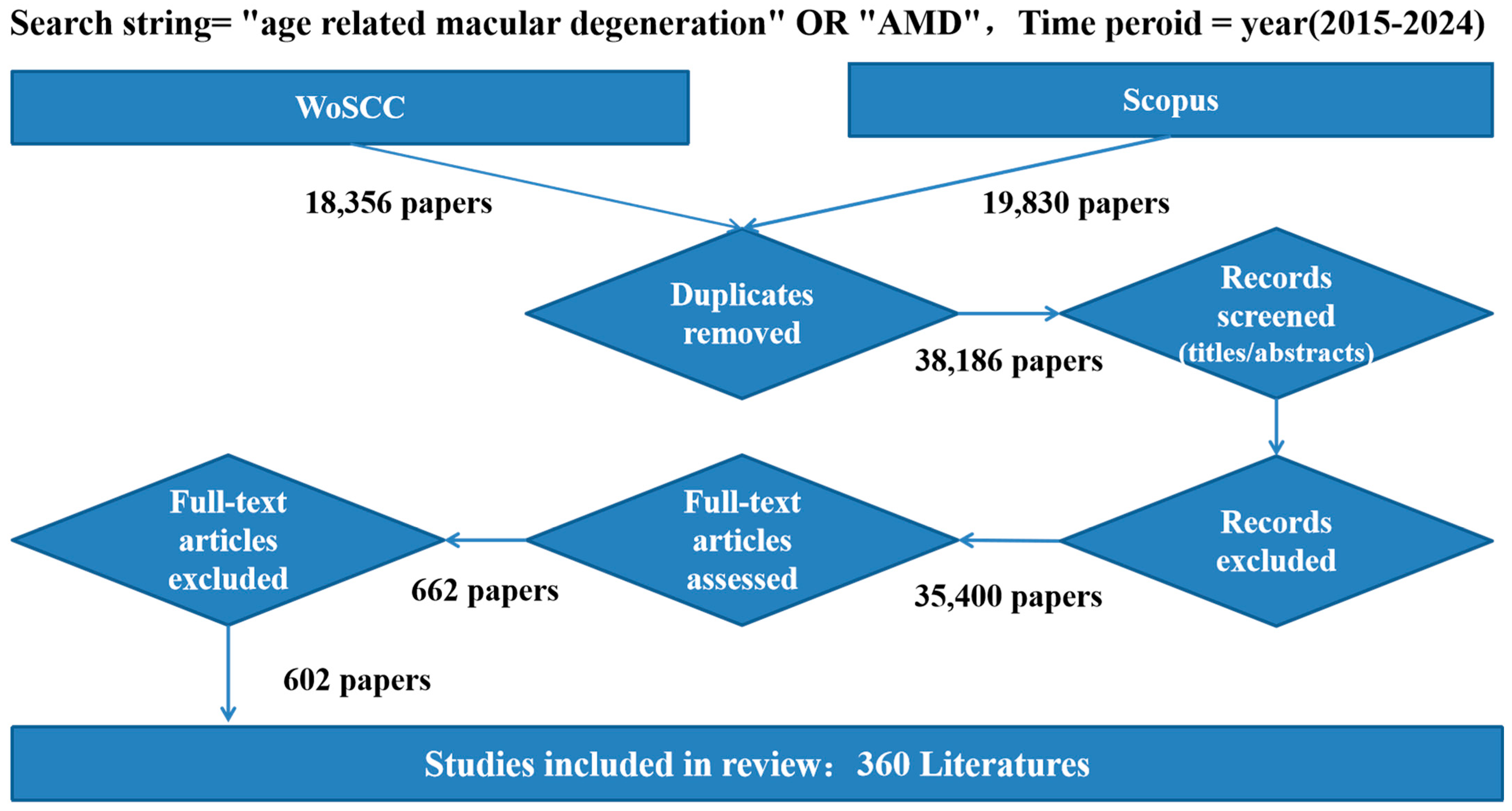
2.1. Data Source
2.2. Analysis Tools
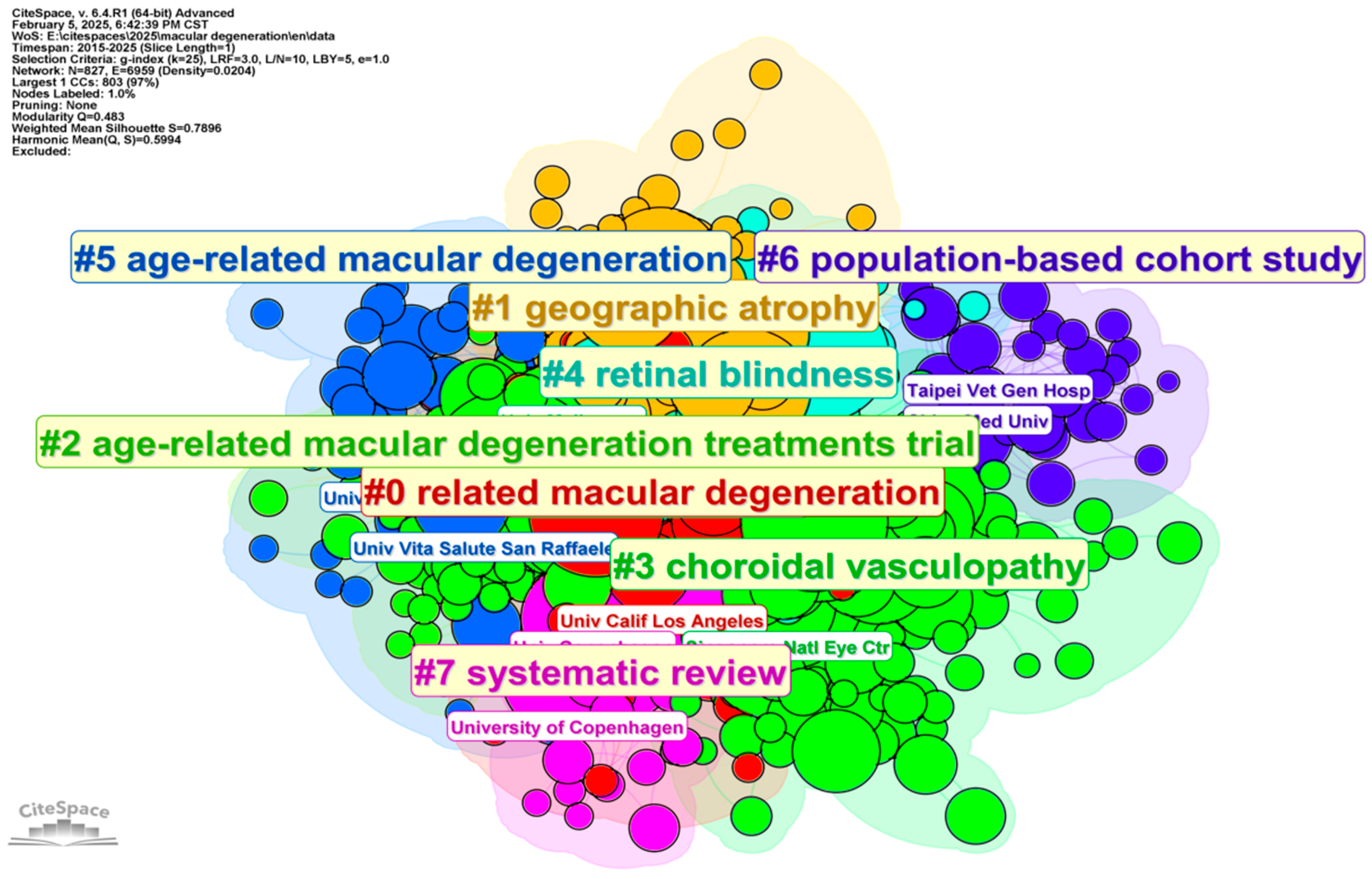
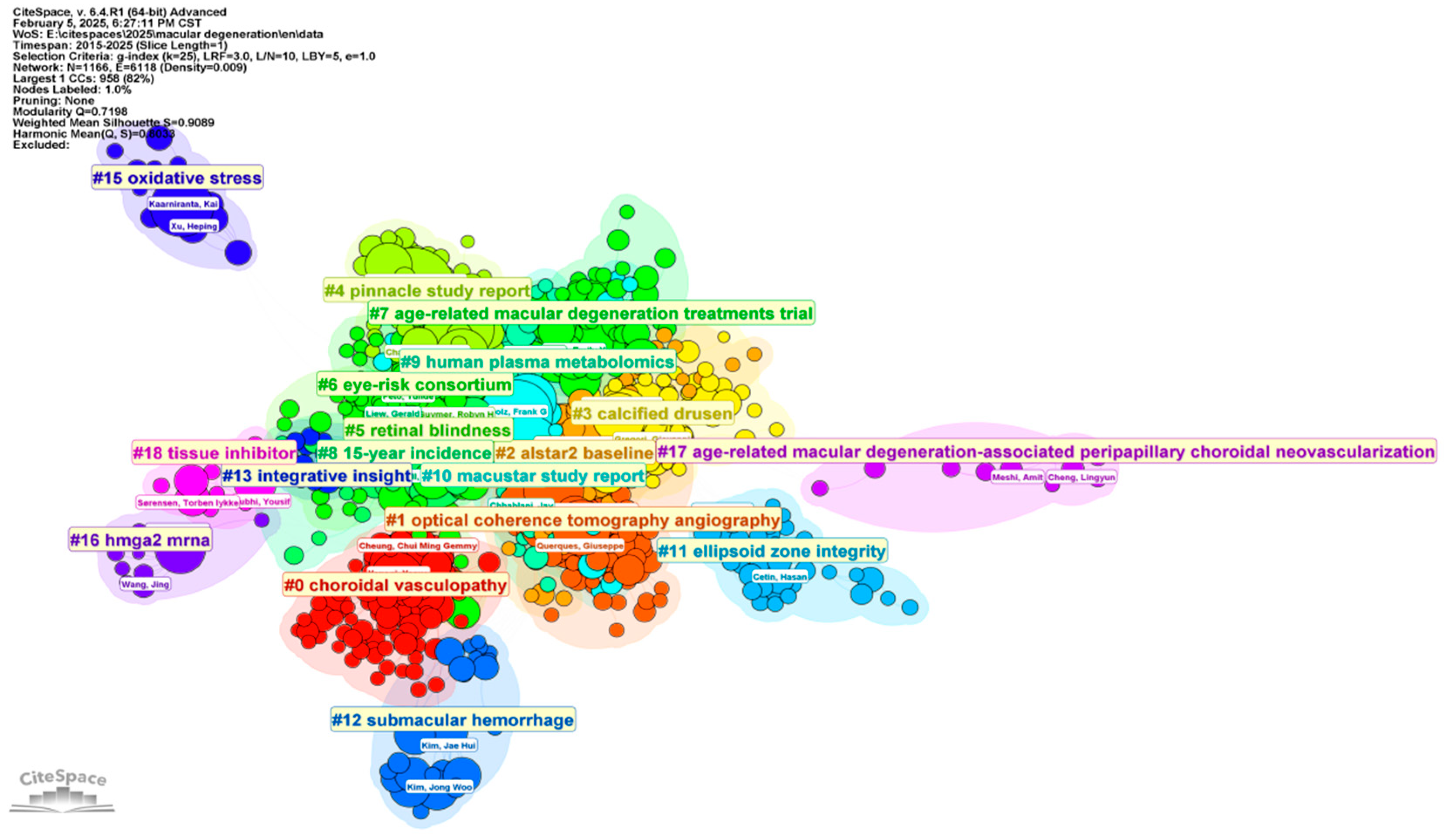
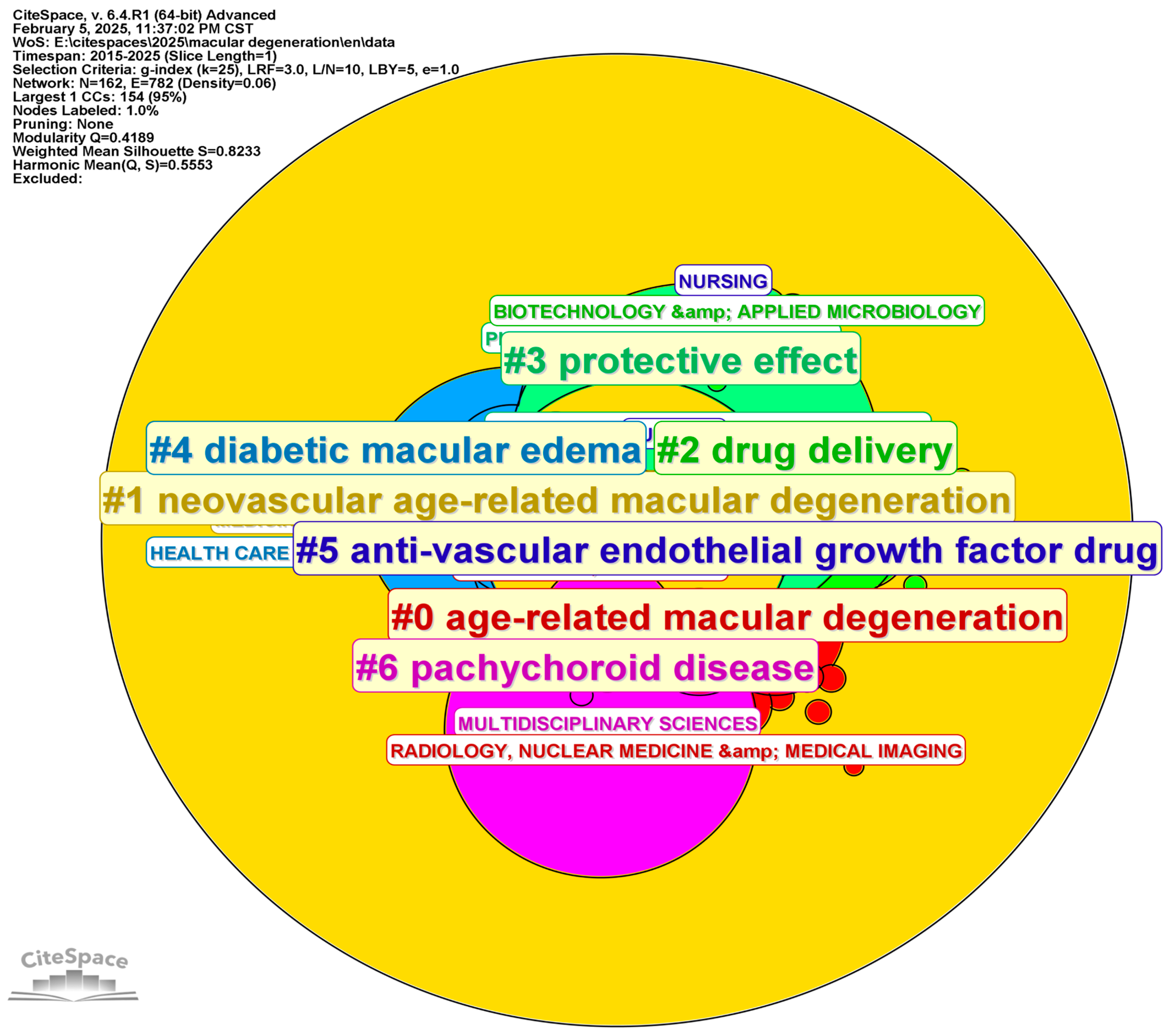
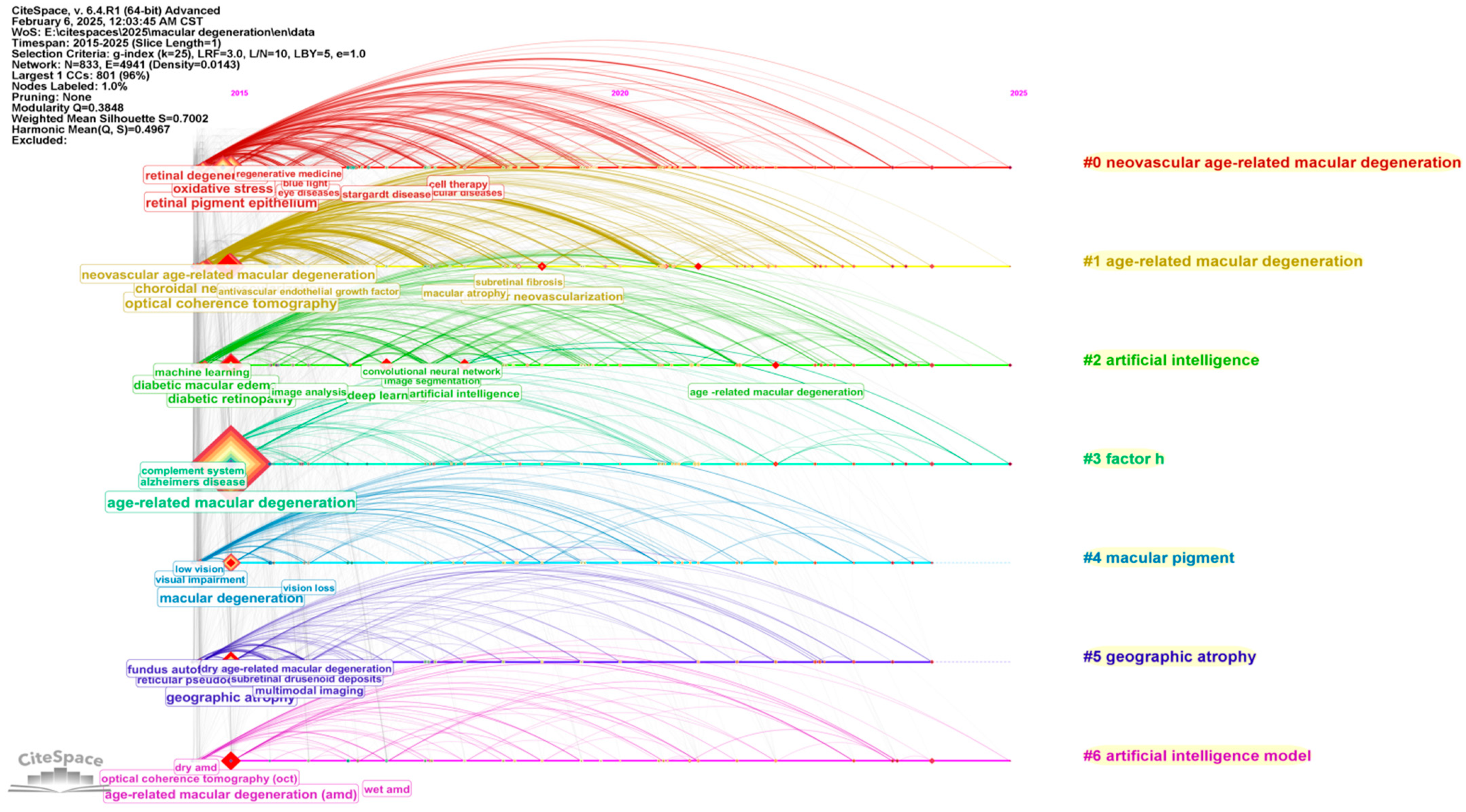
3. Bibliographic Analytic Results
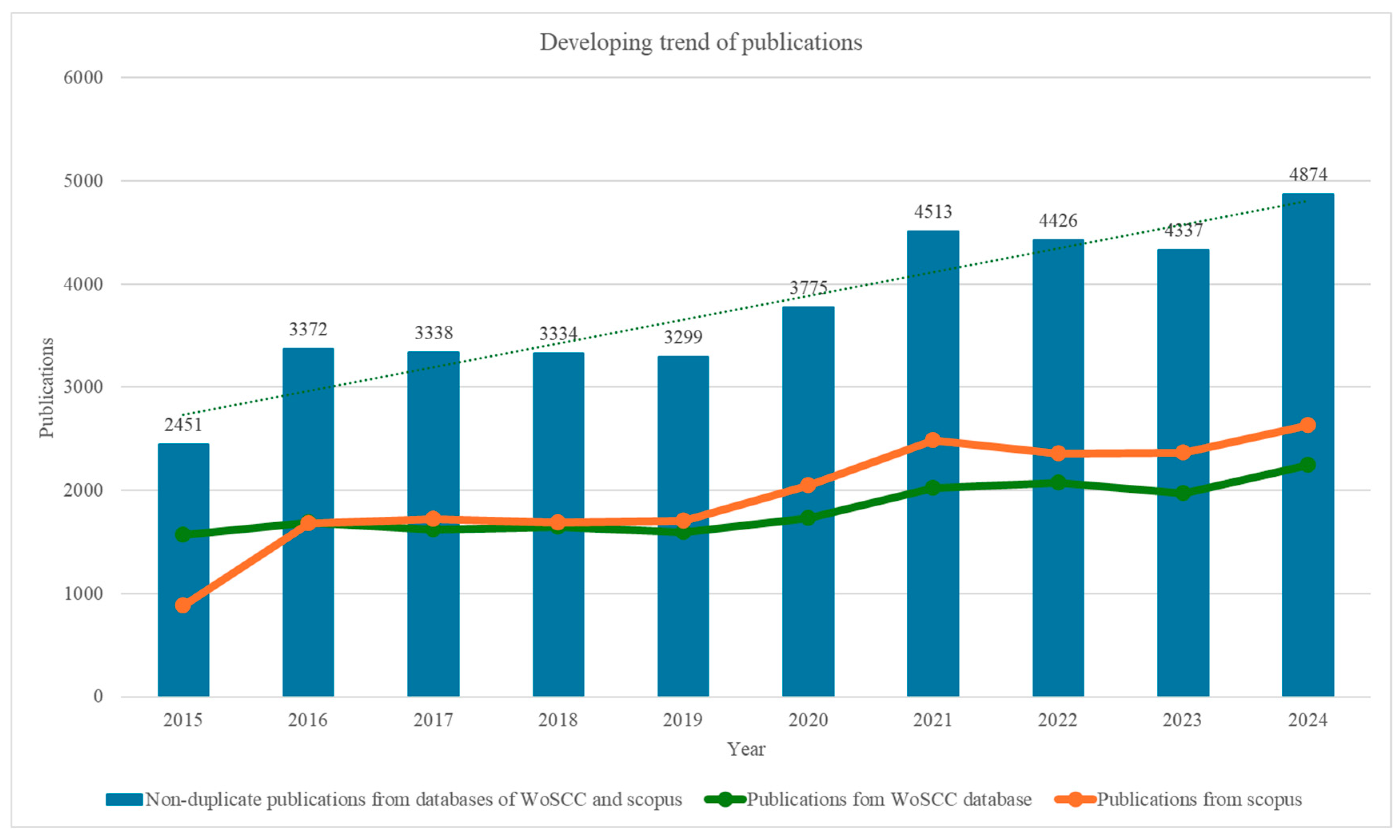
3.1. Developing Trends in Literature
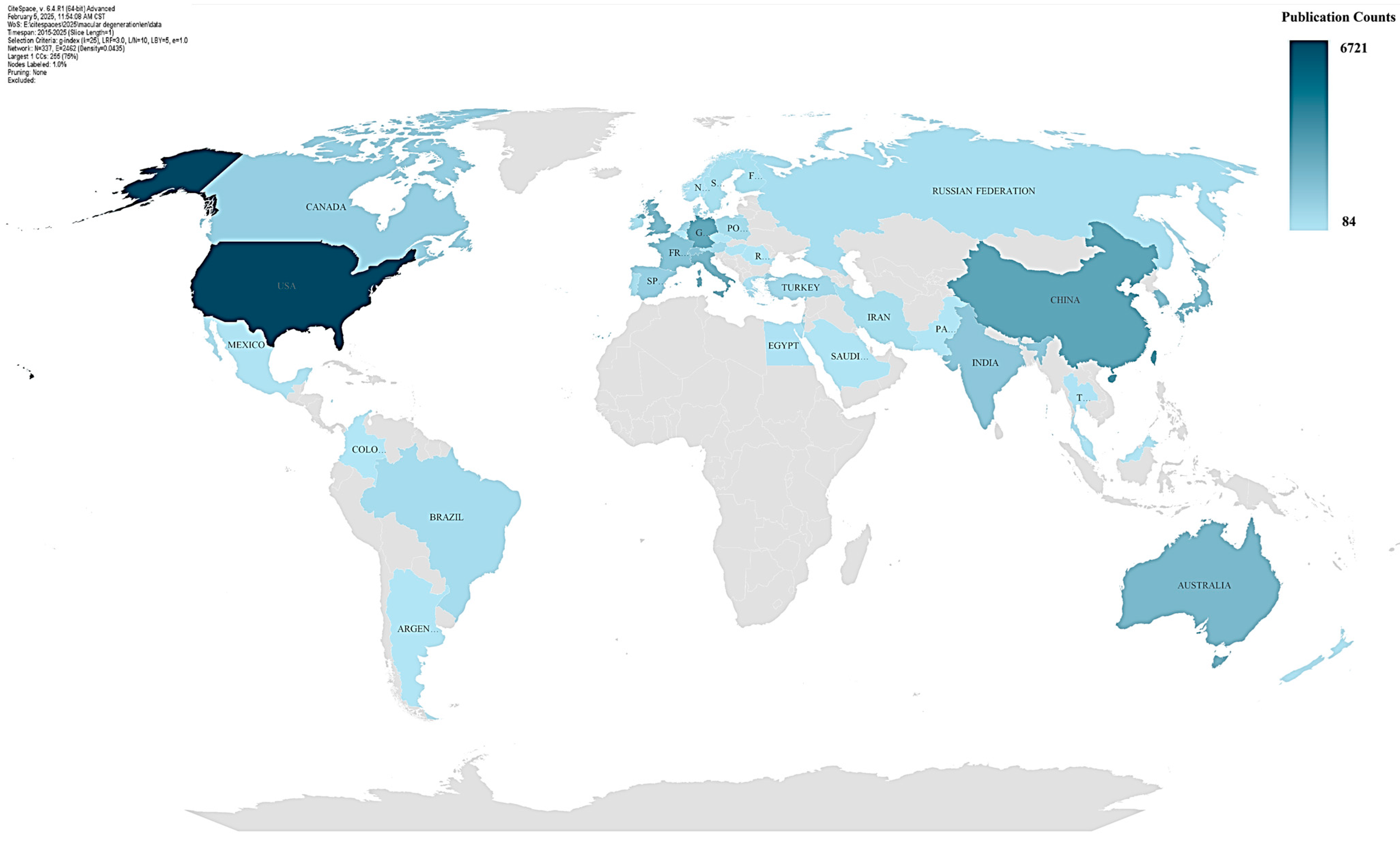
3.2. Analysis of Countries and Institutions
3.3. Analysis of Cited Journals, Cited Authors, and Cited References
| Ranking | Authors | Count | Institution | Involved Publications |
|---|---|---|---|---|
| 1 | Francesco Bandello | 310 | IRCCS San Raffaele Scientific Institute, Milan, Italy | Macular Atrophy in Neovascular Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis [11]. |
| 2 | Ursula Schmidt-Erfurth | 305 | Medical University of Vienna, Vienna, Austria | Features of intermediate and late dry age-related macular degeneration on adaptive optics ophthalmoscopy: Pinnacle Study Report 8 [11]. |
| 3 | Giuseppe Querques | 278 | School of Medicine, Vita-Salute San Raffaele University, Milan, Italy | Delineating the contours of PVAC and TelCaps [12]. |
| 4 | Usha Chakravarthy | 259 | Queen’s University of Belfast, Belfast, United Kingdom | Interplay between Lipids and Complement Proteins—How Multiomics Data Integration Can Help Unravel Age-related Macular Degeneration Pathophysiology: A Proof-of-concept Study [13]. |
| 5 | Frank G. Holz | 233 | 0 | Heterogenous visual function deficits in intermediate age-related macular degeneration—A MACUSTAR report [14]. |
| Year | Reference | Findings | Hotspot | Journal | Number of Cited by (According to Google Scholar) |
|---|---|---|---|---|---|
| 2015 | Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies [15] | This study provides evidence of the medium- to long-term safety of human embryonic stem cell (hESC)-derived retinal pigment epithelium transplants for treating Stargardt’s macular dystrophy and age-related macular degeneration. No adverse proliferation or rejection was observed, and visual acuity improved or remained stable in many patients, indicating potential for hESC-derived cells in regenerative treatments. | Human Embryonic Stem Cells (hESC), Retinal Pigment Epithelium, Regenerative Medicine, Stargardt’s Macular Dystrophy, Age-related Macular Degeneration, Stem Cell Transplantation, Visual Acuity, Safety, Graft Survival, Tissue Repair, Immune Rejection, Subretinal Transplantation, Phase 1/2 Studies, Quality of Life | The Lancet | 1530 |
| 2016 | Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism [16] | This review explores the multifaceted roles of Nrf2 and its target heme oxygenase-1 (HO-1), emphasizing their cytoprotective, anti-aging, and stress-protective effects. Disturbances in HO-1 levels are linked to age-related disorders like neurodegeneration, cancer, and macular degeneration, suggesting a conserved function across species. | Nrf2, Heme Oxygenase-1 (HO-1), Antioxidant, Anti-inflammatory, Detoxification, Aging, Neurodegeneration, Macular Degeneration, Stress Protection, Longevity, Transcription Factors, Cytoprotection, Apoptosis, Angiogenesis, Age-dependent Disorders | Cellular molecular life sciences | 2339 |
| 2017 | Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis [17] | This systematic review and meta-analysis identified major causes of vision impairment and blindness globally, with uncorrected refractive error and cataracts as the leading contributors. The study also projected a significant rise in cases by 2020, underscoring the need for increased eye care to address preventable vision loss, particularly in adults aged 50 and older. | Vision Impairment, Blindness, Uncorrected Refractive Error, Cataract, Age-related Macular Degeneration, Glaucoma, Diabetic Retinopathy, Population-based Studies, Eye Care, Preventable Vision Loss, Systematic Review, Meta-analysis, Global Health | The Lancet Global Health | 3499 |
| 2018 | Identifying medical diagnoses and treatable diseases by image-based deep learning [17] | This research developed an AI system using transfer learning to classify macular degeneration, diabetic retinopathy, and pediatric pneumonia from medical images. The system demonstrated comparable accuracy to human experts and provided interpretable diagnoses, with potential for broad applications in biomedical imaging. | AI, Transfer Learning, Macular Degeneration, Diabetic Retinopathy, Pneumonia Diagnosis, OCT, Chest X-rays, Deep Learning, Clinical Decision Support, Medical Imaging | Cell | 3929 |
| 2019 | Artificial intelligence and deep learning in ophthalmology [21] | Deep learning has shown significant potential in ophthalmology, especially in detecting major eye diseases like diabetic retinopathy, glaucoma, macular oedema, and age-related macular degeneration. However, challenges remain in clinical deployment, including algorithm explainability, medicolegal issues, and acceptance from both physicians and patients. | Deep Learning, Ophthalmology, AI, Image Recognition, Diabetic Retinopathy, Glaucoma, Macular Oedema, Age-related Macular Degeneration, Telemedicine, Algorithm Explainability, Clinical Challenges, Medicolegal Issues, Patient and Physician Acceptance, Ocular Imaging | British Journal of Ophthalmology | 1247 |
