An Evaluation Model for Brain Ischemia Protection in Mice by Low-Intensity Pulsed Ultrasound Stimulation Based on Functional Cortico-Muscular Coupling
Abstract
1. Introduction
2. Materials and Methods
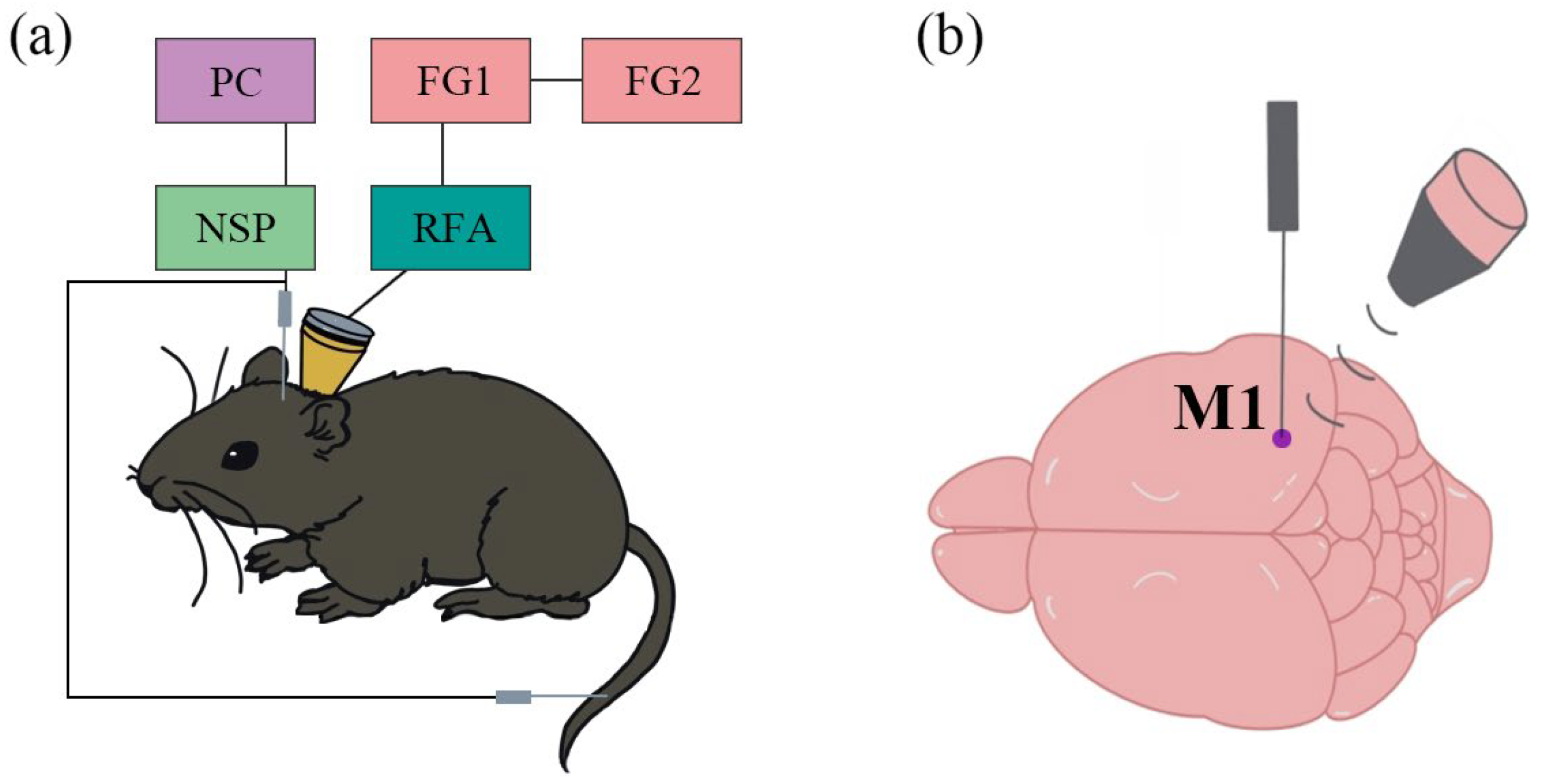
2.1. Animal Anesthesia and Surgery
2.2. BCAO Procedure
2.3. Low-Intensity Pulsed Ultrasound Stimulation System
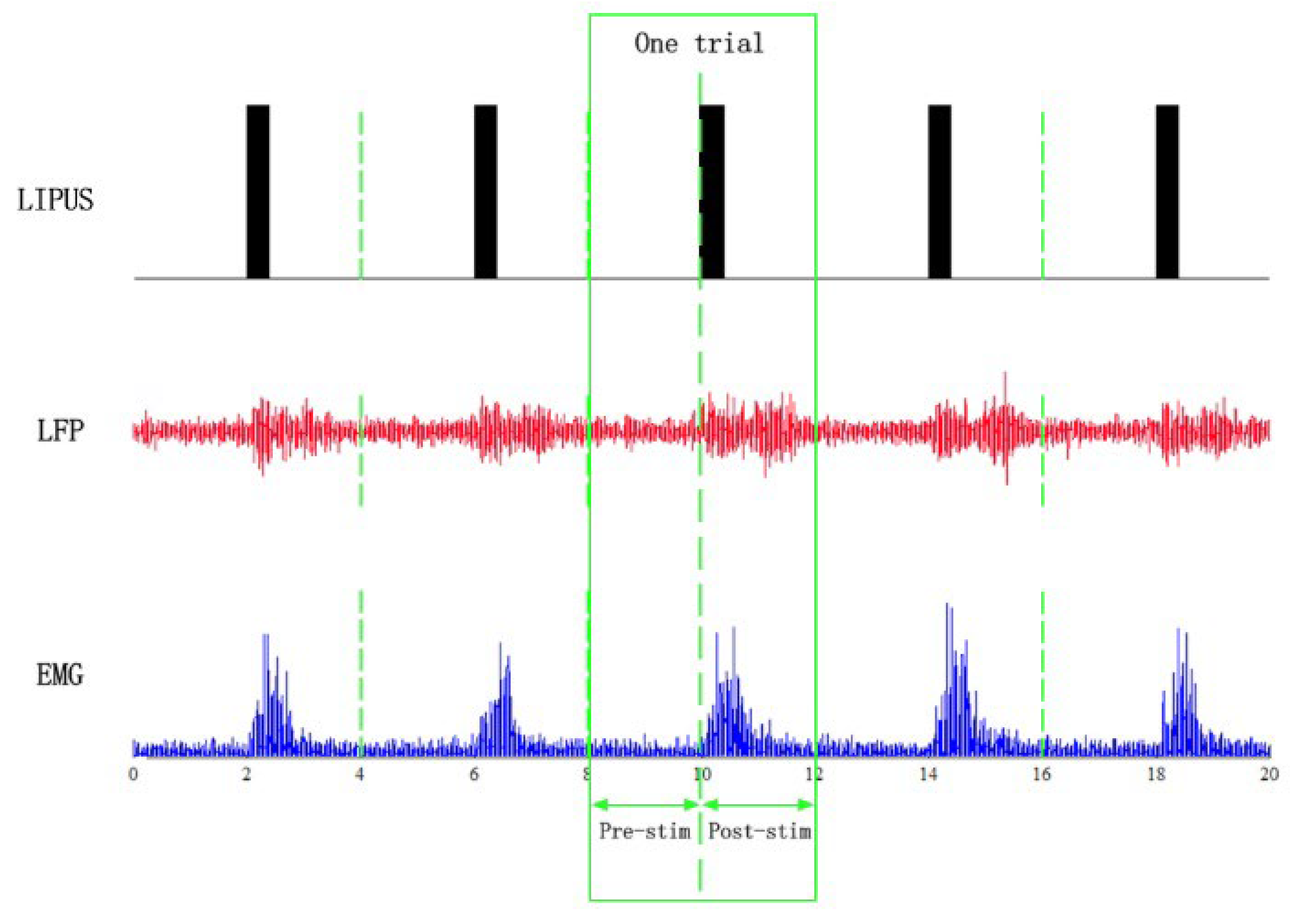
2.4. Data Acquisition
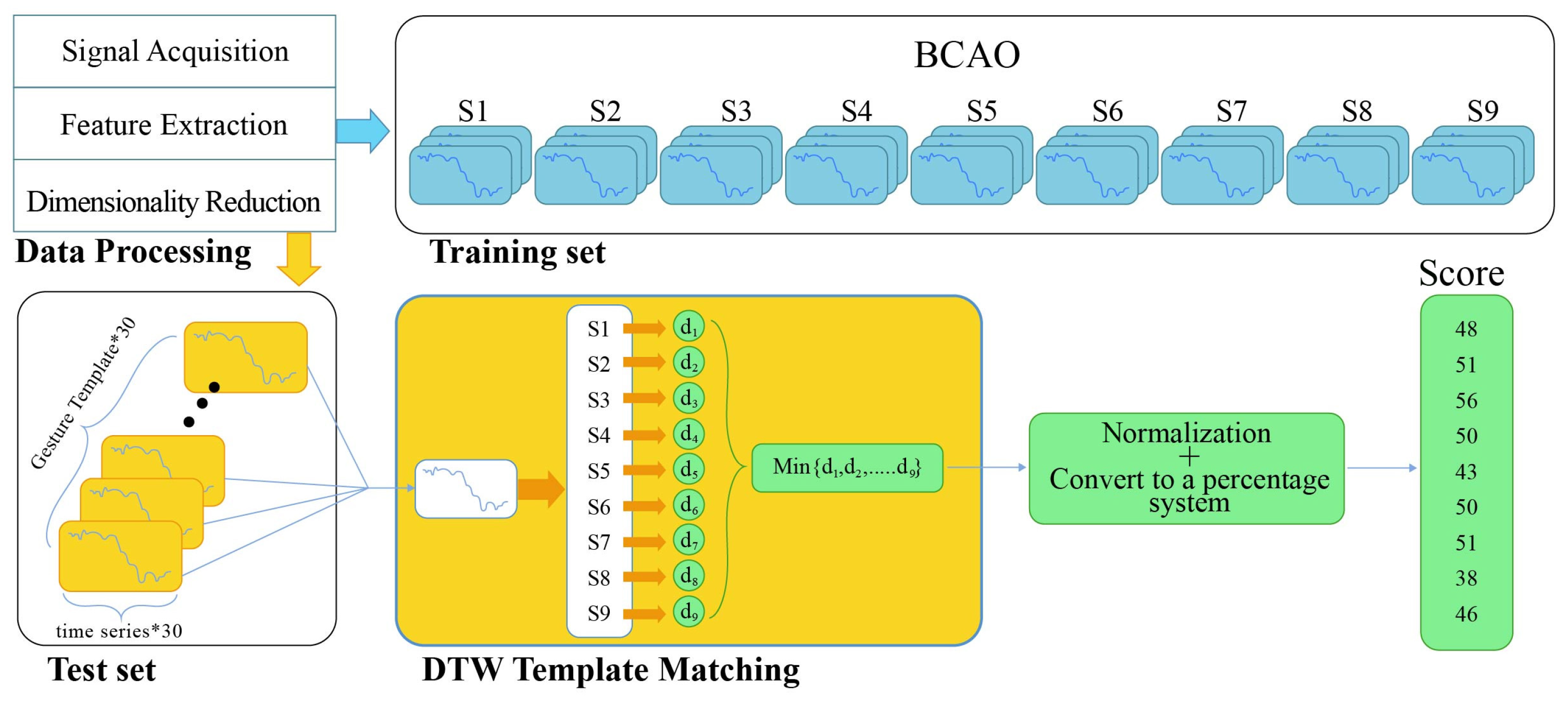
2.5. Data Processing and Statistics
2.6. Dynamic Time Warping Analysis
3. Results
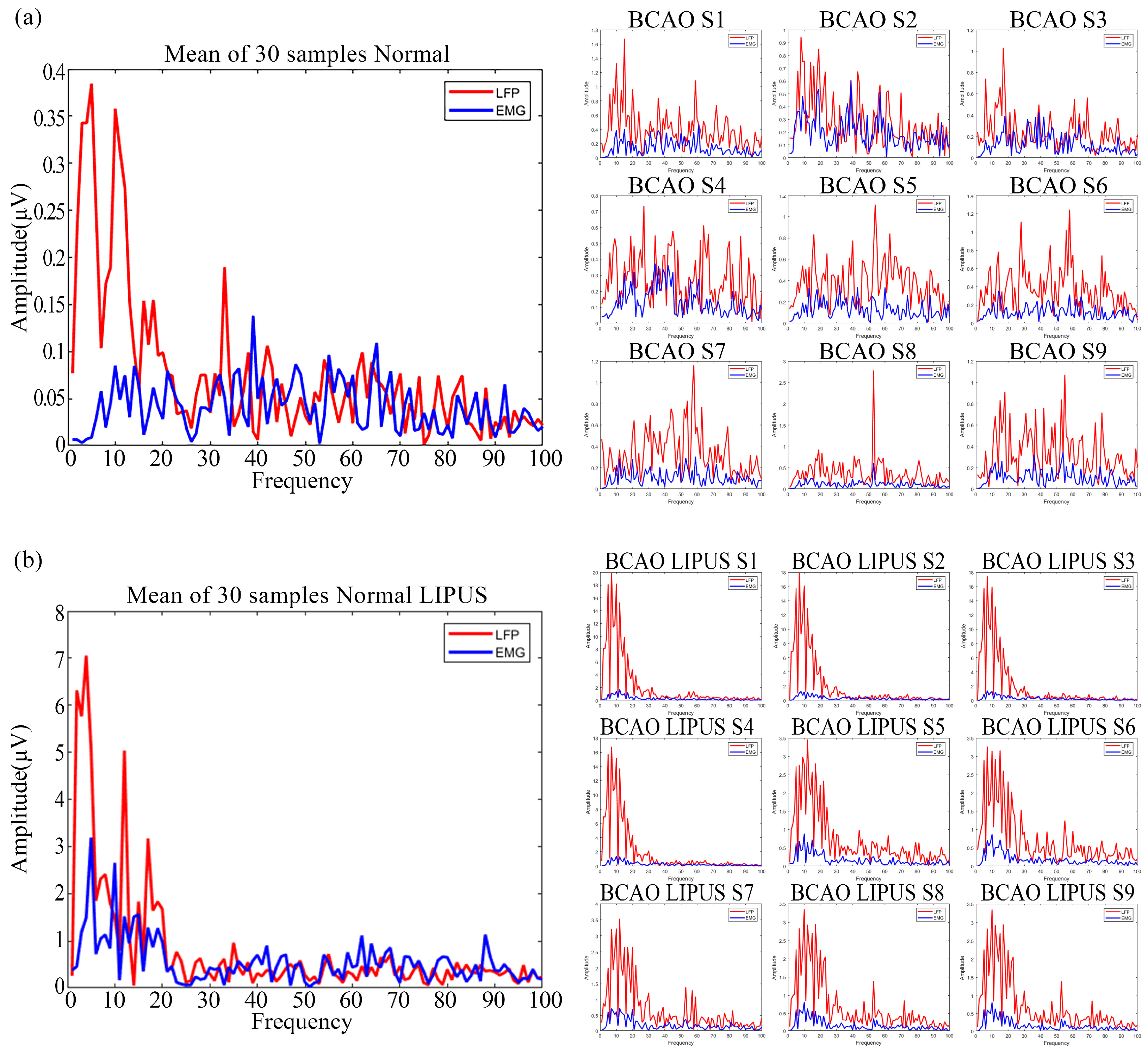
3.1. Preprocessed Data Graphs of Normal and BCAO Mice Before and After LIPUS
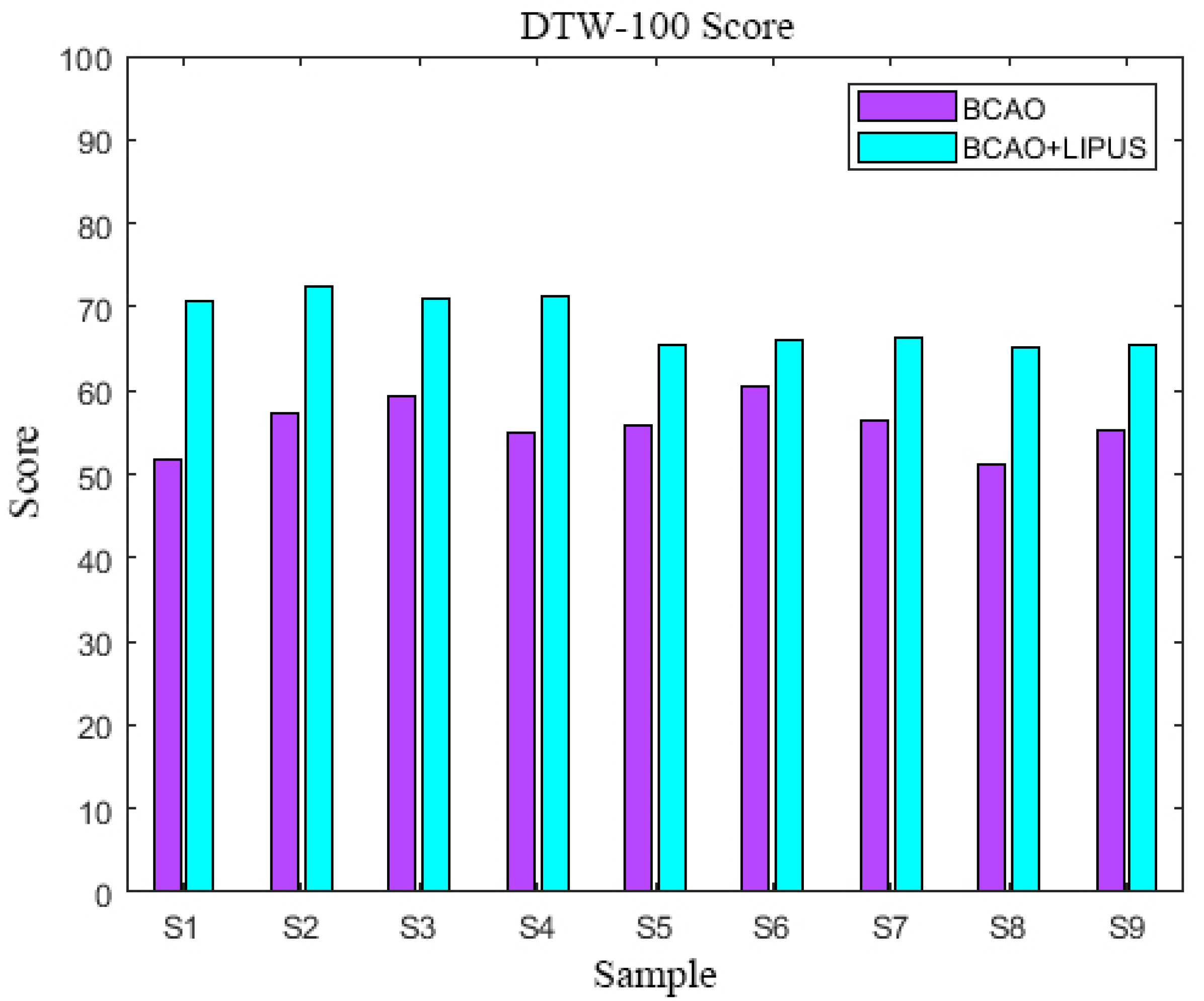
3.2. BCAO Group DTW-100 Scores Before and After LIPUS
3.3. DTW-100 Scores
3.4. Preprocessed Frequency-Domain Data Graphs of Normal and BCAO Mice Before and After LIPUS
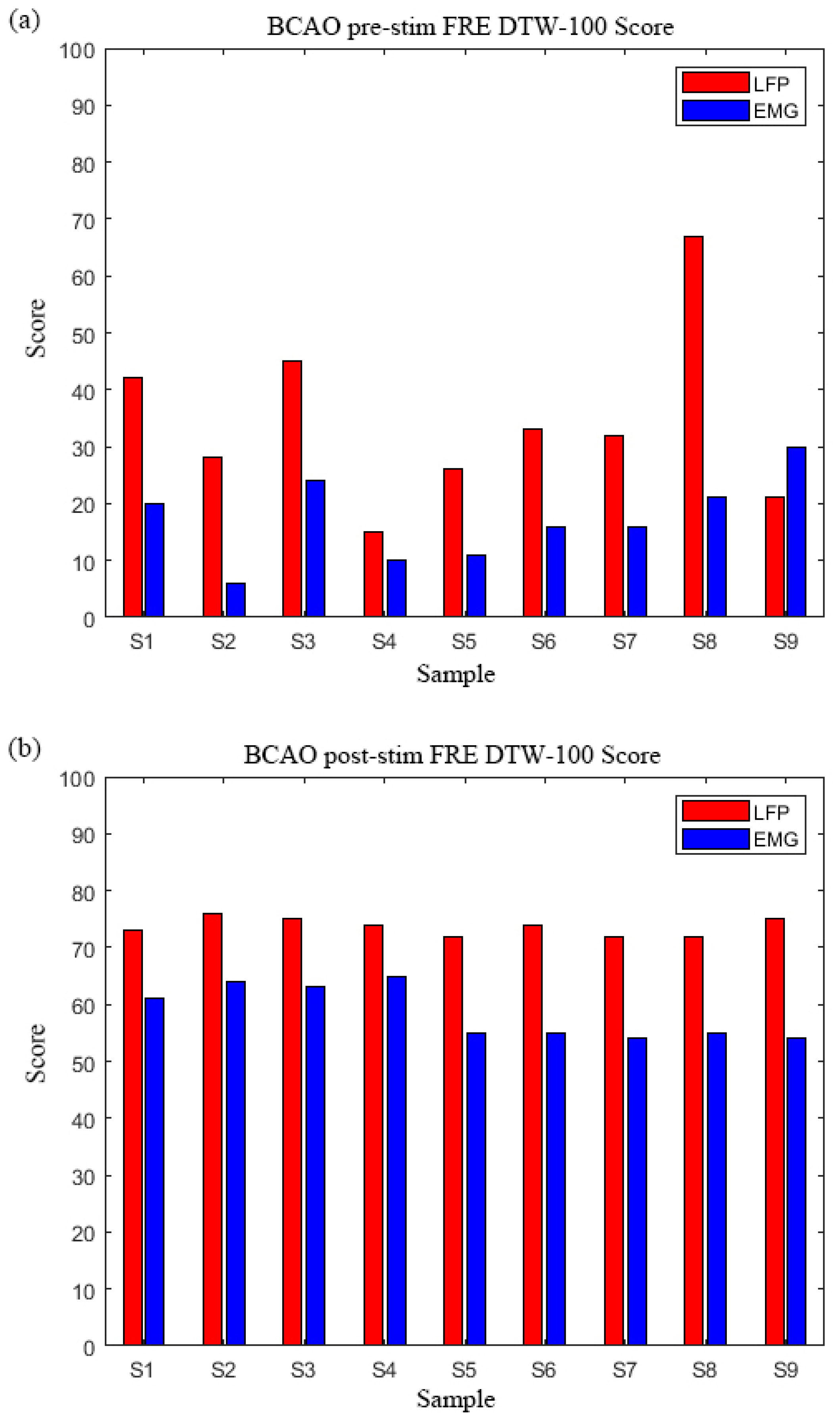
3.5. BCAO Group Frequency-Domain DTW-100 Scores Before and After LIPUS
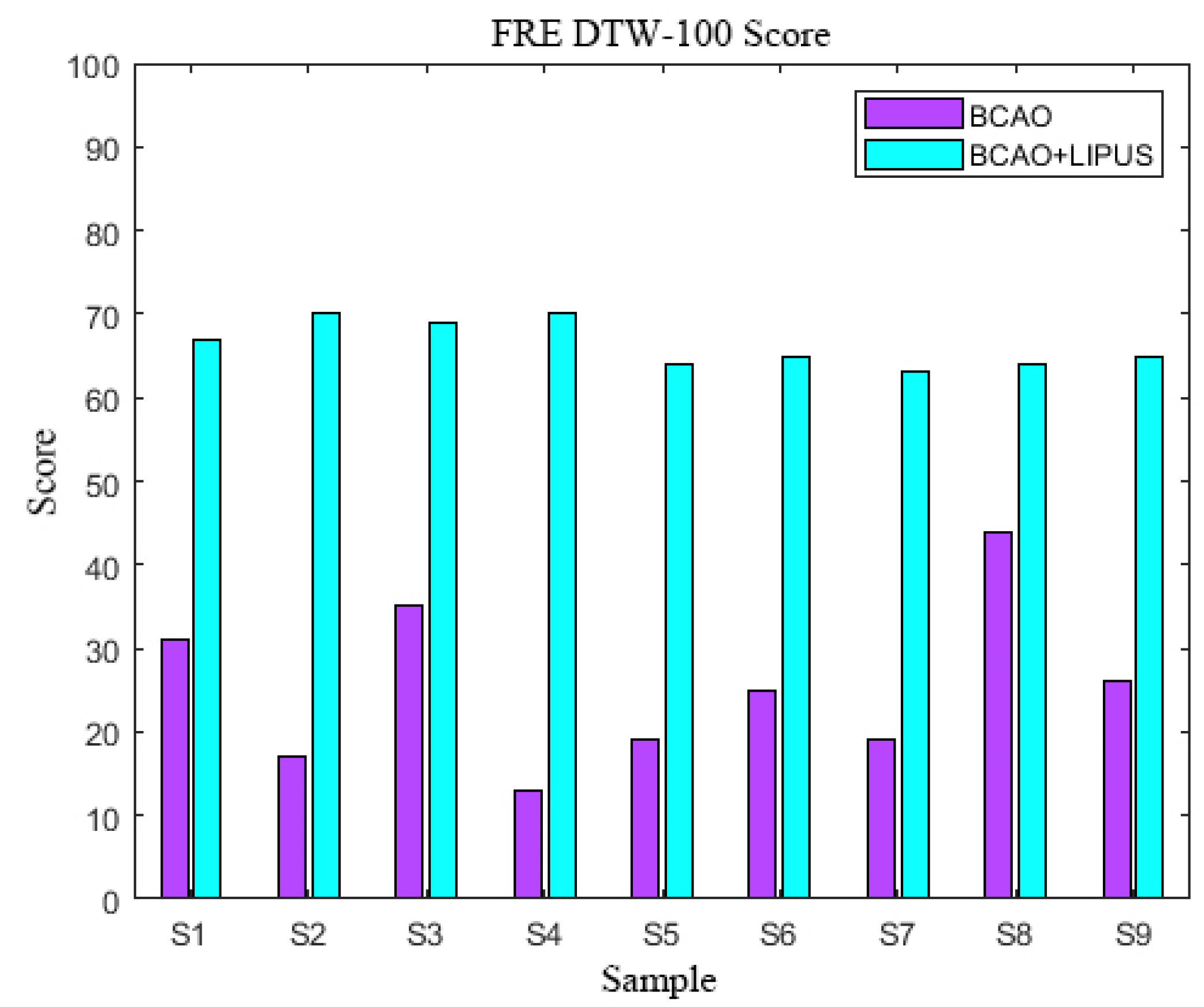
3.6. FRE DTW-100 Scores
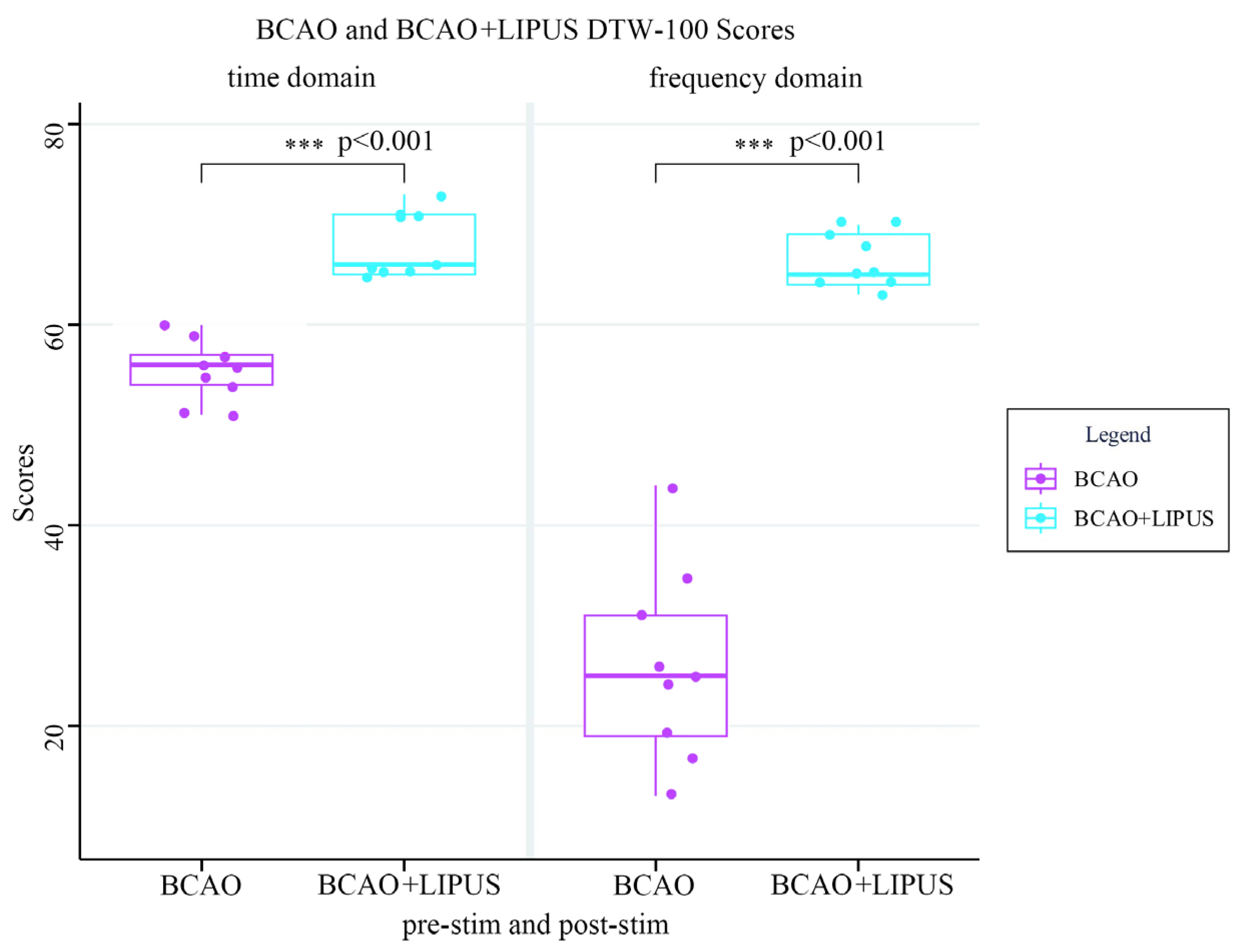
3.7. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LIPUS | Low-Intensity Pulsed Ultrasound Stimulation |
| LFP | Local Field Potential |
| EMG | Electromyography |
| DTW | Dynamic Time Warping |
| BCAO | Bilateral Carotid Artery Occlusion |
| M1 | Primary motor cortex |
| FRE | Frequency |
| FCMC | Functional Cortico-Muscular Coupling |
Appendix A
| BCAO DTW-100 Scores before LIPUS | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
| LFP | 48 | 51 | 56 | 50 | 43 | 50 | 51 | 38 | 46 |
| EMG | 55 | 62 | 63 | 60 | 69 | 71 | 62 | 64 | 65 |
| BCAO DTW-100 Scoresafter LIPUS | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
| LFP | 81 | 82 | 82 | 82 | 78 | 78 | 78 | 77 | 79 |
| EMG | 61 | 63 | 60 | 60 | 53 | 54 | 54 | 53 | 52 |