| 2020 | Ranibizumab and Bevacizumab for Treatment of Neovascular Age-related Macular Degeneration Two-Year Results [19] | Ranibizumab and bevacizumab had similar effects on visual acuity over two years, with monthly treatment resulting in greater improvements than as-needed treatment. Switching from monthly to as-needed treatment led to decreased visual acuity and a lower proportion of patients without fluid, while bevacizumab was associated with a higher rate of systemic serious adverse events compared to ranibizumab. | Ranibizumab, Bevacizumab, Neovascular Age-related Macular Degeneration, Visual Acuity, Monthly Treatment, As-needed Treatment, Clinical Trial, Systemic Adverse Events, Vascular Endothelial Growth Factor, Treatment Regimen, Drug Comparison, Fluid Resolution, Arteriothrombotic Events, Serious Adverse Events | Ophthalmology | It could not find the citation on Google Scholar, according to Web of Science, the citation is 1142. |
| 2021 | Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study [20] | The study found that, despite a reduction in age-standardized rates of avoidable blindness, the target set by the World Health Assembly Global Action Plan was not achieved in the global reduction in avoidable vision impairment (MSVI). The prevalence of avoidable blindness and MSVI increased due to the ageing population, with cataract and undercorrected refractive error being the leading causes of both blindness and MSVI in adults aged 50 and older. | Avoidable Vision Impairment, Blindness, Cataract, Undercorrected Refractive Error, Age-related Macular Degeneration, Diabetic Retinopathy, Glaucoma, Global Action Plan (WHA GAP), Age-standardized Prevalence, Eye Health Services, Systematic Review, Meta-analysis, Ageing Population, Public Health, MSVI | The Lancet Global Health | 1751 |
| 2022 | Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomized, double-masked, phase 3, non-inferiority trials [22] | In two phase 3 trials (TENAYA and LUCERNE), intravitreal faricimab (6.0 mg) administered up to every 16 weeks was shown to be non-inferior to aflibercept (2.0 mg every 8 weeks) in improving best-corrected visual acuity in patients with neovascular age-related macular degeneration. Rates of ocular adverse events were similar for both treatments. | Faricimab, Neovascular Age-Related Macular Degeneration (nAMD), Intravitreal Injections, Best-Corrected Visual Acuity, Phase 3 Trials, Aflibercept, Ocular Adverse Events, Non-Inferiority, Angiopoietin-2, Vascular Endothelial Growth Factor A (VEGF-A), Treatment Regimens, TENAYA, LUCERNE. | The Lancet | 445 |
| 2023 | Endoplasmic reticulum stress: molecular mechanism and therapeutic targets [23] | This review highlights the pivotal role of endoplasmic reticulum stress in various ocular diseases, including glaucoma, diabetic retinopathy, age-related macular degeneration, and cataracts, among others. It discusses how ER stress disrupts proteostasis and triggers adaptive responses that impact transcription and protein processing. Additionally, it examines therapeutic strategies aimed at alleviating ER stress, such as drugs, gene therapy, and stem cell therapy, as potential treatments for these diseases. | Endoplasmic Reticulum (ER), ER Stress, Proteostasis, Ocular Diseases, Glaucoma, Diabetic Retinopathy, Age-Related Macular Degeneration, Retinitis Pigmentosa, Protein Homeostasis, Unfolded Protein Response, Therapeutic Strategies, Gene Therapy, Stem Cell Therapy, Neurotrophic Factors, Autophagy, Ocular Tumors, Myopia. | Signal transduction targeted therapy | |
| 2024 | Age-Related Macular Degeneration A Review [2] | AMD is a major cause of vision impairment in older adults, with increasing global prevalence. It is primarily influenced by genetic and environmental factors like smoking, and its progression is characterized by either geographic atrophy or neovascularization. Treatment with anti-VEGF injections and nutritional supplements has been shown to slow progression and improve visual outcomes in patients with AMD. | AMD, Genetic Factors, Smoking, Geographic Atrophy, Neovascular AMD, Anti-VEGF Injections, Visual Acuity, Optical Coherence Tomography, Nutritional Supplements, Vitamin C, Vitamin E, Zinc, Carotenoids, Heritability, Incidence. 4o | JAMA | 104 |
| 2025 | Recent Advances in Our Understanding of Age-Related Macular Degeneration: Mitochondrial Dysfunction, Redox Signaling, and the Complement System [24] | AMD is a multifactorial retinal disease driven by aging, oxidative stress, inflammation, vascular dysfunction, and complement activation, with a significant global impact. While current treatments target neovascular AMD, recent approvals of therapies for geographic atrophy offer hope. This review highlights the role of oxidative stress, mitochondrial dynamics, and the complement system in AMD pathophysiology and discusses novel therapeutic approaches and emerging treatments. | AMD, Geographic Atrophy (GA), Oxidative Stress, Mitochondrial Dynamics, Complement System, Aging, Inflammation, Redox Status, Choroidal Neovascularization (CNV), Emerging Therapies, Drug Therapies, Retinal Disease, Cell Death Pathways. | Aging disease | 13 |
3.4. Analysis of Categories, Hotspots, and Burst Topic Historic Trends
4. Qualitative Analytic Result
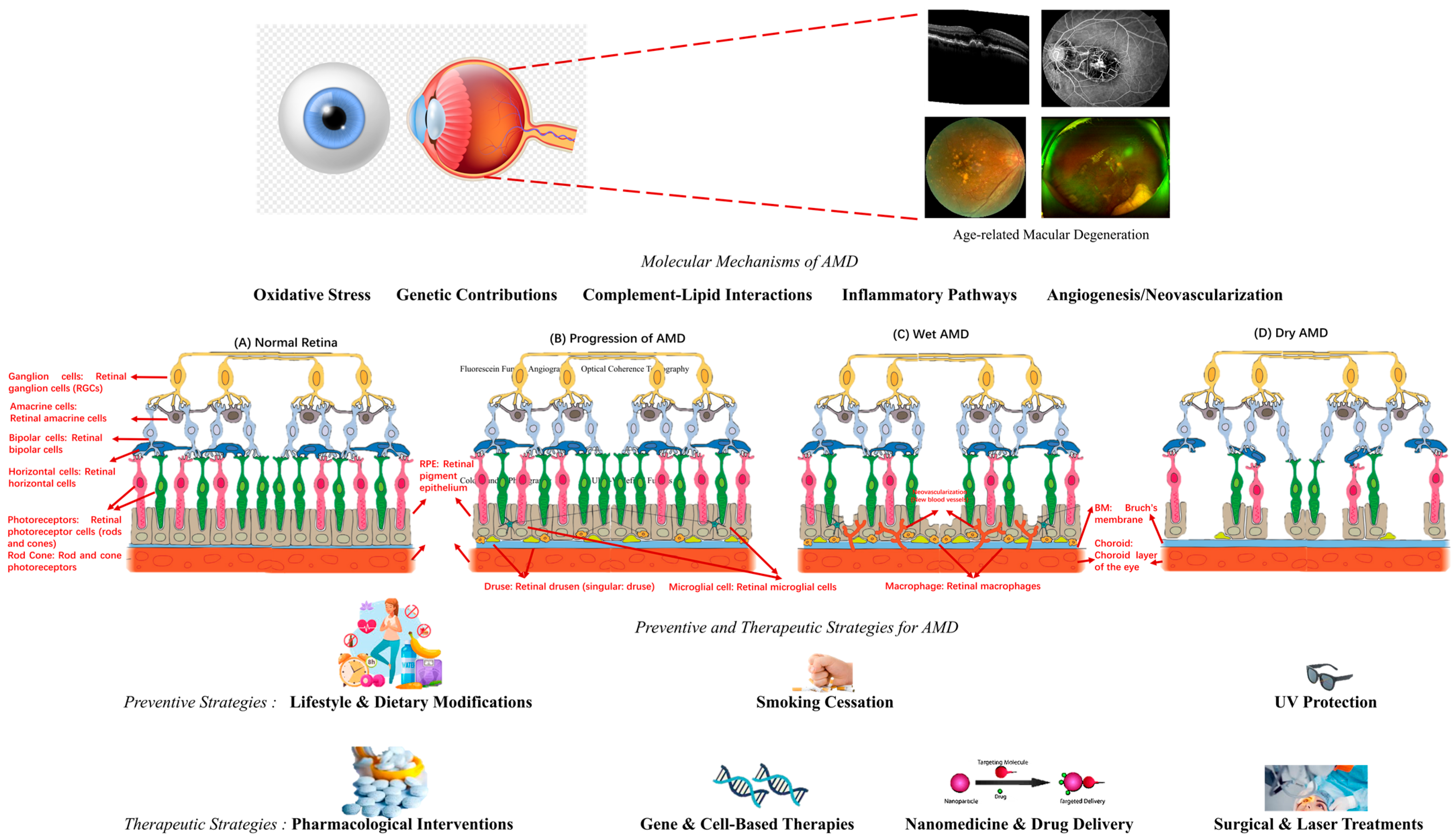
4.1. Molecular Mechanisms of AMD
4.1.1. Genetic Contributions to AMD Pathogenesis
4.1.2. Complement–Lipid Interactions in AMD: Insights from Multiomics Studies
4.1.3. Oxidative Stress and Retinal Pigment Epithelium Degeneration
4.1.4. Inflammatory Pathways in AMD Progression
4.1.5. Angiogenesis and Neovascularization in Wet AMD
4.2. Biomarkers of Evidence of AMD
4.3. Prognosis of AMD Based on Imaging Evidence
4.4. Preventive and Therapeutic Strategies for AMD
4.4.1. Preventive Strategies: Lifestyle and Dietary Modifications
4.4.2. Pharmacological Interventions for AMD
4.4.3. Gene and Cell-Based Therapies
4.4.4. Nanomedicine and Drug Delivery
4.5. Coexisting Ocular Diseases Contributing to AMD Progression and Complications
4.5.1. Diabetic Retinopathy and Its Influence on AMD Progression
4.5.2. Perifoveal Vascular Anomalous Complex and Retinal Capillary Telangiectasia in AMD