| DTW-100 Scores | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
| BCAO | 51 | 57 | 59 | 54 | 56 | 60 | 56 | 51 | 55 |
| BCAO+LIPUS | 71 | 73 | 71 | 71 | 65 | 66 | 66 | 65 | 65 |
| BCAOFRE DTW-100 Scores before LIPUS | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
| LFP | 42 | 28 | 45 | 15 | 26 | 33 | 32 | 67 | 21 |
| EMG | 20 | 6 | 24 | 10 | 11 | 16 | 16 | 21 | 30 |
| BCAOFRE DTW-100 Scoresafter LIPUS | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
| LFP | 73 | 76 | 75 | 74 | 72 | 74 | 72 | 72 | 75 |
| EMG | 63 | 64 | 63 | 65 | 55 | 55 | 54 | 55 | 54 |
| FRE DTW-100 Scores | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
| BCAO | 31 | 17 | 35 | 13 | 19 | 25 | 24 | 44 | 26 |
| BCAO+LIPUS | 68 | 70 | 69 | 70 | 64 | 65 | 63 | 64 | 65 |
References
- Balch, M.H.H.; Harris, H.; Chugh, D.; Gnyawali, S.; Rink, C.; Nimjee, S.M.; Arnold, W.D. Ischemic Stroke-Induced Polyaxonal Innervation at the Neuromuscular Junction Is Attenuated by Robot-Assisted Mechanical Therapy. Exp. Neurol. 2021, 343, 113767–113823. [Google Scholar] [CrossRef]
- Neumann, J.T.; Cohan, C.H.; Dave, K.R.; Wright, C.B.; Perez-Pinzon, M.A. Global Cerebral Ischemia: Synaptic and Cognitive Dysfunction. Curr. Drug Targets 2013, 14, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Donnan, G.A.; Davis, S.M.; Parsons, M.W.; Ma, H.; Dewey, H.M.; Howells, D.W. How to Make Better Use of Thrombolytic Therapy in Acute Ischemic Stroke. Nat. Rev. Neurol. 2011, 7, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bogosavljevic, V.; Leys, D.; Jovanovic, D.; Beslac-Bumbasirevic, L.; Lucas, C. Intravenous Thrombolytic Therapy in Patients with Stroke Mimics: Baseline Characteristics and Safety Profile. Eur. J. Neurol. 2011, 18, 1246–1250. [Google Scholar] [CrossRef]