4.5.3. Glaucoma and Its Impact on Visual Function in Patients with AMD
4.5.4. Choroidal and Vitreoretinal Interface Disorders in AMD Progression
4.6. Structural Alterations in Patients with Age-Related Macular Degeneration
4.6.1. Early-Stage Structural Alterations in Patients with Age-Related Macular Degeneration
4.6.2. Middle-Stage Structural Alterations in Patients with Age-Related Macular Degeneration
4.6.3. Late-Stage Structural Alterations in Patients with Age-Related Macular Degeneration
4.7. Heterogeneous Visual Function Impairment in Age-Related Macular Degeneration
4.7.1. Variability in Visual Function Across AMD Stages
4.7.2. Heterogeneous Functional Deficits in Intermediate AMD
4.7.3. Implications for AMD Diagnosis, Prognosis, and Clinical Trials
4.8. Artificial Intelligence in AMD Diagnosis and Treatment
4.8.1. AI-Assisted Diagnosis and Screening of AMD
4.8.2. AI in AMD Progression Prediction and Risk Assessment
4.8.3. AI-Optimized Treatment Strategies for AMD
4.8.4. AI-Enabled Teleophthalmology and Remote Monitoring
4.8.5. Challenges and Future Directions
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| AMD | Age-related macular degeneration |
| RPE | Retinal pigment epithelium |
| ROS | Reactive oxygen species |
| CFH | Complement factor H |
| ARMS2 | Age-related maculopathy susceptibility 2 |
| GWAS | Genome-wide association studies |
| WoSCC | Web of Science Core Collection |
| NEI | National Eye Institute |
| UCLA | University of California, Los Angeles |
| OCTA | Optical coherence tomography angiography |
| hESC-RPE | Human embryonic stem cell-derived retinal pigment epithelium |
| OCT | Optical coherence tomography |
| BCVA | Best-corrected visual acuity |
| nAMD | Neovascular Age-Related Macular Degeneration |
| ER | Endoplasmic Reticulum |
| VEGF-A | Vascular Endothelial Growth Factor A |
| GA | Geographic Atrophy |
| CNV | Choroidal Neovascularization |
| AOO | Adaptive Optics Ophthalmoscopy |
| GRS | Genetic risk scores |
| RPD | Reticular pseudodrusen |
| LDA | Large drusen area |
| MASP1 | Mannan-binding lectin serine protease 1 |
| CFHR1 | Complement factor H-related protein 1 |
| CPN2 | Carboxypeptidase N subunit 2 |
| VEGF | Vascular endothelial growth factor |
| AREDS | Age-Related Eye Disease Study |
| iPSCs | Induced pluripotent stem cells |
| ESCs | Embryonic stem cells |
| MSCs | Mesenchymal stem cells |
| PEG | Polyethylene glycol |
| BRB | Blood–retinal barrier |
| DR | Diabetic retinopathy |
| DME | Diabetic macular edema |
| PVAC | Perifoveal vascular anomalous complex |
| RCT | Retinal capillary telangiectasia |
| T3 MNV | Type 3 macular neovascularization |
| MacTel | Macular Telangiectasia |
| ICGA | Indocyanine green angiography |
| IOP | Intraocular pressure |
| PCV | Polypoidal choroidal vasculopathy |
| VMT | Vitreomacular traction |
| ERM | Epiretinal membranes |
| iAMD | Intermediate AMD |
| CMD | Cystoid Macular Degeneration |
| CME | Cystoid macular edema |
| RIT | Rod Intercept Time |
| DL | Deep learning |
| ML | Machine learning |
| EHRs | Electronic health records |
| AI | Artificial intelligence |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open Access Journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-related macular degeneration: A review. JAMA 2024, 331, 147–157. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.K.; Geerlings, M.J.; den Hollander, A.I. Age-related macular degeneration. In Genetics and Genomics of Eye Disease; Academic Press: Cambridge, MA, USA, 2020; pp. 155–180. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace. 2024. Available online: https://sourceforge.net/projects/citespace/ (accessed on 1 March 2025).
- Chen, C. Searching for intellectual turning points: Progressive knowledge domain visualization. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. 1), 5303–5310. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Xu, C.; Wu, F.; Chiu, Y.-R. Advancements and trends in cooperative economy research—A Knowledge Map analysis based on CiteSpace and Bibliometrix. Heliyon 2025, 11, e41095. [Google Scholar] [CrossRef]
- Corradetti, G.; Corvi, F.; Nguyen, T.V.; Sadda, S.R. Management of neovascular age-related macular degeneration during the COVID-19 pandemic. Ophthalmol. Retin. 2020, 4, 757–759. [Google Scholar] [CrossRef]
- Dow, E.R.; Keenan, T.D.; Lad, E.M.; Lee, A.Y.; Lee, C.S.; Loewenstein, A.; Eydelman, M.B.; Chew, E.Y.; Keane, P.A.; Lim, J.I. From data to deployment: The collaborative community on ophthalmic imaging roadmap for artificial intelligence in age-related macular degeneration. Ophthalmology 2022, 129, e43–e59. [Google Scholar] [CrossRef]
- Querques, G.; Sacconi, R.; Gelormini, F.; Borrelli, E.; Prascina, F.; Zucchiatti, I.; Querques, L.; Bandello, F. Subthreshold laser treatment for reticular pseudodrusen secondary to age-related macular degeneration. Sci. Rep. 2021, 11, 2193. [Google Scholar] [CrossRef]
- Berni, A.; Coletto, A.; Li, J.; Shen, M.; Bandello, F.; Reibaldi, M.; Borrelli, E. Macular Atrophy in Neovascular Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Ophthalmol. Retin. 2025, 1, 1–20. [Google Scholar] [CrossRef]
- Querques, G. Delineating the contours of PVAC and TelCaps. Eye 2025, 39, 803. [Google Scholar] [CrossRef]