- Marshall, R.S. Progress in Intravenous Thrombolytic Therapy for Acute Stroke. JAMA Neurol. 2015, 72, 928. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.M.; Schumacher, H.C.; Connolly, E.S.; Heyer, E.J.; Gray, W.A.; Higashida, R.T. Current Status of Endovascular Stroke Treatment. Circulation 2011, 123, 2591–2601. [Google Scholar] [CrossRef]
- Ding, D. Endovascular Mechanical Thrombectomy for Acute Ischemic Stroke: A New Standard of Care. J. Stroke 2015, 17, 123. [Google Scholar] [CrossRef]
- Przybylowski, C.J. Evolution of Endovascular Mechanical Thrombectomy for Acute Ischemic Stroke. World J. Clin. Cases 2014, 2, 614. [Google Scholar] [CrossRef]
- Ovbiagele, B.; Kidwell, C.S.; Starkman, S.; Saver, J.L. Neuroprotective Agents for the Treatment of Acute Ischemic Stroke. Curr. Neurol. Neurosci. Rep. 2003, 3, 9–20. [Google Scholar] [CrossRef]
- Fisher, M. New Approaches to Neuroprotective Drug Development. Stroke 2011, 42, S24–S27. [Google Scholar] [CrossRef]
- Kikuchi, K.; Uchikado, H.; Morioka, M.; Murai, Y.; Tanaka, E. Clinical Neuroprotective Drugs for Treatment and Prevention of Stroke. Int. J. Mol. Sci. 2012, 13, 7739–7761. [Google Scholar] [CrossRef]
- Jiang, X.; Savchenko, O.; Li, Y.; Qi, S.; Yang, T.; Zhang, W.; Chen, J. A Review of Low-Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans. Bio-Med. Eng. 2019, 66, 2704–2718. [Google Scholar] [CrossRef]
- Chen, C.-M.; Wu, C.-T.; Yang, T.-H.; Liu, S.-H.; Yang, F.-Y. Preventive Effect of Low Intensity Pulsed Ultrasound against Experimental Cerebral Ischemia/Reperfusion Injury via Apoptosis Reduction and Brain-Derived Neurotrophic Factor Induction. Sci. Rep. 2018, 8, 5568–5579. [Google Scholar] [CrossRef]
- Wu, C.-T.; Yang, T.-H.; Chen, M.-C.; Chung, Y.-P.; Guan, S.-S.; Long, L.-H.; Liu, S.-H.; Chen, C.-M. Low Intensity Pulsed Ultrasound Prevents Recurrent Ischemic Stroke in a Cerebral Ischemia/Reperfusion Injury Mouse Model via Brain-Derived Neurotrophic Factor Induction. Int. J. Mol. Sci. 2019, 20, 5169. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Zhang, C.; Wang, W.; Chen, R.; Jiao, H.; Li, L.; Zhang, L.; Cui, L. Anti-Inflammation of Natural Components from Medicinal Plants at Low Concentrations in Brain via Inhibiting Neutrophil Infiltration after Stroke. Mediat. Inflamm. 2016, 2016, 9537901. [Google Scholar] [CrossRef]
- Guo, T.; Li, H.; Lv, Y.; Lu, H.; Niu, J.; Sun, J.; Yang, G.-Y.; Ren, C.; Tong, S. Pulsed Transcranial Ultrasound Stimulation Immediately after the Ischemic Brain Injury Is Neuroprotective. IEEE Trans. Bio-Med. Eng. 2015, 62, 2352–2357. [Google Scholar] [CrossRef]
- Su, W.-S.; Wu, C.-H.; Chen, S.-F.; Yang, F.-Y. Low-Intensity Pulsed Ultrasound Improves Behavioral and Histological Outcomes after Experimental Traumatic Brain Injury. Sci. Rep. 2017, 7, 15524. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of Healthy Motor Modules Predicts Reduced Locomotor Performance and Muscle Coordination Complexity Post-Stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef]
- Arya, K.N.; Pandian, S. Interlimb Neural Coupling: Implications for Poststroke Hemiparesis. Ann. Phys. Rehabil. Med. 2014, 57, 696–713. [Google Scholar] [CrossRef]
- Akbas, T.; Neptune, R.R.; Sulzer, J. Neuromusculoskeletal Simulation Reveals Abnormal Rectus Femoris-Gluteus Medius Coupling in Post-Stroke Gait. Front. Neurol. 2019, 10, 301. [Google Scholar] [CrossRef]
- Levin, M.F.; Kleim, J.A.; Wolf, S.L. What Do Motor “Recovery” and “Compensation” Mean in Patients Following Stroke? Neurorehabil. Neural Repair 2009, 23, 313–319. [Google Scholar] [CrossRef]
- Cai, S.; Lu, Z.; Chen, B.; Guo, L.; Qing, Z.; Yao, L. Dynamic Gesture Recognition of A-Mode Ultrasonic Based on the DTW Algorithm. IEEE Sens. J. 2022, 22, 17924–17931. [Google Scholar] [CrossRef]