- Nusinovici, S.; Zhou, L.; Raghavan, L.; Tham, Y.C.; Li, H.; Cheung, D.; Wang, X.; Cheung, C.M.G.; Wong, T.Y.; Chakravarthy, U.; et al. Interplay between Lipids and Complement Proteins—How Multiomics Data Integration Can Help Unravel Age-related Macular Degeneration Pathophysiology: A Proof-of-concept Study. Ophthalmol. Sci. 2025, 5, 100629. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, H.M.; Crabb, D.P.; Behning, C.; Binns, A.M.; Abdirahman, A.; Terheyden, J.H.; Finger, R.P.; Leal, S.; Tufail, A.; Holz, F.A.; et al. Heterogenous visual function deficits in intermediate age-related macular degeneration–A MACUSTAR report. Ophthalmol. Sci. 2025, 5, 100708. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Kermany, D.S.; Goldbaum, M.; Cai, W.; Valentim, C.C.; Liang, H.; Baxter, S.L.; McKeown, A.; Yang, G.; Wu, X.; Yan, F.; et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell 2018, 172, 1122–1131. [Google Scholar] [CrossRef]
- Wang, M.H.; Pan, Y.; Jiang, X.; Lin, Z.; Liu, H.; Liu, Y.; Cui, J.; Tan, J.; Gong, C.; Hou, G.; et al. Leveraging Artificial Intelligence and Clinical Laboratory Evidence to Advance Mobile Health Applications in Ophthalmology: Taking the Ocular Surface Disease as a Case Study. iLABMED 2025, 3, 64–85. [Google Scholar] [CrossRef]
- Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.-s.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L., 3rd. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and Bevacizumab for Treatment of Neovascular Age-related Macular Degeneration: Two-Year Results. Ophthalmology 2020, 127, S135–S145. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Bourne, R.R.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Pasquale, L.R.; Peng, L.; Campbell, J.P.; Lee, A.Y.; Raman, R.; Tan, G.S.W.; Schmetterer, L.; Keane, P.A.; Wong, T.Y. Artificial intelligence and deep learning in ophthalmology. Br. J. Ophthalmol. 2019, 103, 167–175. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Ruiz, C.Q.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef]
- Buonfiglio, F.; Korb, C.A.; Stoffelns, B.; Pfeiffer, N.; Gericke, A. Recent Advances in Our Understanding of Age-Related Macular Degeneration: Mitochondrial Dysfunction, Redox Signaling, and the Complement System. Aging Dis. 2025, 2025, 1–41. [Google Scholar] [CrossRef]
- Sayegh, R.R.; Vitale, S.; Agrón, E.; Farrar, J.T.; Asbell, P.A.; Chew, E.Y.; Group, A.R. Prevalence and risk factors for the development of chronic postoperative pain after cataract surgery in the Age-related Eye Disease Study (AREDS). J. Pain 2025, 28, 104790. [Google Scholar] [CrossRef] [PubMed]
- Garzone, D.; Imtiaz, M.A.; Mauschitz, M.M.; Aziz, N.A.; Holz, F.G.; Breteler, M.M.; Finger, R.P. Age-Related Macular Degeneration and Its Genetic Risk: A Population-based Study. Curr. Eye Res. 2025, 50, 82–86. [Google Scholar] [CrossRef]
- Wang, H. A survey of AI to AMD and quantitative analysis of AMD pathology based on medical images. Artif. Intell. Robot. Res. 2022, 11, 143–157. [Google Scholar] [CrossRef]
- Wang, M.H.; Yu, X. A bibliographic study of “liver-eye” related research: A correlation function analytic research between age-related macular degeneration (AMD) and traditional chinese medicine (TCM) liver wind internal movement syndrome. Adv. Clin. Med. 2023, 13, 6342. [Google Scholar] [CrossRef]
- Chiang, B.; Chung, Y.G.; Prausnitz, M.R. Suprachoroidal drug delivery for VEGF suppression in wet AMD and other diseases with choroidal neovascularization. Am. J. Ophthalmol. 2025. [Google Scholar] [CrossRef]
- Wang, M.H.; Zhou, J.; Huang, C.; Tang, Z.; Yu, X.; Hou, G.; Yang, J.; Yuan, Q.; Chong, K.K.; Huang, L. Fusion learning methods for the age-related macular degeneration diagnosis based on multiple sources of ophthalmic digital images. In Proceedings of the Second International Conference on Electrical, Electronics, and Information Engineering (EEIE 2023), Wuhan, China, 2–4 November 2023; SPIE: Bellingham, WA, USA, 2023; pp. 470–492. [Google Scholar]
- Yang, J.; Fong, S.; Wang, H.; Hu, Q.; Lin, C.; Huang, S.; Shi, J.; Lan, K.; Tang, R.; Wu, Y.; et al. Artificial intelligence in ophthalmopathy and ultra-wide field image: A survey. Expert Syst. Appl. 2021, 182, 115068. [Google Scholar] [CrossRef]
- Eandi, C.M.; Ciardella, A.; Parravano, M.; Missiroli, F.; Alovisi, C.; Veronese, C.; Morara, M.C.; Grossi, M.; Virgili, G.; Ricci, F. Indocyanine green angiography and optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3690–3696. [Google Scholar] [CrossRef]
- Pole, C.; Ameri, H. Fundus autofluorescence and clinical applications. J. Ophthalmic Vis. Res. 2021, 16, 432. [Google Scholar] [CrossRef]
- Gonzalez, C. AMD: Analysis, evaluation, evolution and prognosis of amd atrophy complication: Study with oct and automatic oct segmentation. Acta Ophthalmol. 2025, 103, 553. [Google Scholar] [CrossRef]
- Crincoli, E.; Parravano, M.C.; Sacconi, R.; Costanzo, E.; Polito, M.S.; Desideri, L.F.; Querques, G. Updates on novel and traditional OCT and OCTA biomarkers in nAMD. Eye 2025, 1, 1–11. [Google Scholar] [CrossRef]
- Yu, T.; Shao, A.; Wu, H.; Su, Z.; Shen, W.; Zhou, J.; Lin, X.; Shi, D.; Grzybowski, A.; Wu, J.; et al. A Systematic Review of Advances in AI-Assisted Analysis of Fundus Fluorescein Angiography (FFA) Images: From Detection to Report Generation. Ophthalmol. Ther. 2025, 14, 599–619. [Google Scholar] [CrossRef]
- Arrigo, A.; Aragona, E.; Bordato, A.; Amato, A.; Borghesan, F.; Bandello, F.; Battaglia Parodi, M. Morphological and functional relationship between OCTA and FA/ICGA quantitative features in AMD-related macular neovascularization. Front. Med. 2021, 8, 758668. [Google Scholar] [CrossRef]
- Wang, H. A Bibliographic Study and Quantitative Analysis of Age-related Macular Degeneration and Fundus Images. Ann. Ophthalmol. Vis. Sci. 2022, 5, 1–8. [Google Scholar]
- Rodrigo-Diaz, E.; Tahir, H.J.; Kelly, J.M.; Parry, N.R.; Aslam, T.; Murray, I.J. The light and the dark of early and intermediate AMD: Cone-and rod-mediated changes are linked to fundus photograph and FAF abnormalities. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5070–5079. [Google Scholar] [CrossRef]