- Permanasari, Y.; Harahap, E.H.; Ali, E.P. Speech Recognition Using Dynamic Time Warping (DTW). J. Phys. Conf. Ser. 2019, 1366, 12091. [Google Scholar] [CrossRef]
- Mohan, B.J.; Babu, N.R. Speech Recognition Using MFCC and DTW. In Proceedings of the 2014 International Conference on Advances in Electrical Engineering (ICAEE), Vellore, India, 9–11 January 2014; IEEE: Vellore, India, 2014; pp. 1–4. [Google Scholar]
- Zhi-Qiang, H.; Jia-Qi, Z.; Xin, W.; Zi-Wei, L.; Yong, L. Improved Algorithm of DTW in Speech Recognition. IOP Conf. Ser.Mater. Sci. Eng. 2019, 563, 52072. [Google Scholar] [CrossRef]
- Yadav, M.; Alam, M.A. Dynamic Time Warping (Dtw) Algorithm in Speech: A Review. Int. J. Res. Electron. Comput. Eng. 2018, 6, 524–528. [Google Scholar]
- Yuan, Y.; Chen, Y.-P.P.; Ni, S.; Xu, A.G.; Tang, L.; Vingron, M.; Somel, M.; Khaitovich, P. Development and Application of a Modified Dynamic Time Warping Algorithm (DTW-S) to Analyses of Primate Brain Expression Time Series. BMC Bioinf. 2011, 12, 347. [Google Scholar] [CrossRef]
- Cavill, R.; Kleinjans, J.; Briedé, J.-J. DTW4Omics: Comparing Patterns in Biological Time Series. PLoS ONE 2013, 8, e71823. [Google Scholar] [CrossRef]
- Furlanello, C.; Merler, S.; Jurman, G. Combining Feature Selection and DTW for Time-Varying Functional Genomics. IEEE Trans. Signal Process. 2006, 54, 2436–2443. [Google Scholar] [CrossRef]
- Han, T.; Peng, Q.; Zhu, Z.; Shen, Y.; Huang, H.; Abid, N.N. A Pattern Representation of Stock Time Series Based on DTW. Phys. A 2020, 550, 124161. [Google Scholar] [CrossRef]
- Han, M. Systematic Financial Risk Detection Based on DTW Dynamic Algorithm and Sensor Network. Meas. Sens. 2024, 34, 101257. [Google Scholar] [CrossRef]