- Frank-Publig, S.; Birner, K.; Riedl, S.; Reiter, G.S.; Schmidt-Erfurth, U. Artificial intelligence in assessing progression of age-related macular degeneration. Eye 2025, 39, 262–273. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Hu, A.; Boyer, D.; Cousins, S.W.; Waheed, N.K.; Rosenfeld, P.J.; Brown, D.; Kaiser, P.K.; Abbruscato, A.; Gao, G.; et al. ReCLAIM-2: A Randomized Phase II Clinical Trial Evaluating Elamipretide in Age-related Macular Degeneration, Geographic Atrophy Growth, Visual Function, and Ellipsoid Zone Preservation. Ophthalmol. Sci. 2025, 5, 100628. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Kim, Y.; Han, K.; Kim, J.H. Prevalence and risk factors of age-related macular degeneration in South Korea: Korea National Health and Nutrition Examination Survey. Ophthalmic Epidemiol. 2025, 32, 34–43. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Wang, S.; Yu, C.; Guo, Z.; Huang, S.; Cai, P.; Miao, Y.; Li, S.; Chen, Q. Real world pharmacovigilance assessment of drug related macular degeneration risks. Sci. Rep. 2025, 15, 1220. [Google Scholar] [CrossRef]
- Jamil, M.U.; Waheed, N.K. Gene therapy for geographic atrophy in age-related macular degeneration: Current insights. Eye 2025, 39, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, J.C.; Parkinson, D.H.; Balikov, D.A.; Rao, R.C. AMD and Stem Cell-Based Therapies. Int. Ophthalmol. Clin. 2024, 64, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhang, Z.; Wang, J.; Yang, Y.; Wang, J.; Chen, T.; Wang, Y.; Xiao, H.; Gu, P.; Wang, Z.; et al. Enhanced activation of retinal progenitor cells by zinc silicate bioceramics for effective functional restoration in retinal degenerative diseases. View 2025, 6, 20240068. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, Y.; Xu, Y.; Shao, A.; Han, H.; Ye, J. Supramolecular Engineering of Nanoceria for Management and Amelioration of Age-Related Macular Degeneration via the Two-Level Blocking of Oxidative Stress and Inflammation. Adv. Sci. 2025, 12, 2408436. [Google Scholar] [CrossRef]
- Nikolaidou, A.; Spyratou, E.; Sandali, A.; Gianni, T.; Platoni, K.; Lamprogiannis, L.; Efstathopoulos, E.P. Utilization of Nanoparticles for Treating Age-Related Macular Degeneration. Pharmaceuticals 2025, 18, 162. [Google Scholar] [CrossRef]
- Bai, L.; Wang, Y. Mesenchymal stem cells-derived exosomes alleviate senescence of retinal pigment epithelial cells by activating PI3K/AKT-Nrf2 signaling pathway in early diabetic retinopathy. Exp. Cell Res. 2024, 441, 114170. [Google Scholar] [CrossRef]
- Hasan, M.M.; Phu, J.; Wang, H.; Sowmya, A.; Kalloniatis, M.; Meijering, E. OCT-based diagnosis of glaucoma and glaucoma stages using explainable machine learning. Sci. Rep. 2025, 15, 3592. [Google Scholar] [CrossRef]
- Ghanchi, F.; Bourne, R.; Downes, S.M.; Gale, R.; Rennie, C.; Tapply, I.; Sivaprasad, S. An update on long-acting therapies in chronic sight-threatening eye diseases of the posterior segment: AMD, DMO, RVO, uveitis and glaucoma. Eye 2022, 36, 1154–1167. [Google Scholar] [CrossRef]
- Crincoli, E.; Ferrara, S.; Miere, A.; Sacconi, R.; Battista, M.; Catania, F.; Souied, E.H.; Querques, G. Correlation between AI-measured lacquer cracks extension and development of myopic choroidal neovascularization. Eye 2023, 37, 2963–2968. [Google Scholar] [CrossRef]
- Taylor, T.R.; Menten, M.J.; Rueckert, D.; Sivaprasad, S.; Lotery, A.J. The role of the retinal vasculature in age-related macular degeneration: A spotlight on OCTA. Eye 2024, 38, 442–449. [Google Scholar] [CrossRef]
- Hagag, A.M.; Holmes, C.; Raza, A.; Riedl, S.; Anders, P.; Kaye, R.; Prevost, T.; Fritsche, L.G.; Rueckert, D.; Bogunović, H.; et al. Features of intermediate and late dry age-related macular degeneration on adaptive optics ophthalmoscopy: Pinnacle Study Report 8. Eye 2025, 39, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Xing, L.; Pan, Y.; Gu, F.; Fang, J.; Yu, X.; Pang, C.P.; Chong, K.K.-L.; Cheung, C.Y.-L.; Liao, X.; et al. AI-based Advanced approaches and dry eye disease detection based on multi-source evidence: Cases, applications, issues, and future directions. Big Data Min. Anal. 2024, 7, 445–484. [Google Scholar] [CrossRef]
- Wang, M.H.; Chong, K.K.-l.; Lin, Z.; Yu, X.; Pan, Y. An explainable artificial intelligence-based robustness optimization approach for age-related macular degeneration detection based on medical IOT systems. Electronics 2023, 12, 2697. [Google Scholar] [CrossRef]
- Mukherjee, S.; De Silva, T.; Duic, C.; Jayakar, G.; Keenan, T.D.; Thavikulwat, A.T.; Chew, E.; Cukras, C. Validation of Deep Learning–Based Automatic Retinal Layer Segmentation Algorithms for Age-Related Macular Degeneration with 2 Spectral-Domain OCT Devices. Ophthalmol. Sci. 2025, 5, 100670. [Google Scholar] [CrossRef]
- Nielsen, C.; Ahsan, A.O.; Wilms, M.; Forkert, N.D. (Eds.) A Novel Multimodal Deep Learning Fusion Framework for Predicting Neovascular Activity Evolution in Exudative Age-Related Macular Degeneration. In International Challenge on Device-Independent Diabetic Macular Edema Onset Prediction, International Challenge on Monitoring Age-Related Macular Degeneration Progression in Optical Coherence Tomography; Springer: Cham, Switzerland, 2025; pp. 182–191. [Google Scholar]
- Gim, N.; Ferguson, A.; Blazes, M.; Soundarajan, S.; Gasimova, A.; Jiang, Y.; Gutiérrez, C.S.; Zalunardo, L.; Corradetti, G.; Elze, T.; et al. Publicly Available Imaging Datasets for Age-related Macular Degeneration: Evaluation according to the Findable, Accessible, Interoperable, Reusable (FAIR) Principles. Exp. Eye Res. 2025, 255, 110342. [Google Scholar] [CrossRef]