- Tsinaslanidis, P.E.; Alexandridis, A.; Zapranis, A.; Livanis, E. Dynamic Time Warping as a Similarity Measure: Applications in Finance. In Proceedings of the Hellenic Finance and Accounting Association, Volos, Greece, 12–13 December 2014. [Google Scholar]
- Zhang, Z.; Tang, P.; Duan, R. Dynamic Time Warping under Pointwise Shape Context. Inf. Sci. 2015, 315, 88–101. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Gong, Y.; Cheng, Y.; Gao, X.; Chen, X. Motor Function Evaluation of Hemiplegic Upper-Extremities Using Data Fusion from Wearable Inertial and Surface EMG Sensors. Sensors 2017, 17, 582. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Li, J.; Wang, T.; Wang, J.; Pi, S.; Li, H.; Xi, X. Evaluation of Movement Functional Rehabilitation after Stroke: A Study via Graph Theory and Corticomuscular Coupling as Potential Biomarker. Math. Biosci. Eng. 2023, 20, 10530–10551. [Google Scholar] [CrossRef] [PubMed]
- Strojnik, V.; Komi, P.V. Neuromuscular Fatigue after Maximal Stretch-Shortening Cycle Exercise. J. Appl. Physiol. 1998, 84, 344–350. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, X.; Du, Z.; Yuan, Y.; Li, X.; Xie, P. Multi-Scale Coupling between LFP and EMG in Mice by Low-Intensity Pulsed Ultrasound Stimulation with Different Number of Tone-Burst. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 33, 1118–1125. [Google Scholar] [CrossRef]
- Kim, E.; Kum, J.; Lee, S.H.; Kim, H. Development of a Wireless Ultrasonic Brain Stimulation System for Concurrent Bilateral Neuromodulation in Freely Moving Rodents. Front. Neurosci. 2022, 16, 1011699. [Google Scholar] [CrossRef]
- Li, X.-H.; Guo, D.; Chen, L.-Q.; Chang, Z.-H.; Shi, J.-X.; Hu, N.; Chen, C.; Zhang, X.-W.; Bao, S.-Q.; Chen, M.-M.; et al. Low-Intensity Ultrasound Ameliorates Brain Organoid Integration and Rescues Microcephaly Deficits. Brain 2024, 147, 3817–3833. [Google Scholar] [CrossRef]
- Song, D.; Chen, X.; Zhou, N.; Yuan, Y.; Geng, S.; Zhang, C.; Zhao, Z.; Wang, X.; Bao, X.; Lan, X.; et al. Low-Intensity Pulsed Ultrasound Triggers a Beneficial Neuromodulation in Dementia Mice with Chronic Cerebral Hypoperfusion via Activation of Hippocampal Fndc5/Irisin Signaling. J. Transl. Med. 2023, 21, 139. [Google Scholar] [CrossRef]
- Remsik, A.B.; Williams, L.; Gjini, K.; Dodd, K.; Thoma, J.; Jacobson, T.; Walczak, M.; McMillan, M.; Rajan, S.; Young, B.M.; et al. Ipsilesional Mu Rhythm Desynchronization and Changes in Motor Behavior Following Post Stroke BCI Intervention for Motor Rehabilitation. Front. Neurosci. 2019, 13, 53. [Google Scholar] [CrossRef]
- Wang, I.-T.J.; Allen, M.; Goffin, D.; Zhu, X.; Fairless, A.H.; Brodkin, E.S.; Siegel, S.J.; Marsh, E.D.; Blendy, J.A.; Zhou, Z. Loss of CDKL5 Disrupts Kinome Profile and Event-Related Potentials Leading to Autistic-like Phenotypes in Mice. Proc. Natl. Acad. Sci. USA 2012, 109, 21516–21521. [Google Scholar] [CrossRef]
- Heinemann, U.; Buchheim, K.; Gabriel, S.; Kann, O.; Kovacs, R.; Schuchmann, S. Cell Death and Metabolic Activity during Epileptiform Discharges and Status Epilepticus in the Hippocampus. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2002; Volume 135, pp. 197–210. ISBN 978-0-444-50814-0. [Google Scholar]
- Reed, E.S. Neural Regulation of Adaptive Behavior. Ecol. Psychol. 1989, 1, 97–117. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Kamesar, A. Heksor: The Central Nervous System Substrate of an Adaptive Behaviour. J. Physiol. 2022, 600, 3423–3452. [Google Scholar] [CrossRef]
- Voss, P.; Thomas, M.E.; Cisneros-Franco, J.M.; De Villers-Sidani, É. Dynamic Brains and the Changing Rules of Neuroplasticity: Implications for Learning and Recovery. Front. Psychol. 2017, 8, 1657. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; Sur, M.; Dobkin, B.H.; O’Brien, C.; Sanger, T.D.; Trojanowski, J.Q.; Rumsey, J.M.; Hicks, R.; Cameron, J.; Chen, D.; et al. Harnessing Neuroplasticity for Clinical Applications. Brain 2011, 134, 1591–1609. [Google Scholar] [CrossRef]