- Wang, M.; Lin, Z.; Zhou, J.; Xing, L.; Zeng, P. (Eds.) Applications of Explainable Artificial Intelligent Algorithms to Age-related Macular Degeneration Diagnosis: A Case Study Based on CNN, Attention, and CAM Mechanism. In Proceedings of the 2023 IEEE International Conference on Contemporary Computing and Communications (InC4), Bangalore, India, 21–22 April 2023; IEEE: New York, NY, USA, 2023; pp. 1–5. [Google Scholar]
- Han Wang, M.; Jiang, X.; Zeng, P.; Li, X.; Chong, K.K.-L.; Hou, G.; Fang, X.; Yu, Y.; Yu, X.; Fang, J.; et al. Balancing Accuracy and User Satisfaction: The Role of Prompt Engineering in AI-Driven Healthcare Solutions. Front. Artif. Intell. 2025, 8, 1517918. [Google Scholar] [CrossRef] [PubMed]
- Han Wang, M.; Cui, J.; Lee, S.M.-Y.; Lin, Z.; Zeng, P.; Li, X.; Liu, H.; Liu, Y.; Xu, Y.; Wang, Y.; et al. Applied Machine Learning in Intelligent Systems: Knowledge Graph-Enhanced Ophthalmic Contrastive Learning with “Clinical Profile” Prompts. Front. Artif. Intell. 2025, 8, 1527010. [Google Scholar] [CrossRef]

| Ranking | Country | Publications Count | Centrality | Role in Research Network |
|---|---|---|---|---|
| 1 | USA | 6721 | 0.03 | Leading Contributor |
| 2 | United States | 6458 | 0.08 | Leading Contributor |
| 3 | China | 2863 | 0.04 | Emerging Leader |
| 4 | Germany | 2716 | 0.06 | Strong Collaborator |
| 5 | Peoples R China | 2337 | 0.02 | Emerging Leader |
| 6 | Australia | 2009 | 0.04 | Strong Contributor |
| 7 | Italy | 2007 | 0.04 | Strong Contributor |
| 8 | United Kingdom | 1878 | 0.05 | Global Hub |
| 9 | England | 1667 | 0.02 | Global Hub |
| 10 | Japan | 1647 | 0.01 | Regional Leader |
| 11 | South Korea | 1629 | 0.02 | Regional Leader |
| 12 | Switzerland | 1555 | 0.06 | Key Hub |
| 13 | France | 1539 | 0.06 | Key Hub |
| 14 | India | 1390 | 0.03 | Regional Contributor |
| 15 | Spain | 1049 | 0.05 | Key Collaborator |
| 16 | Canada | 1047 | 0.11 | Key Hub |
| 17 | Singapore | 935 | 0.04 | Key Collaborator |
| 18 | Netherlands | 753 | 0.02 | Moderate Contributor |
| Ranking | Institutions | Publications Count | Centrality | Role in Research Network |
|---|---|---|---|---|
| 1 | University of Melbourne | 430 | 0 | Significant contributor to global research output, leading in impactful publications. |
| 2 | University of California | 409 | 0 | Prominent role in advancing interdisciplinary research within the network. |
| 3 | University of Sydney | 392 | 0 | Major contributor to research initiatives, particularly in collaboration with local and global institutions. |
| 4 | National Eye Institute | 392 | 0 | Key player in fostering innovation and collaboration in vision-related research. |
| 5 | University of California, Los Angeles | 383 | 0 | Leading institution in producing high-impact research, especially in healthcare and technology. |
| 6 | Harvard Medical School | 349 | 0 | Highly influential in medical research, facilitating widespread collaborations globally. |
| 7 | University College London (UCL) | 300 | 0 | Renowned for its pioneering research, with strong inter-institutional ties. |
| 8 | Duke University | 289 | 0 | Contributes significantly to collaborative research projects and knowledge dissemination. |
| 9 | National Institutes of Health (NIH) | 285 | 0 | Major hub for funding and facilitating groundbreaking biomedical research. |
| 10 | Moorfields Eye Hospital NHS Foundation | 285 | 0 | Key institution in ophthalmic research, connecting niche areas of expertise globally. |
| 11 | Sun Yat-Sen University | 227 | 0 | A prominent Asian institution showcasing increasing contributions to global research and collaboration. |
| 12 | Shanghai Jiao Tong University | 193 | 0 | Emerging as a significant research hub in Asia, contributing to advancements in interdisciplinary fields. |
| Ranking | Journal Name | Record Count | Percentage of Total | Impact Factor (2023) |
|---|---|---|---|---|
| 1 | Investigative Ophthalmology & Visual Science (IOVS) | 6137 | 18.43% | 3.241 |
| 2 | Retina: The Journal of Retinal and Vitreous Diseases | 1129 | 3.39% | 3.944 |
| 3 | Ophthalmology | 1033 | 3.10% | 8.462 |
| 4 | American Journal of Ophthalmology | 817 | 2.45% | 5.711 |
| 5 | British Journal of Ophthalmology | 721 | 2.17% | 2.601 |
| 6 | Graefe’s Archive for Clinical and Experimental Ophthalmology | 700 | 2.10% | 2.467 |
| 7 | Eye | 615 | 1.85% | 2.806 |
| 8 | Acta Ophthalmologica | 604 | 1.81% | 2.623 |
| 9 | Experimental Eye Research | 495 | 1.49% | 3.291 |
| 10 | PLOS ONE | 466 | 1.40% | 3.752 |
| 11 | Archives of Ophthalmology | 446 | 1.34% | 5.201 |
| 12 | Scientific Reports | 429 | 1.29% | 4.011 |
| 13 | Clinical and Experimental Ophthalmology | 347 | 1.04% | 2.034 |
| 14 | Clinical Ophthalmology | 346 | 1.04% | 1.952 |
| 15 | European Journal of Ophthalmology | 316 | 0.95% | 2.098 |
| 16 | Ophthalmologica | 310 | 0.93% | 1.365 |
| 17 | International Journal of Molecular Sciences | 308 | 0.93% | 5.923 |
| 18 | Ophthalmology Retina | 304 | 0.91% | 5.305 |
| 19 | JAMA Ophthalmology | 290 | 0.87% | 7.492 |
| 20 | Ophthalmologe | 248 | 0.75% | 1.452 |
| Ranking | Category | Publications Count | Centrality | Role in Research Network |
|---|---|---|---|---|
| 1 | Ophthalmology | 10,170 | 0.07 | Central |
| 2 | Biochemistry & Molecular Biology | 1285 | 0.18 | Central |
| 3 | Pharmacology & Pharmacy | 995 | 0.15 | Central |
| 4 | Multidisciplinary Sciences | 919 | 0 | Moderate |
| 5 | Medicine, Research & Experimental | 903 | 0.19 | Central |
| 6 | Medicine, General & Internal | 859 | 0.04 | Moderate |
| 7 | Cell Biology | 843 | 0.07 | Moderate |
| 8 | Chemistry, Multidisciplinary | 460 | 0.04 | Moderate |
| 9 | Neurosciences | 443 | 0.16 | Central |
| 10 | Genetics & Heredity | 441 | 0.03 | Moderate |
| 11 | Engineering, Biomedical | 377 | 0.18 | Central |
| 12 | Biotechnology & Applied Microbiology | 292 | 0.15 | Moderate |