- Bottasso, E. Toward the Existence of a Sympathetic Neuroplasticity Adaptive Mechanism Influencing the Immune Response. A Hypothetical View—Part II. Front. Endocrinol. 2019, 10, 633. [Google Scholar] [CrossRef]
- Tamiya, S.; Yoshida, Y.; Harada, S.; Nakamoto, K.; Tokuyama, S. Establishment of a Central Post-Stroke Pain Model Using Global Cerebral Ischaemic Mice. J. Pharm. Pharmacol. 2013, 65, 615–620. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Chen, X.; Du, Z.; Yuan, Y.; Li, X.; Xie, P. An Evaluation Model for Brain Ischemia Protection in Mice by Low-Intensity Pulsed Ultrasound Stimulation Based on Functional Cortico-Muscular Coupling. Bioengineering 2025, 12, 541. https://doi.org/10.3390/bioengineering12050541
Jin Z, Chen X, Du Z, Yuan Y, Li X, Xie P. An Evaluation Model for Brain Ischemia Protection in Mice by Low-Intensity Pulsed Ultrasound Stimulation Based on Functional Cortico-Muscular Coupling. Bioengineering. 2025; 12(5):541. https://doi.org/10.3390/bioengineering12050541
Chicago/Turabian StyleJin, Ziqiang, Xiaoling Chen, Zechuan Du, Yi Yuan, Xiaoli Li, and Ping Xie. 2025. "An Evaluation Model for Brain Ischemia Protection in Mice by Low-Intensity Pulsed Ultrasound Stimulation Based on Functional Cortico-Muscular Coupling" Bioengineering 12, no. 5: 541. https://doi.org/10.3390/bioengineering12050541
APA StyleJin, Z., Chen, X., Du, Z., Yuan, Y., Li, X., & Xie, P. (2025). An Evaluation Model for Brain Ischemia Protection in Mice by Low-Intensity Pulsed Ultrasound Stimulation Based on Functional Cortico-Muscular Coupling. Bioengineering, 12(5), 541. https://doi.org/10.3390/bioengineering12050541