| 13 | Geriatrics & Gerontology | 290 | 0.08 | Moderate |
| 14 | Immunology | 273 | 0.07 | Moderate |
| 15 | Chemistry, Medicinal | 254 | 0.02 | Moderate |
| 16 | Health Care Sciences & Services | 237 | 0.02 | Low |
| 17 | Radiology, Nuclear Medicine & Medical Imaging | 233 | 0.08 | Moderate |
| 18 | Nutrition & Dietetics | 220 | 0.02 | Low |
| 19 | Optics | 195 | 0.09 | Moderate |
| 20 | Food Science & Technology | 195 | 0.04 | Low |
| Ranking | Keyword | Count | Category | Insight |
|---|---|---|---|---|
| 1 | human | 15,607 | General Theme | Focus on human-centered studies |
| 2 | age-related macular degeneration | 11,950 | Disease Focus | Focus on age-related eye diseases |
| 3 | humans | 11,594 | General Theme | Human studies in general |
| 4 | article | 10,165 | General Theme | General term for academic articles |
| 5 | macular degeneration | 10,097 | Disease Focus | Focus on macular degeneration pathologies |
| 6 | male | 7756 | Demographic Focus | Demographic analysis |
| 7 | female | 7479 | Demographic Focus | Demographic analysis |
| 8 | aged | 6800 | Demographic Focus | Demographic analysis |
| 9 | optical coherence tomography | 6578 | Methodology | Use of advanced imaging techniques |
| 10 | controlled study | 6238 | Methodology | Reliance on structured methodologies |
| 11 | visual acuity | 5391 | Clinical Focus | Intersection of clinical treatments |
| 12 | retinal pigment epithelium | 4750 | Clinical Focus | Focus on retinal pathologies |
| 13 | ranibizumab | 4316 | Clinical Focus | Key treatment for AMD |
| 14 | diabetic retinopathy | 3493 | Clinical Focus | Focus on diabetic eye diseases |
| 15 | genetics | 2272 | Molecular Mechanism | Exploration of genetic factors |
| 16 | angiogenesis inhibitors | 2687 | Molecular Mechanism | Study of angiogenesis-related therapies |
| 17 | oxidative stress | 2608 | Molecular Mechanism | Study of oxidative stress mechanisms |
| 18 | deep learning | 1940 | Emerging Technology | Adoption of AI techniques |
| 19 | optical coherence tomography angiography | 1129 | Emerging Technology | Use of advanced imaging in ophthalmology |
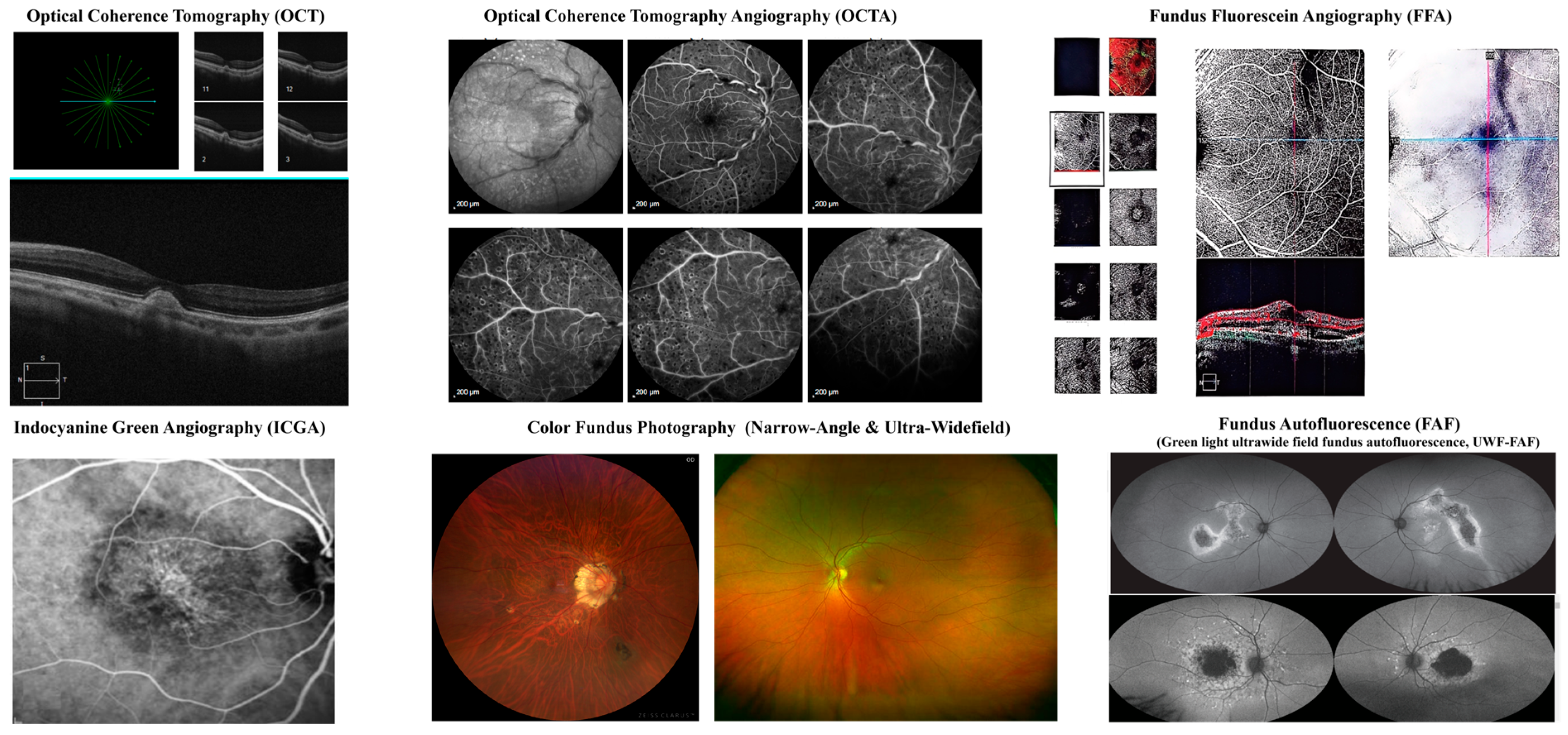
| Imaging Modality | Key Biomarkers |
|---|---|
| OCT | Drusen, SRF, IRF, PED, Hyperreflective foci, Ellipsoid zone loss, GA, Fibrovascular scarring, Choroidal thickness changes [34] |
| OCTA | CNV, Capillary dropout, Choriocapillaris flow deficits, Dark halo sign, Vascular density changes [35] |
| FFA | Classic/Occult CNV, Window defects, Pooling in PED, Staining of fibrosis, Blocked fluorescence [36] |
| ICGA | Polypoidal lesions, Branching vascular networks, Hypofluorescence in GA, Late leakage from CNV [37] |
| Fundus Photography | Drusen, GA, Pigmentary changes, Subretinal hemorrhage, Exudates, Fibrotic scars [38] |
| FAF | Hyperautofluorescent drusen, Hypoautofluorescence in GA, Speckled autofluorescence, Ring of increased FAF [39] |
| Image Type | Biomarker | Before Treatment (Signs of Active AMD) | After Treatment (Effective Response) |
|---|---|---|---|
| Optical Coherence Tomography (OCT) | Drusen | Large, confluent soft drusen | Reduction in drusen size or resorption |
| Subretinal Fluid (SRF) | Hyporeflective spaces above the RPE | Decreased or complete resolution of SRF | |
| Intraretinal Fluid (IRF) | Cystoid spaces in the retina | Reduction or disappearance of IRF | |
| Retinal Pigment Epithelium Detachment (PED) | Elevated RPE with fluid accumulation | Flattening or decreased height of PED | |
| Hyperreflective Foci | Bright spots in retinal layers | Reduction in number or disappearance | |
| Ellipsoid Zone Disruption | Loss of photoreceptor integrity | Partial or full recovery of the ellipsoid zone | |
| Geographic Atrophy (GA) | Atrophic retinal areas with RPE loss | Stabilization (no further progression) | |
| Fibrovascular Scarring | Hyperreflective fibrotic tissue in the macula | No further growth of the scar | |
| Choroidal Thickness | Thinning (dry AMD) or thickening (wet AMD) | Stabilization or normalization | |
| Optical Coherence Tomography Angiography (OCTA) | Choroidal Neovascularization (CNV) | Active CNV with abnormal vessels | Reduction in CNV size, decreased vascular density |
| Capillary Dropout | Loss of retinal capillary network | Improved vascular perfusion or stability | |
| Choriocapillaris Flow Deficits | Non-perfused areas | Reduction in ischemic areas | |
| Dark Halo Sign | Hypointense ring around CNV | Decrease or disappearance | |
| Vascular Density Changes | Increased neovascularization or reduced capillary density | Stabilization or improvement in normal vasculature | |
| Fundus Fluorescein Angiography (FFA) | Classic CNV | Well-defined hyperfluorescent lesion | Decrease or disappearance of leakage |
| Occult CNV | Diffuse hyperfluorescence with leakage | Reduced fluorescence or stability | |
| Window Defects | Hyperfluorescence due to RPE atrophy | No expansion of defects | |
| Pooling in PED | Accumulation of dye in PED | Decreased pooling | |
| Staining of Fibrotic Scars | Persistent fluorescence without leakage | No further increase in staining | |
| Blocked Fluorescence | Hemorrhage or lipid exudates blocking fluorescence | Absorption of hemorrhage and reduced blockage | |
| Indocyanine Green Angiography (ICGA) | Polypoidal Lesions | Active, round, hyperfluorescent structures | Decrease in lesion size or complete resolution |
| Branching Vascular Networks | Abnormal choroidal vasculature | Reduction in complexity of networks | |
| Hypofluorescence in GA | Large areas of non-perfusion | No further enlargement of hypofluorescent zones | |
| Late Leakage from CNV | Persistent fluorescence in late phases | Decrease or stabilization | |
| Color Fundus Photography (Ultra-Widefield & Narrow-Angle) | Drusen | Soft, large drusen in macula | Reduction in drusen size or disappearance |
| Geographic Atrophy (GA) | Large atrophic regions | No further spread of GA | |
| Pigmentary Changes | RPE hyperpigmentation or hypopigmentation | Stabilization of pigmentary abnormalities | |
| Subretinal Hemorrhage | Red patches in wet AMD | Absorption of hemorrhage | |
| Exudates | Lipid deposits near the macula | Reduction in exudates | |
| Fibrotic Scars | White lesions due to fibrosis | No further growth of scar tissue | |
| Fundus Autofluorescence (FAF) | Hyperautofluorescent Drusen | Increased metabolic activity in RPE | Reduced autofluorescence, indicating drusen regression |
| Hypoautofluorescence in GA | Large areas of RPE loss | No expansion of atrophic regions | |
| Speckled Autofluorescence | Irregular FAF pattern, suggesting stressed RPE | Normalization or stability | |
| Ring of Increased FAF around GA | Predicts future atrophy expansion | Ring disappears or remains stable | |
| Patchy Hypoautofluorescence | Advanced disease with fibrosis | No worsening of patchy areas |
| Studies | Data Source | Sample Size | Algorithm Architecture | Validation Methods | Performance Metrics |
|---|---|---|---|---|---|
| AMD detection [56] | OCT & FAF: Kaggle open-source datasets CFP (45°): Open-source + real-world data from Zhuhai People’s Hospital UWF (200°): In-house dataset from Shenzhen Aier Hospital | OCT: 32,347 images (AMD + normal) FAF: 1947 AMD + 2874 normal images CFP: 4445 AMD (445 open-source + 4000 real-world) 4874 normal (2000 open-source + 2874 real-world) UWF: 2300 images (1100 AMD + 1200 normal) | VGG16 and Improved VGG16 (VGG16 + Skip-attention) | Accuracy for the Unseen Testing Dataset Sensitivity Specificity AUC XAI Indicator Test Time/Second (Per Image) | Accuracy (Unseen Testing Dataset): Original VGG16: 57–97% (avg: 82%); Improved VGG16: 90.6–100% (avg: 96.62%) Sensitivity: Original VGG16: 61–94% (avg: 80%); Improved VGG16: 91–100% (avg: 96%) Specificity: Original VGG16: 46–95% (avg: 74%); Improved VGG16: 89.1–100% (avg: 96%) AUC (Area Under the Curve): Original VGG16: 80.88–91.01% (avg: 90.77%); Improved VGG16: 84.25–99.75% (avg: 94.58%) XAI Indicator (Explainability Score): Original VGG16: 0.3–1.0 (avg: 0.64); Improved VGG16: 0.6–1.0 (avg: 0.84) Test Time per Image (seconds): Original VGG16: 0.067–0.117 s (avg: 0.084 s); Improved VGG16: 0.07–0.45 s (avg: 18.50% increase overall) |
| The segmentations of the internal limiting membrane (ILM), retinal pigment epithelium (RPE), and RPE to Bruch’s membrane region [57] | Rear-word data from individuals participating in a prospective longitudinal study on AMD at the National Eye Institute, National Institutes of Health | Total OCT volumes: 402 (201 Spectralis for training, 201 Spectralis + 201 Cirrus for testing); Total B-scans: 25,728 (201 × 128) | U-Net and DeepLabV3 | Mean absolute error (MAE) and mean squared error (MSE) | Mean Absolute Error (MAE): ILM: 1.82 ± 0.24 pixels (≈ 7.0 ± 0.9 μm); RPE: 2.46 ± 0.66 pixels (≈ 9.5 ± 2.6 μm) Dice Similarity Coefficient (RPEDC region): Dice: 0.87 ± 0.01 |
| Classify changes in consecutive OCT B-scans; Predict 3-month structural evolution from a single time point [58] | MARIO Challenge dataset (MICCAI 2024) Includes OCT B-scans, infrared fundus localizer images, and clinical variables | Not explicitly stated, but based on official MARIO challenge dataset (participant-limited, multi-time-point OCT volumes) | Multimodal fusion deep learning framework; Feature extractors: Finetuned RETFound + EfficientNetV2; Input modalities: OCT B-scans + infrared fundus + clinical data | Evaluated on MARIO challenge benchmark tasks; Task-specific performance comparison using standard challenge metrics | F1 Score (Task 1—change detection): 0.851 F1 Score (Task 2–3-month prediction): 0.703 |
| Evaluation of findable, accessible, interoperable, reusable (FAIR) principle compliance in public OCT datasets for AMD research [59] | Source Type: Public, open-access datasets Search Platforms: Google Dataset Search National Library of Medicine (NLM) Dataset Catalog PubMed Inclusion Criteria: AMD-specific research purpose Human-derived OCT images Open-access availability | Google Dataset Search: 42 datasets NLM Dataset Catalog: 142 datasets PubMed: 131 publications Final included datasets for analysis: 16 non-duplicate OCT datasets | None applied (focus was on dataset FAIR compliance assessment, not algorithmic modeling) | Evaluation against FAIR principles (Findable, Accessible, Interoperable, Reusable); Manual checklist-based analysis based on metadata and documentation review | Findable: 5%; Accessible: 82%; Interoperable: 73%; Reusable: 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.H. Integrating Artificial Intelligence and Precision Therapeutics for Advancing the Diagnosis and Treatment of Age-Related Macular Degeneration. Bioengineering 2025, 12, 548. https://doi.org/10.3390/bioengineering12050548
Wang MH. Integrating Artificial Intelligence and Precision Therapeutics for Advancing the Diagnosis and Treatment of Age-Related Macular Degeneration. Bioengineering. 2025; 12(5):548. https://doi.org/10.3390/bioengineering12050548
Chicago/Turabian StyleWang, Mini Han. 2025. "Integrating Artificial Intelligence and Precision Therapeutics for Advancing the Diagnosis and Treatment of Age-Related Macular Degeneration" Bioengineering 12, no. 5: 548. https://doi.org/10.3390/bioengineering12050548
APA StyleWang, M. H. (2025). Integrating Artificial Intelligence and Precision Therapeutics for Advancing the Diagnosis and Treatment of Age-Related Macular Degeneration. Bioengineering, 12(5), 548. https://doi.org/10.3390/bioengineering12050548






